1. Introduction
Deception is a ubiquitous feature of life [
1,
2]; evolutionarily speaking, deception has been selected to improve escape from natural enemies [
1,
3] and increase reproduction (as in pollination by deceit [
1]) or food acquisition [
1]. A fascinating example of interspecific deception is aggressive mimicry, which occurs when “the mimic signals a fitness benefit to the receiver and the mimic’s signal is deceptive” ([
4], p. 4). Aggressive mimicry as an aid to capture prey has been described in a wide range of animals, including spiders [
5], aphids [
6], katydids [
7], fish [
8,
9], and
Homo sapiens [
10]. In North America, predatory firefly females from species belonging to three genera of the subfamily Photurinae (
Bicellonycha,
Crematogaster, and
Photuris) emit bioluminescent responses to the flashing signals of males of other firefly species (several species of
Photinus are common prey, although males from other genera, including other
Photuris species, are also hunted); in this way these “femmes fatales” attract the males and then try to capture them and feed on them [
11,
12]. By feeding on the males of other species, predatory females not only obtain nutrients and energy but also defensive steroid pyrones called lucibufagins (molecules that
Photuris cannot produce) that protect the female [
13] and her eggs [
14] from predators. Thus, firefly femmes fatales [
11,
12,
15] are classical and extreme examples of aggressive mimicry: classical because the femme fatale sends fake signals indicating the presence of a sexually receptive female and extreme because it can result in the deceived individual being killed.
In many cases,
Photuris females mimic the specific response signals of the females of the prey species [
12,
15], and individual females of some species have been shown to mimic the specific response of females from several species depending on the identity of the potential prey [
12,
15,
16]. These studies led the most important researcher of Photurinae and femme fatale behavior, the late Prof. James Lloyd, to think that femmes fatales are the source of selective pressures that have been crucial for the evolutionary divergence of bioluminescent signals in prey firefly species of the American continent ([
15]; see also [
12,
15,
17,
18]). Females of predatory
Photuris have morphological adaptations apparently designed to subdue and consume their prey [
19], and recent molecular studies have identified genes involved in resistance to lucibufagins [
20] and several candidate genes that could represent diverse physiological adaptations for the consumption of potentially toxic prey [
21]. Thus, it is expected that
Photuris predation is the source of selective pressures promoting the evolution of countermeasures in their prey fireflies [
15,
18], which resulted in a coevolutionary process that could be responsible for the complexity and plasticity frequently observed in the communication systems of fireflies [
12,
16,
18,
22,
23,
24,
25,
26]. Since, in many places, several potential prey firefly species coexist, sometimes with more than one species of femmes fatales, the coevolutionary process, rather than a product of pairwise interactions, is more likely to be of the type known as “diffuse coevolution” [
27,
28]. In this context, some prey counter-adaptations could select for an increased degree of deceptiveness in the signals produced by
Photuris females. This has led to the study of the match between the response signals produced by females of prey species and the deceptive responses emitted by femmes fatales, implicitly considering the degree of match as a measure of the degree of deceptiveness (see review in [
12]). However, a perfect copy of the signal is not necessary for successful mimicry; instead, only the components used by the receiver to assess the signal need to be mimicked [
4].
