Antennal Sensitivity of Spotted Lanternflies, Lycorma delicatula: Differential Electrophysiological Responses of Males and Females to Compounds Derived from Host Plants and Conspecifics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Chemicals
2.3. Electroantennogram (EAG) Preparation and Recording
2.4. Antennal Sensitivity Recording
2.4.1. Fixed-Dose Relative Response
2.4.2. Dose–Response
2.5. Statistical Analysis
3. Results
3.1. Antennal Sensitivity
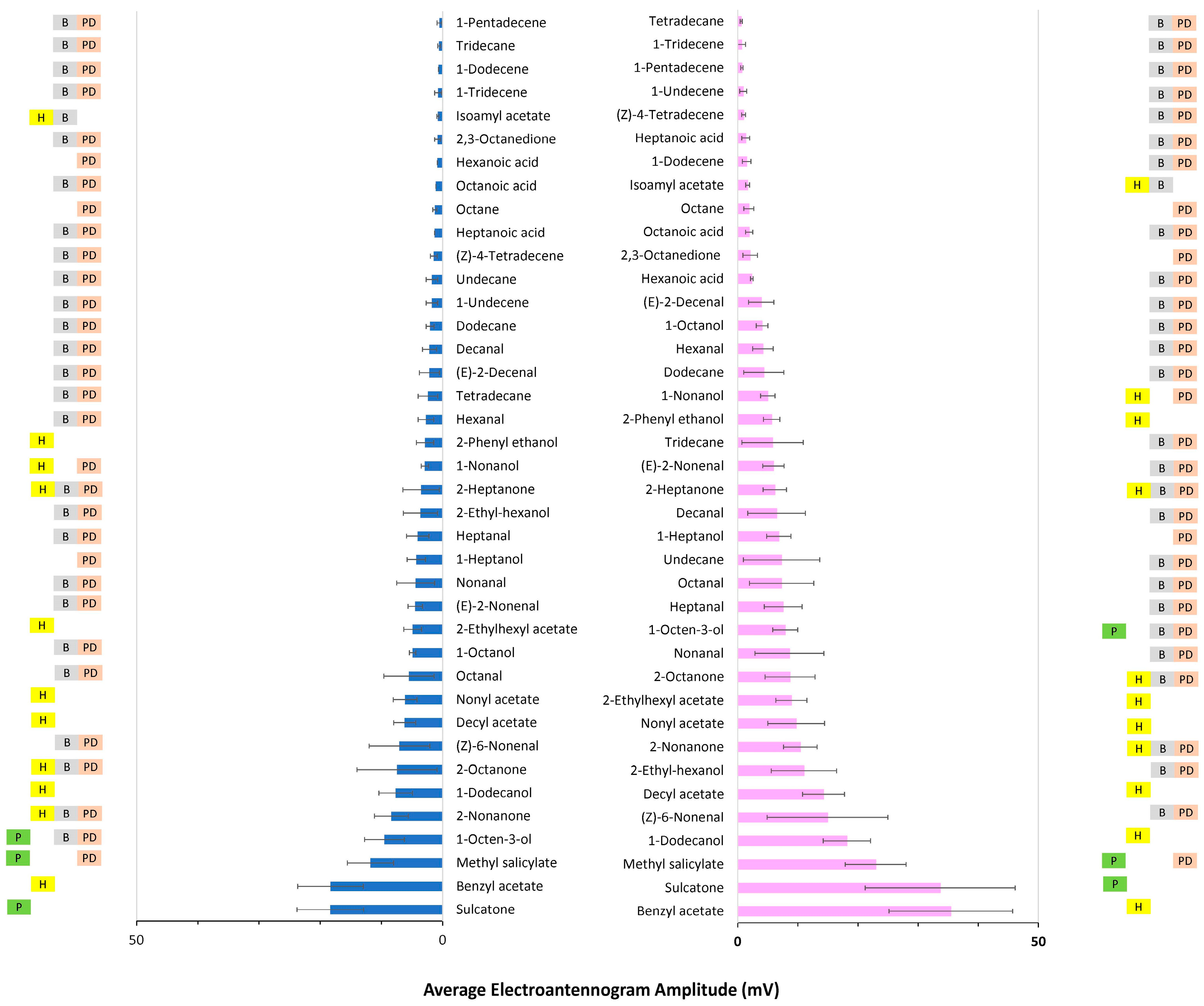
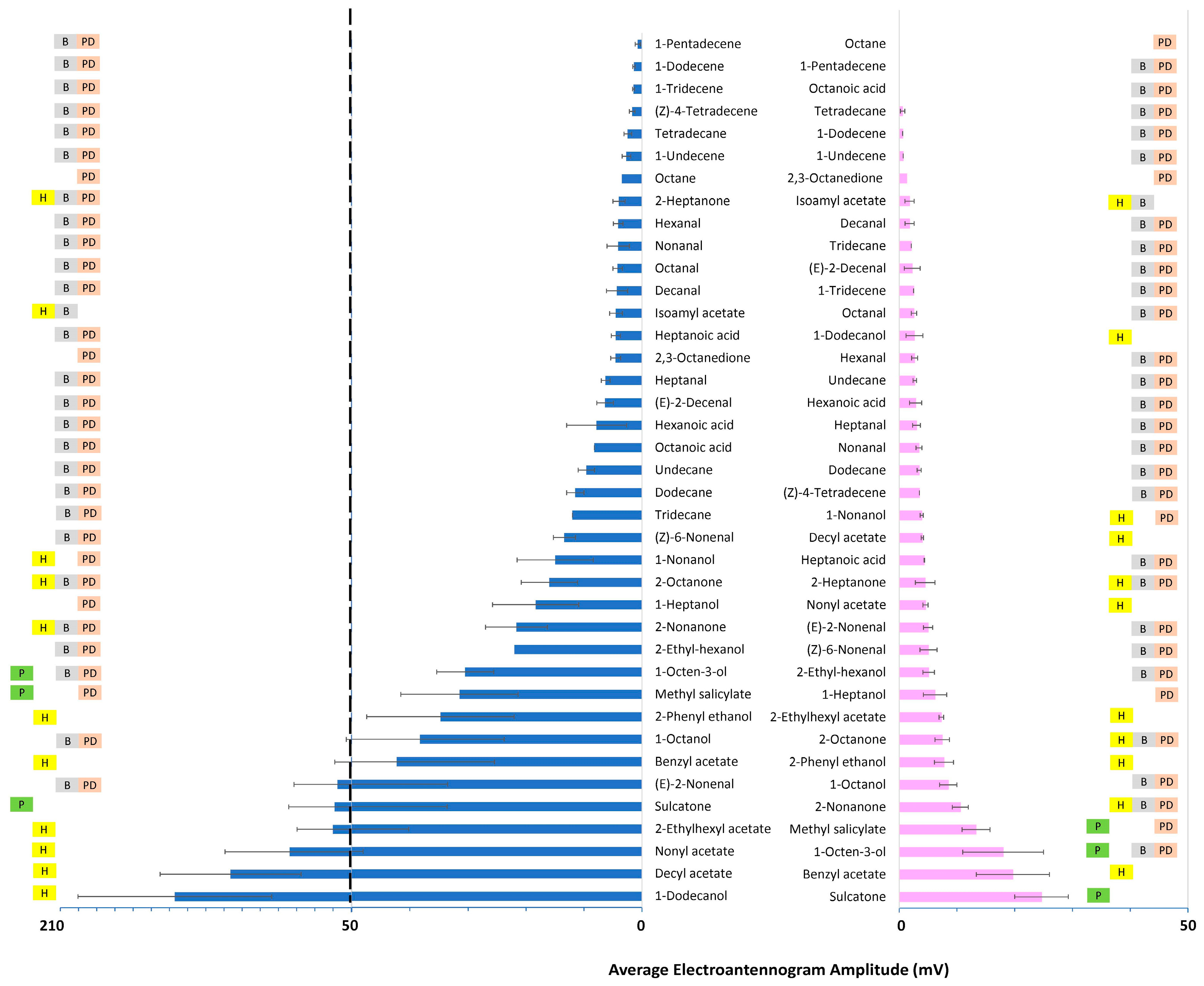
3.1.1. Fixed-Dose Relative Response
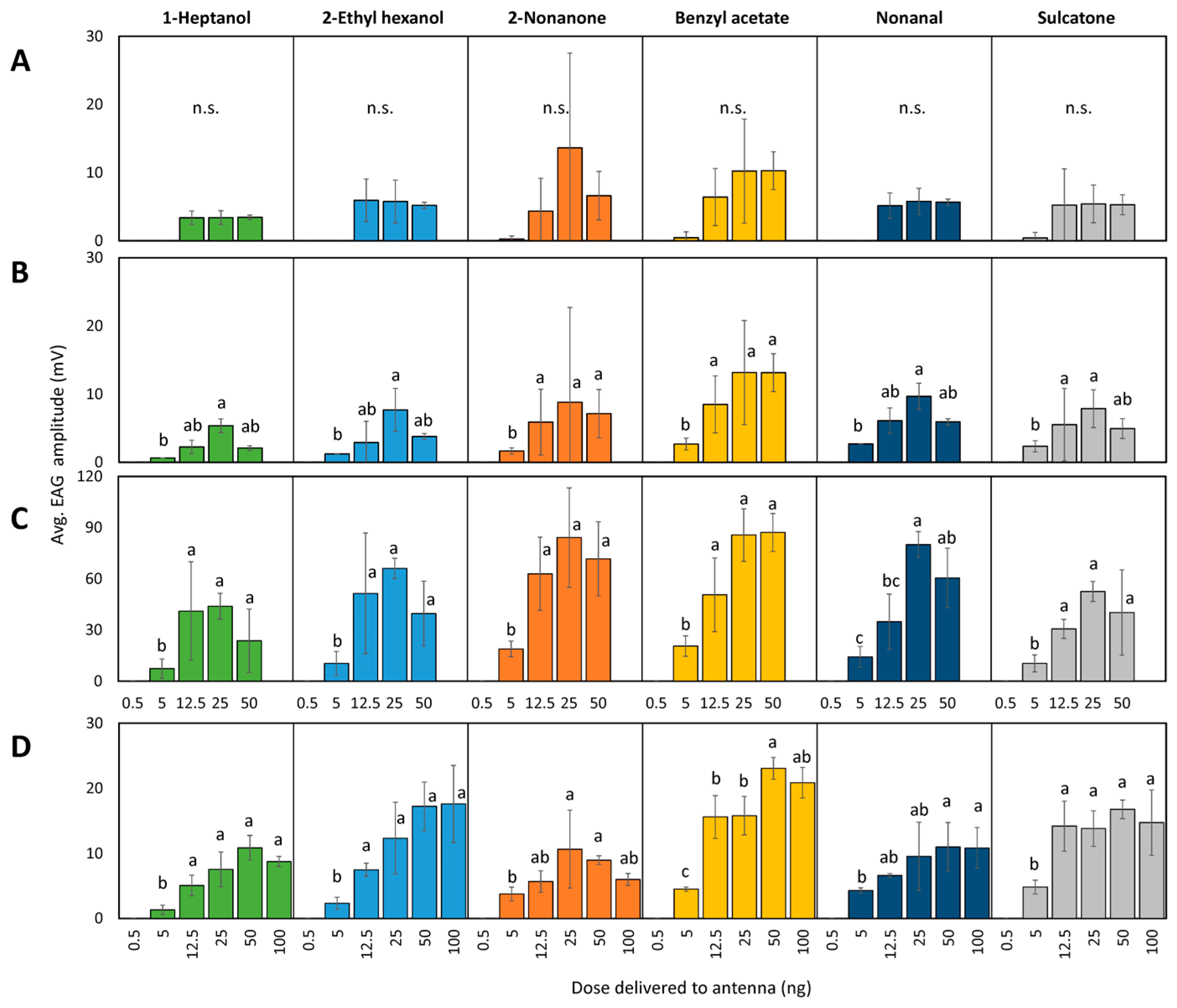
3.1.2. Dose–Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barringer, L.E.; Donovall, L.R.; Spichiger, S.-E.; Lynch, D.; Henry, D. The first new world record of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae). Entomol. News 2015, 125, 20–23. [Google Scholar] [CrossRef]
- Barringer, L.; Ciafré, C.M. Worldwide feeding host plants of spotted lanternfly, with significant additions from North America. Environ. Entomol. 2020, 49, 999–1011. [Google Scholar] [CrossRef]
- Nixon, L.J.; Jones, S.K.; Tang, L.; Urban, J.; Felton, K.; Leskey, T.C. Survivorship and development of the invasive Lycorma delicatula (Hemiptera: Fulgoridae) on wild and cultivated temperate host plants. Environ. Entomol. 2022, 51, 222–228. [Google Scholar] [CrossRef]
- Urban, J.M. Perspective: Shedding light on spotted lanternfly impacts in the USA. Pest Manag. Sci. 2020, 76, 10–17. [Google Scholar] [CrossRef]
- Urban, J.M.; Leach, H. Biology and management of the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae), in the united states. Annu. Rev. Entomol. 2023, 68, 151–167. [Google Scholar] [CrossRef]
- Nation Sr, J.L. Insect Physiology and Biochemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2022; p. 555. [Google Scholar]
- Suh, E.; Bohbot, J.D.; Zwiebel, L.J. Peripheral olfactory signaling in insects. Curr. Opin. Insect Sci. 2014, 6, 86–92. [Google Scholar] [CrossRef]
