Histone Acetylation Enhancing Host Melanization in Response to Parasitism by an Endoparasitoid Wasp
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Analysis of LysAc Modification by Western Blot
2.3. Analysis of LysAc Modification through the Combination of 4D Label-Free Proteomics and LysAc Modification Omics
2.4. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.5. Verification of Protein Expression and the LysAc Modification Level in Histone Proteins by Western Blot and qRT-PCR
2.6. RNA Interference
2.7. Phenotype Analysis
2.8. Capacity Assay of Clearance of Pathogen
2.9. Statistical Analysis
3. Results
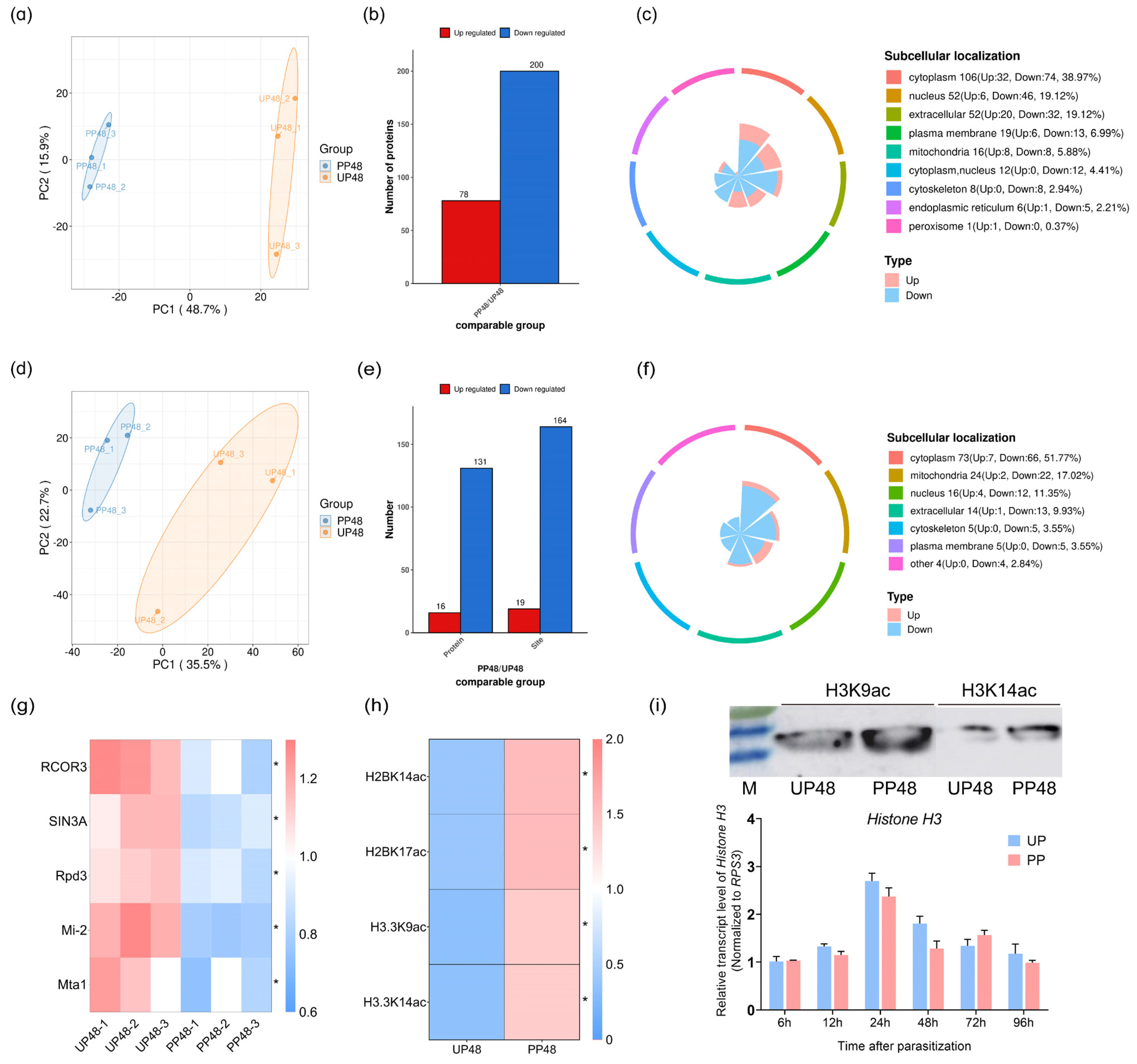
3.1. Initial Analysis of Regulation of Host Protein LysAc through the Parasitism of T. brontispae
3.2. Dynamic Changes in Proteome and LysAc Modifications in Host Pupae after Parasitization
3.3. Expression Analysis of Host HATs and HDACs Induced by the Parasitism of T. brontispae
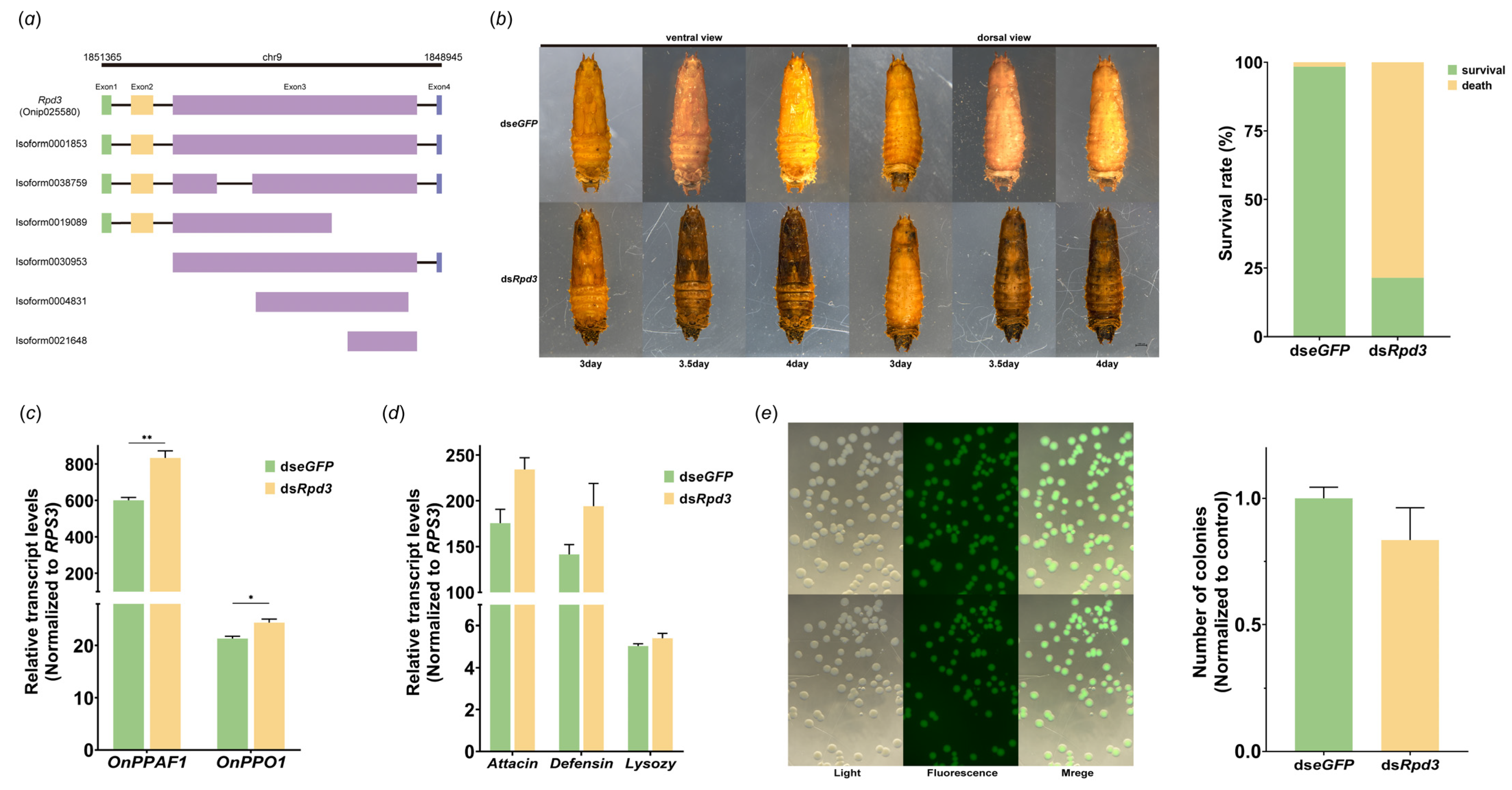
3.4. Role of Rpd3 in Prophenoloxidase Cascade
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Moreau, S.J.M.; Asgari, S. Venom proteins from parasitoid wasps and their biological functions. Toxins 2015, 7, 2385–2412. [Google Scholar] [CrossRef]