On the other hand, since aggressive mimicry selects for male prey counterstrategies to avoid being attracted and captured, a more direct way of measuring the efficiency of the deceptive signals of
Photuris females is measuring their success in attracting, capturing, and consuming prey. These measurements are more directly related to the selective pressures exerted and the adaptations developed by both predator and prey. When the courting areas of femmes fatales and prey fireflies do not overlap, it is reasonable to expect that hunting females move to places where the probability of finding prey is higher and then start to attract and attempt to capture prey. Previous studies (summarized in [
12]) and a recent study of
Photuris lugubris [
29] indicate that mated females move from their own courtship display areas to, or close to, the courtship display areas of their prey fireflies. However, the consequences of this movement for prey encounters, although apparently obvious, have not been quantified. Similarly, rates of prey attraction and capture by firefly femmes fatales are rarely reported. Lloyd [
16] studied
Photuris versicolor hunting two species of
Photinus and two species of
Photuris in the field and observed wide interspecific variation in the rates of capture of the different prey species (4%, 9%, 20%, and 54%). However, Lloyd did not report or discuss the causes of this variation. In a laboratory experiment, Lewis et al. [
30] observed large differences in the consumption rates of females of several
Photuris species confined with males from eight species of Lampyridae, including five species of
Photinus (one of them,
P. carolinus, a synchronous firefly). The causes of this variation were not determined, but the fact that the species with low consumption rates showed evidence of having been attacked suggests between-prey differences in defensive chemistry, nutritional value, or ability to escape once captured. Furthermore, since only 11 females from at least three
Photuris species were studied, interspecific differences in hunting ability cannot be ruled out. Finally, Lloyd [
12] also reported an observation of one female
Photuris carrorum that answered the signals of twenty-five male
Photinus macdermotti before capturing one (a success rate of 3.8%), although he did not mention if the twenty-five males that were not captured approached the female or if they were unsuccessfully attacked.
In this paper, we report field measurements of rates of attraction and capture for male
Photinus palaciosi, a synchronous firefly from the mountains of Central Mexico [