- El-Shafie, H.A.F.; Faleiro, J.R. Semiochemicals and their potential use in pest management. In Biological Control of Pest and Vector Insects; Intech: London, UK, 2017; Volume 5772. [Google Scholar]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Olsson, S.B.; Hansson, B.S. Electroantennogram and single sensillum recording in insect antennae. In Pheromone Signaling. Methods in Molecular Biology, vol 1068; Humana Press: Totowa, NJ, USA, 2013; pp. 157–177. [Google Scholar]
- Kaissling, K.-E. Pheromone reception in insects: The example of silk moths. In Neurobiology of Chemical Communication; Mucignat-Caretta, C., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 99–146. [Google Scholar]
- Yan, X.; Wang, Z.; Xie, J.; Deng, C.; Sun, X.; Hao, C. Glomerular organization of the antennal lobes of the diamondback moth, Plutella xylostella L. Front. Neuroanat. 2019, 13, 4. [Google Scholar] [CrossRef]
- Ngumbi, E.; Chen, L.; Fadamiro, H. Electroantennogram (EAG) responses of Microplitis croceipes and Cotesia marginiventris and their lepidopteran hosts to a wide array of odor stimuli: Correlation between EAG response and degree of host specificity? J. Insect Physiol. 2010, 56, 1260–1268. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Wickham, J.D.; Warden, M.L. Factors guiding the orientation of nymphal spotted lanternfly, Lycorma delicatula. Insects 2023, 14, 279. [Google Scholar] [CrossRef]
- Faal, H.; Cooperband, M.F.; Canlas, I.; Carrillo, D. Evidence of pheromone use in a fulgorid, spotted lanternfly. Forests 2022, 13, 1639. [Google Scholar] [CrossRef]
- Faal, H.; Meier, L.R.; Canlas, I.J.; Murman, K.; Wallace, M.; Carrillo, D.; Cooperband, M.F. Volatiles from male honeydew excretions attract conspecific male spotted lanternflies, Lycorma delicatula (Hemiptera: Fulgoridae). Front. Insect Sci. 2022, 2, 982965. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Murman, K. Responses of adult spotted lanternflies to artificial aggregations composed of all males or females. Front. Insect Sci. 2022, 2, 981832. [Google Scholar] [CrossRef]