- Corley, L.S.; Strand, M.R. Evasion of encapsulation by the polyembryonic parasitoid Copidosoma floridanum is mediated by a polar body-derived extraembryonic membrane. J. Invertebr. Pathol. 2003, 83, 86–89. [Google Scholar] [CrossRef]
- Hu, J.; Xu, Q.; Hu, S.; Yu, X.; Liang, Z.; Zhang, W. Hemomucin, an O-glycosylated protein on embryos of the wasp Macrocentrus cingulum that protects it against encapsulation by Hemocytes of the host Ostrinia furnacalis. J. Innate Immun. 2014, 6, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Rivers, D.B. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Annu. Rev. Entomol. 2011, 56, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Beckage, N.E. Polydnaviruses as endocrine regulators. In Parasitoid Viruses; Academic Press: Cambridge, MA, USA, 2012; pp. 163–168. [Google Scholar] [CrossRef]
- Strand, M.R. Polydnavirus gene products that interact with the host immune system. In Parasitoid Viruses; Academic Press: Cambridge, MA, USA, 2012; pp. 149–161. [Google Scholar] [CrossRef]
- Strand, M.R. Teratocytes and their functions in parasitoids. Curr. Opin. Insect Sci. 2014, 6, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Q.; Shi, M.; Huang, J.H.; Chen, X.X. Parasitoid polydnaviruses and immune interaction with secondary hosts. Dev. Comp. Immunol. 