31], by
Photuris lugubris femmes fatales [
29]. This study is part of a broader project aimed at understanding the evolution of the femme fatale behavior of Photurinae fireflies.
3. Results
A total of 92 observations of Photuris females were made. Since the females were not marked, it is not known how many different females were studied. Some females were observed in, or very close to, places where females were observed on previous nights, and for this reason, we suspect that at least some females were observed on more than one night. The total time looking for females was 153.2 h, and the amount of time observing Photuris females was 56.8 h, for a total of 210 h of fieldwork.
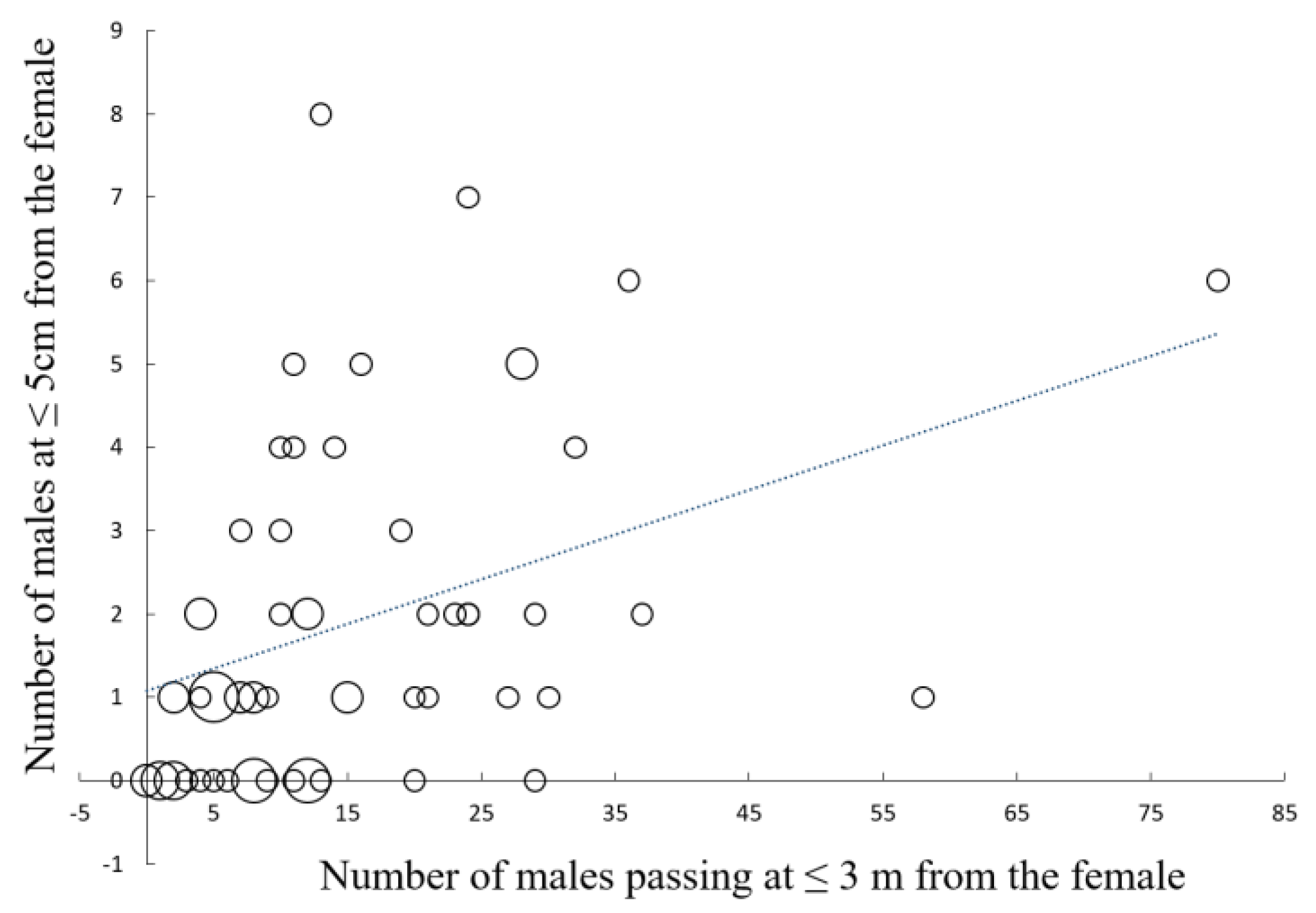
Prey density, measured as the number of male
Photinus observed flying at 3 m or less from the female
Photuris, had a positive effect on the number of males that approached the female close enough to risk being attacked (≤5 cm) (
Figure 1;
r = 0.46,
p < 0.00005,
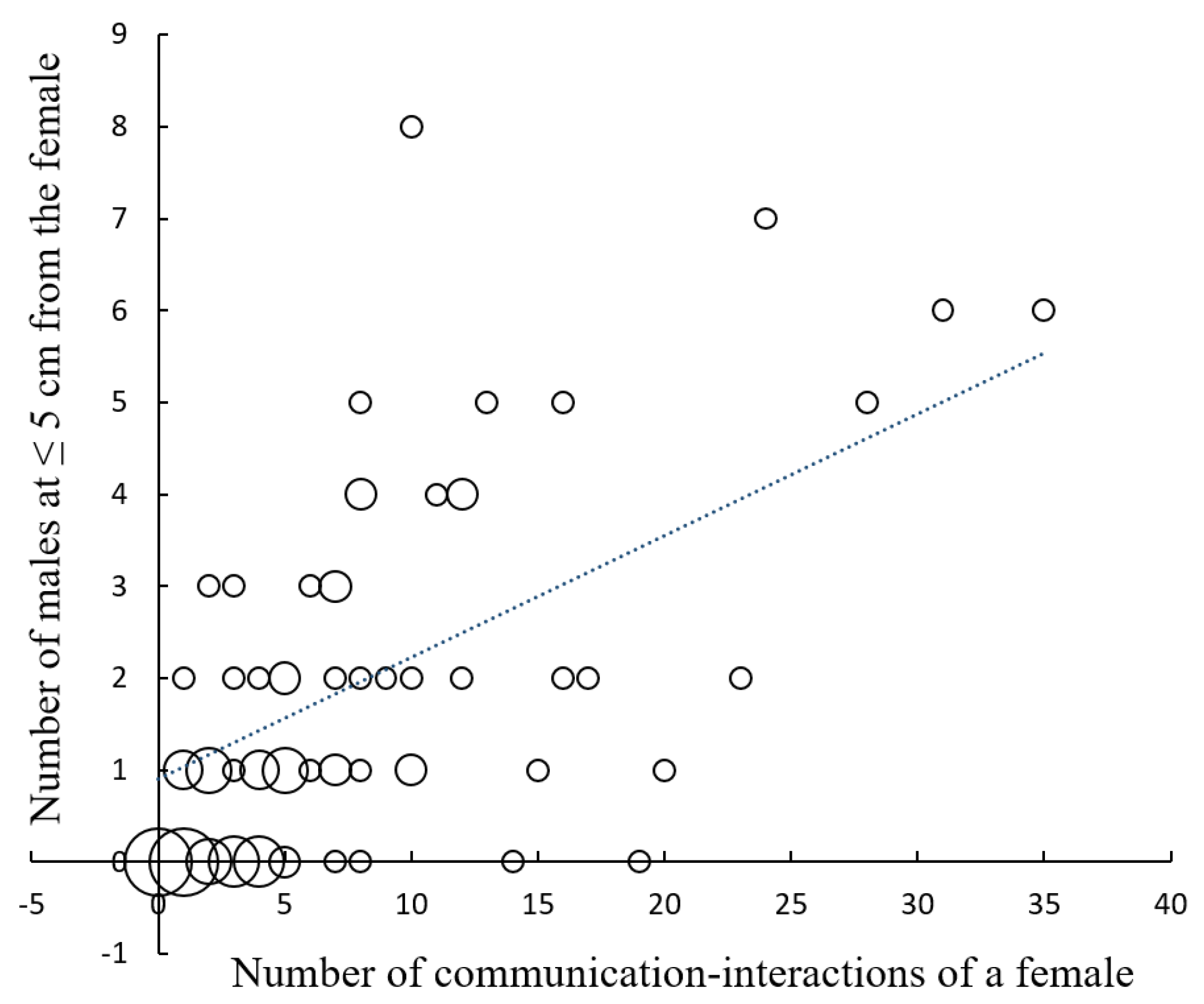
n = 71; sample size is less than 92 because one of the observers did not record the number of males passing at a distance ≤ 3 m). The number of communication events, a direct measure of aggressive mimicry, also had a positive effect on the number of males that approached a female