- Derstine, N.T.; Meier, L.; Canlas, I.; Murman, K.; Cannon, S.; Carrillo, D.; Wallace, M.; Cooperband, M.F. Plant volatiles help mediate host plant selection and attraction of the spotted lanternfly (Hemiptera: Fulgoridae): A generalist with a preferred host. Environ. Entomol. 2020, 49, 1049–1062. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Wickham, J.; Cleary, K.; Spichiger, S.-E.; Zhang, L.; Baker, J.; Canlas, I.; Derstine, N.; Carrillo, D. Discovery of three kairomones in relation to trap and lure development for spotted lanternfly (Hemiptera: Fulgoridae). J. Econ. Entomol. 2019, 112, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Faal, H.; Canlas, I.J.; Cossé, A.; Jones, T.H.; Carrillo, D.; Cooperband, M.F. Investigating photo-degradation as a potential pheromone production pathway in spotted lanternfly, Lycorma delicatula. Insects 2023, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Murman, K.; Setliff, G.P.; Pugh, C.V.; Toolan, M.J.; Canlas, I.; Cannon, S.; Abreu, L.; Fetchen, M.; Zhang, L.; Warden, M.L. Distribution, survival, and development of spotted lanternfly on host plants found in North America. Environ. Entomol. 2020, 49, 1270–1281. [Google Scholar] [CrossRef]
- Sant’ana, J.; Dickens, J.C. Comparative electrophysiological studies of olfaction in predaceous bugs, Podisus maculiventris and P. nigrispinus. J. Chem. Ecol. 1998, 24, 965–984. [Google Scholar] [CrossRef]
- Keena, M.A.; Hamilton, G.; Kreitman, D. The potential climatic range of spotted lanternfly may be broader than previously predicted. Front. Insect Sci. 2023, 3, 1092189. [Google Scholar] [CrossRef]
- Wang, R.-R.; Liu, J.-J.; Li, X.-Y.; Liang, A.-P.; Bourgoin, T. Relating antennal sensilla diversity and possible species behaviour in the planthopper pest Lycorma delicatula (Hemiptera: Fulgoromorpha: Fulgoridae). PLoS ONE 2018, 13, e0194995. [Google Scholar] [CrossRef]