Photuris at attack distance (
Figure 2;
r = 0.65,
p < 0.00001,
n = 71). Although in some cases males approached females without a previous communication event (for example, sometimes they appeared to be attracted by the flashes of other males close to the female), in 83 of the 92 observations (90.2%), there was at least one communication event between a female
Photuris and a male
Photinus.
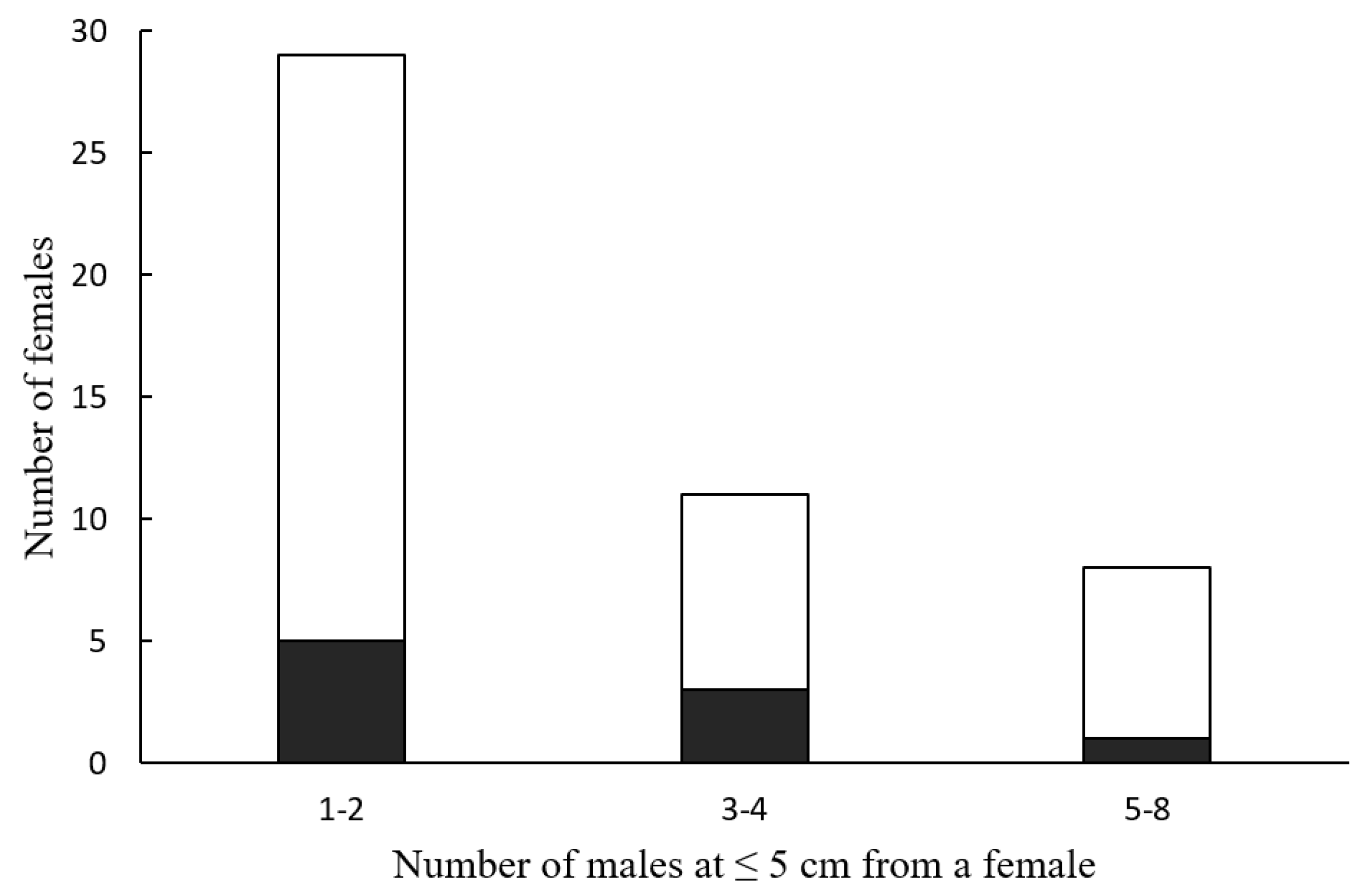
Only 9 of the 92 observations (9.8%) ended in successful hunting. The number of successful attacks was independent of the number of
Photinus males that approached a female
Photuris at an attack distance (≤5 cm) (
Figure 3; Fisher’s exact probability test,
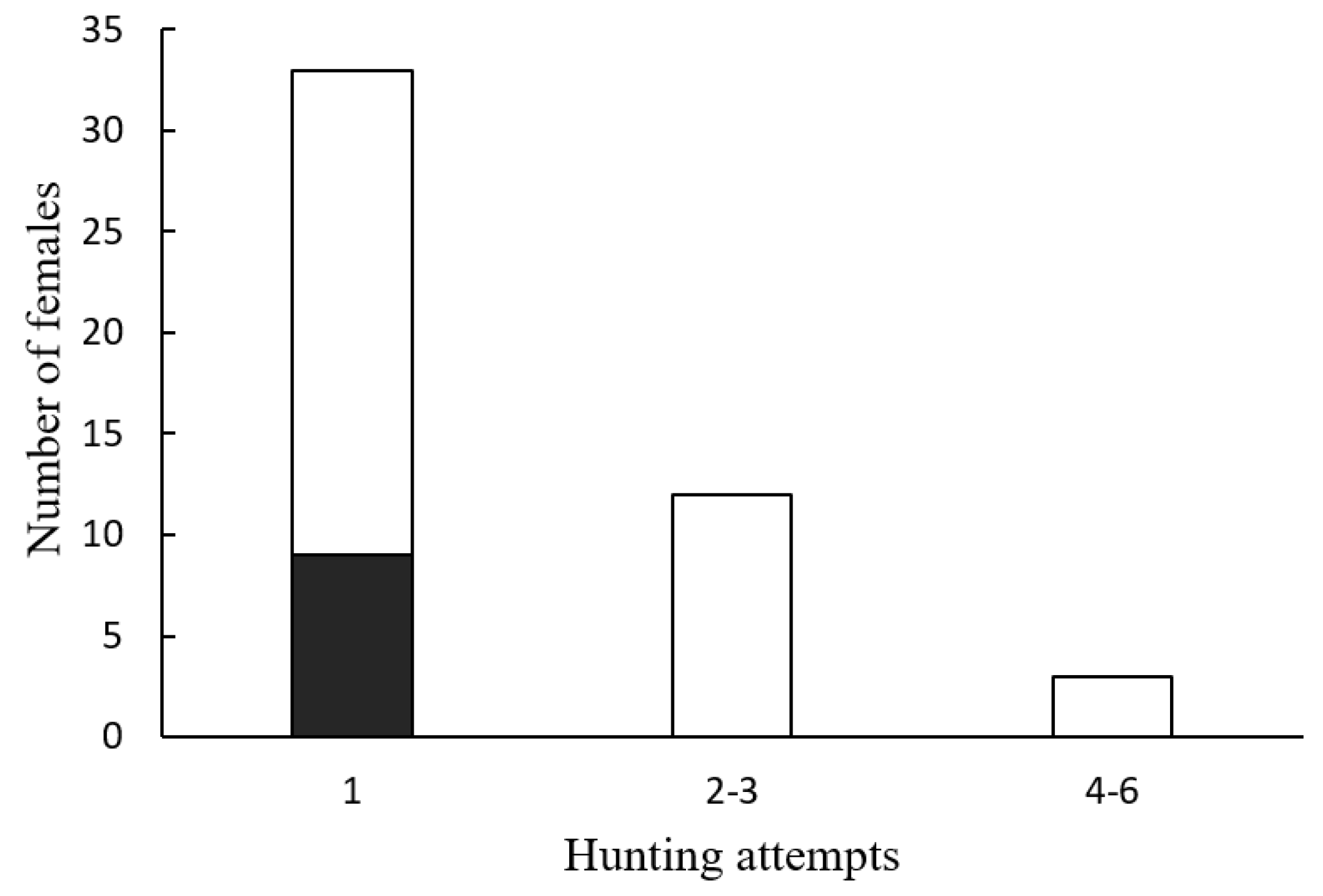
p = 1). Although females were observed attacking males up to six times in a night, all nine successful hunts occurred in the first attack of the night (
Figure 4; Fisher’s exact probability test,
p = 0.041).
The behavior frequently observed during Photuris–Photinus interactions helps us understand the low rates of hunting success. Photinus males attracted by Photuris females behave as if they are “hesitant” (or very “cautious”) to approach Photuris females because they frequently remain in flight exchanging signals for several minutes at a relatively close range (<1 m) but without further approaching. This behavior is different from the direct male approaches observed when Photinus males court females of their own species (personal observations). Also, males frequently, upon alighting, approach the females slowly, in contrast to the faster approximations observed in sexual interactions between male and female Photinus (personal observations). Generally, when a female Photuris approaches a Photinus male that is flying or perching close to her, he suddenly “drops” several centimeters, possibly to avoid contact; in these cases, the female does not follow the male. On the other hand, hunting females appear to partially conceal their lanterns by pushing them against the surfaces of their perches (usually leaves), which makes sense considering that female Photuris are much larger than Photinus females, although this behavior could also help them to mimic the response pattern. Additionally, sometime before an attack, females usually turn off their lanterns. This behavior led one of the reviewers of this paper to the reasonable suggestion that the hunting behavior of Photuris is best described as a combination of aggressive mimicry and, in the instants before the attack, stalking.