- Beck, J.J.; Light, D.M.; Gee, W.S. Electrophysiological responses of male and female Amyelois transitella antennae to pistachio and almond host plant volatiles. Entomol. Exp. Appl. 2014, 153, 217–230. [Google Scholar] [CrossRef]
- Fraser, A.M.; Mechaber, W.L.; Hildebrand, J.G. Electroantennographic and behavioral responses of the sphinx moth Manduca sexta to host plant headspace volatiles. J. Chem. Ecol. 2003, 29, 1813–1833. [Google Scholar] [CrossRef]
- Pérez-Aparicio, A.; Torres-Vila, L.M.; Gemeno, C. EAG responses of adult Lobesia botrana males and females collected from Vitis vinifera and Daphne gnidium to larval host-plant volatiles and sex pheromone. Insects 2019, 10, 281. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Il’Ichev, A.L.; Gut, L.J. Antennal and behavioral responses of virgin and mated oriental fruit moth (Lepidoptera: Tortricidae) females to their sex pheromone. Ann. Entomol. Soc. Am. 2006, 99, 898–904. [Google Scholar] [CrossRef]
- Raguso, R.A.; Light, D.M.; Pickersky, E. Electroantennogram responses of Hyles lineata (Sphingidae: Lepidoptera) to volatile compounds from Clarkia breweri (Onagraceae) and other moth-pollinated flowers. J. Chem. Ecol. 1996, 22, 1735–1766. [Google Scholar] [CrossRef]
- Spaethe, J.; Brockmann, A.; Halbig, C.; Tautz, J. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften 2007, 94, 733–739. [Google Scholar] [CrossRef]
- Selzer, R. On the specificities of antennal olfactory receptor cells of Periplaneta americana. Chem. Senses 1984, 8, 375–395. [Google Scholar] [CrossRef]
- Vermeulen, A.; Rospars, J.-P. Why are insect olfactory receptor neurons grouped into sensilla? The teachings of a model investigating the effects of the electrical interaction between neurons on the transepithelial potential and the neuronal transmembrane potential. Eur. Biophys. J. 2004, 33, 633–643. [Google Scholar] [CrossRef]