Besides the nine females observed hunting and feeding on their prey, four other
Photuris females were found already feeding on a male
Photinus. Most of the observations of successful hunting occurred during the first 10 days of observation (10/13;
Figure 5), approximately corresponding to the first half of the sampling period. Since there were fewer observers per night in the second half of the study season (
Figure 5), this number could be an underestimate, although the observers had the impression that the number of predatory females was decreasing during the second half of the study period. Most of the successful attacks (8/9) were observed before 21:00 h (
Figure 5), which is consistent with the observation that successful attacks were the first attacks of the night (
Figure 4).
4. Discussion
Since the courting and mating area of
Photuris in the study site is very small and barely overlaps with the courting area of
Photinus, hunting females move to, or close to, the courting areas of their prey species [
29]. The observations reported here indicate that the relocation of
Photuris females during the hunting period is important to attracting more potential prey (
Figure 1). Previous studies (summarized in [
12]) indicate that mated females of other predatory firefly species also move from their own courtship display areas to, or close to, the courtship display areas of their prey fireflies; however, the quantitative data (see
Supplementary Materials) presented here are the first documenting a possible advantage in female relocation. The location of females within, beside, or between the congregation sites of
Photinus males—as well as variations in the number of males in these congregations and in the number of responding
Photinus females and other “competing”
Photuris females—could result in variations in the number of males interacting with females. The results also provide one of the few published quantitative measures of the importance of firefly aggressive mimicry for attracting prey since
Photuris females attract
Photinus males mainly by responding to their flashing signals (
Figure 2). However, males apparently are also attracted to other males flashing at
Photuris females in close range (see [
35] for a similar observation). We observed that the glowing responses of
Photuris females to males of their own species are different from the signals used to attract
Photinus males (personal observations). It is also interesting that
Photuris females apparently partially conceal their lanterns by pushing them against the surfaces of their perches as if trying to look smaller, like
Photinus females. It will be interesting to test this hypothesis. These observations are consistent with the existence of aggressive mimicry in this species. Detailed studies comparing the courtship signals of both species and the hunting signals of
Photuris are currently underway.
Although
Photuris femmes fatales attract a considerable number of potential prey at attack distance (
Figure 2), their hunting success is low, as only 10% of the females were observed capturing and feeding on a male
Photinus (
Figure 3). These observations are consistent with low success rates documented in most of the few previous studies on attraction and hunting success in
Photuris, as noted in the Introduction section. Some species of
Photuris femmes fatales have been observed employing alternative hunting tactics [
12,
17,
30,
36,
37], and thus, if
P. lugubris females also exhibit some of these behaviors, it is possible that the low success rate documented in this study could be an underestimation of the actual rates of capture. Two of these strategies involve eavesdropping on the bioluminescent signals produced by their prey: “stalking” and “aerial hawking” [
12,
17,
30,
36]. As mentioned in the Results section, in the moments before attacking a deceptively attracted
Photinus, female
Photuris turn off their lanterns and attack, a behavior that led one of the reviewers of this paper to suggest that the hunting tactic of
P. lugubris is a combination of aggressive mimicry and stalking. However, we have never observed a female
Photuris stalking a prey firefly that she did not attract after it responded to her signals. It can be argued that documenting this behavior requires the continuous observation of perched signaling
Photinus. During the mating season of 2021, we made observations of female
Photinus to describe their courtship behavior and never observed a case of stalking by
Photuris; similar observations of perched males flashing were not made, although this male behavior is relatively rare. With respect to “aerial hawking”, in three consecutive mating seasons (2021–2023), we never observed flying
Photuris females pursuing and capturing
Photinus males in flight. However, in 2023, two local tour guides described one observation each of behavior suggesting that this “aerial hawking” strategy might be employed by some females, although probably sporadically. It must be mentioned that the tour guides did not confirm that these observations represented examples of aerial hunting. A third “hunting” strategy reported in
Photuris fireflies is the stealing of fireflies trapped in spiderwebs (kleptoparasitism) [
37]. Although it is common to observe
Photinus trapped in spiderwebs (but never
Photuris), frequently with their lanterns on, a case of kleptoparasitism, or even a
Photuris female close to a spiderweb with
Photinus trapped, has never been observed. However, with the information available, it is not possible to discard this hunting strategy or consider it rare. Thus, further studies are necessary to determine if female
Photuris employ other hunting strategies in “Rancho del Valle” and, in case they do, how much prey they obtain in these other ways. What is clear is that many
Photuris females behave as femmes fatales in our study area and that this strategy has a high rate of failure.
Another intriguing observation of the present study is the fact that all successful hunts occurred during the first attack of the femme fatale, while other females failed after several attacks. This could be the result of individual variation in the ability to deceive and capture male prey by female Photuris. Unfortunately, given that female Photuris were not individually identified, it is not possible to assess this idea. Studying the possible existence of this variation and its correlates (genetic, developmental, phenotypic, environmental) seems a promising line of research.
One reviewer of this paper pointed out two potential biases in the prey capture estimates. First, the observation that most successful attacks occurred early in the mating period could bias down the prey capture estimate because, later in the night, successful femmes fatales would be hard to detect because they would be feeding instead of interacting with prey. However, we think this possible bias is not very important because
Photuris females always produce glows, albeit at low rates, when feeding. In fact, thanks to these glows, females already feeding were detected in this study and in a previous one [
29]. Second, if there is individual variation in hunting ability (a reasonable expectation), hunting females observed late in the night would tend to be unsuccessful females that would bias down the overall capture rate estimate. This is true; some successful femmes fatales were probably missed early in the night given the limited number of field researchers.
As mentioned in the Introduction, the aggressive mimicry of female
Photuris is considered a major selective pressure on the communication systems of fireflies in the American continent, and thus, counter-adaptations in prey species are expected to evolve [
18]. The low hunting success of
Photuris femmes fatales found in this and previous studies could be explained by countermeasures that coevolved in the prey [
15,
18]. Although the existence of a very efficient predatory
Photuris species cannot be discarded, the general pattern suggests that an asymmetry in the strength of the selective pressures experienced by predators and prey could explain the low hunting success of
Photuris females and the relatively good ability to escape of the prey fireflies. This asymmetry is known as the “life–dinner principle” [
38], which proposes that selective pressures are stronger in the prey species than in the predator because an unsuccessful predator loses a dinner but unsuccessful prey loses its life. In the present study, although
Photinus males probably paid time, energy (especially when they spent long periods in flight communicating with the predatory females [
36]), and opportunity costs by being attracted by
Photuris females, they were reasonably good at avoiding predation. On the other hand, exploring the possibility of individual variation in
Photinus males in their ability to avoid being deceived and captured by femmes fatales seems an interesting and complementary line of research for the future.
Although a detailed study of the behavioral strategies used by
P. palaciosi males to reduce predation by
P. lugubris females remains to be performed, the “hesitant” and “dropping-when-approached” behaviors observed in males and the “concealing strategies” of females are consistent with the idea of the late Prof. J. Lloyd [
18,
25] that prey fireflies should evolve a variety of strategies aimed at uncovering the identity of the female they are interacting with. In this context, the absence of “reflex bleeding”, a defense strategy present in several fireflies [
39], is intriguing. In three consecutive mating seasons (2021–2023), reflex bleeding was never observed when
Photuris females attacked males, either in the field or in an experimental setting [
29] or when researchers manipulated individuals (both males and females) by hand. In previous studies involving the manipulation of
Photinus males and females by hand in localities where
Photuris is absent [
31,
40] but other predators are present, in the states of Tlaxcala, Puebla, and Estado de México, reflex bleeding was also never observed.
The aggressive mimicry of sexual signals seems to be rare across the tree of life, but the range of organisms exhibiting this behavior is broad. An incomplete list includes female bolas spiders (
Mastophora sp.) mimicking the sexual pheromones of its moth prey
Spodoptera frugiperda [
5] and males and females of the katydid orthopteran
Chlorobalius leucoviridis attracting and capturing males of several species of cicadas by responding with sound signals like those of its prey’s females [
7]. Pollination via sexual deceit in orchids and other flowering plants is similar because of the imitation of sexual signals; however, successful deception does not mean the death of the cheated receiver [
41].