- Pellegrino, M.; Nakagawa, T.; Vosshall, L.B. Single sensillum recordings in the insects Drosophila melanogaster and Anopheles gambiae. JoVE (J. Vis. Exp.) 2010, 36, e1725. [Google Scholar]
- Hao, Y.; Dietrich, C.H.; Dai, W. Structure and sensilla of the mouthparts of the spotted lanternfly Lycorma delicatula (Hemiptera: Fulgoromorpha: Fulgoridae), a polyphagous invasive planthopper. PLoS ONE 2016, 11, e0156640. [Google Scholar] [CrossRef]
- Syed, Z. Chemical ecology and olfaction in arthropod vectors of diseases. Curr. Opin. Insect Sci. 2015, 10, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Van Giessen, W.A.; Fescemyer, H.W.; Burrows, P.M.; Peterson, J.K.; Barnett, O.W. Quantification of electroantennogram responses of the primary rhinaria of Acyrthosiphon pisum (Harris) to C4-C8 primary alcohols and aldehydes. J. Chem. Ecol. 1994, 20, 909–927. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.S.; Mankin, R.W.; Lemire, G.F. Quantitation of the insect electroantennogram: Measurement of sensillar contributions, elimination of background potentials, and relationship to olfactory sensation. J. Insect Physiol. 1984, 30, 757–763. [Google Scholar] [CrossRef]
- Jacob, V.E.J.M. Current source density analysis of electroantennogram recordings: A tool for mapping the olfactory response in an insect antenna. Front. Cell. Neurosci. 2018, 12, 287. [Google Scholar] [CrossRef]

| Stage | Sex | Compound | p-Value | F-Ratio | d.f. | N |
|---|---|---|---|---|---|---|
| Fourth instar | Male | 1-Heptanol | 0.956 | 0.05 | 2, 9 | 12 |
| 2-Ethyl-hexanol | 0.980 | 0.02 | 2, 9 | 12 | ||
| 2-Nonanone | 0.255 | 1.61 | 3, 9 | 13 | ||
| Benzyl acetate | 0.081 | 3.11 | 3, 9 | 13 | ||
| Nonanal | 0.786 | 0.25 | 2, 9 | 12 | ||
| Sulcatone | 0.403 | 1.09 | 3, 9 | 13 | ||
| Female | 1-Heptanol | 0.013 | 5.80 | 3, 11 | 15 | |
| 2-Ethyl-hexanol | 0.012 | 5.93 | 3, 11 | 15 | ||
| 2-Nonanone | <0.001 | 14.72 | 3, 12 | 16 | ||
| Benzyl acetate | <0.001 | 28.23 | 3, 12 | 16 | ||
| Nonanal | 0.039 | 3.85 | 3, 12 | 16 | ||
| Sulcatone | 0.002 | 8.96 | 3, 12 | 16 | ||
| Adult | Male | 1-Heptanol | 0.002 | 8.31 | 3, 13 | 17 |
| 2-Ethyl-hexanol | 0.001 | 12.34 | 3, 12 | 16 | ||
| 2-Nonanone | <0.001 | 21.75 | 3, 13 | 17 | ||
| Benzyl acetate | <0.001 | 19.19 | 3, 13 | 17 | ||
| Nonanal | <0.001 | 15.04 | 3, 13 | 17 | ||
| Sulcatone | <0.001 | 12.90 | 3, 13 | 17 | ||
| Female | 1-Heptanol | <0.001 | 20.42 | 4, 10 | 15 | |
| 2-Ethyl-hexanol | <0.001 | 17.87 | 4, 10 | 15 | ||
| 2-Nonanone | 0.026 | 4.43 | 4, 10 | 15 | ||
| Benzyl acetate | <0.001 | 67.05 | 4, 10 | 15 | ||
| Nonanal | 0.034 | 4.03 | 4, 10 | 15 | ||
| Sulcatone | 0.001 | 13.43 | 4, 10 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faal, H.; Cooperband, M.F. Antennal Sensitivity of Spotted Lanternflies, Lycorma delicatula: Differential Electrophysiological Responses of Males and Females to Compounds Derived from Host Plants and Conspecifics. Insects 2024, 15, 162. https://doi.org/10.3390/insects15030162
Faal H, Cooperband MF. Antennal Sensitivity of Spotted Lanternflies, Lycorma delicatula: Differential Electrophysiological Responses of Males and Females to Compounds Derived from Host Plants and Conspecifics. Insects. 2024; 15(3):162. https://doi.org/10.3390/insects15030162
Chicago/Turabian StyleFaal, Hajar, and Miriam F. Cooperband. 2024. "Antennal Sensitivity of Spotted Lanternflies, Lycorma delicatula: Differential Electrophysiological Responses of Males and Females to Compounds Derived from Host Plants and Conspecifics" Insects 15, no. 3: 162. https://doi.org/10.3390/insects15030162
APA StyleFaal, H., & Cooperband, M. F. (2024). Antennal Sensitivity of Spotted Lanternflies, Lycorma delicatula: Differential Electrophysiological Responses of Males and Females to Compounds Derived from Host Plants and Conspecifics. Insects, 15(3), 162. https://doi.org/10.3390/insects15030162