2018, 83, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K. Environmental epigenetics and a unified theory of the molecular aspects of evolution: A neo-lamarckian concept that facilitates neo-Darwinian evolution. Genome Biol. Evol. 2015, 7, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Bonduriansky, R.; Crean, A.J.; Day, T. The implications of nongenetic inheritance for evolution in changing environments. Evol. Appl. 2012, 5, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33 (Suppl. S3), 245–254. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.K.; Tsai, C.H.; Wu, C.P.; Lin, Y.H.; Wei, S.C.; Lu, Y.H.; Li, C.H.; Wu, Y.L. MicroRNAs from Snellenius manilae bracovirus regulate innate and cellular immune responses of its host Spodoptera litura. Commun. Biol. 2021, 4, 52. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Ye, X.Q.; Shi, M.; Li, F.; Wang, Z.H.; Zhou, Y.N.; Gu, Q.J.; Wu, X.T.; Yin, C.L.; Guo, D.H.; et al. Parasitic insect-derived miRNAs modulate host development. Nat. Commun. 2018, 9, 2205. [Google Scholar] [CrossRef] [PubMed]
- Martinson, E.O.; Martinson, V.G.; Edwards, R.; Mrinalini; Werren, J.H. Laterally transferred gene recruited as a venom in parasitoid wasps. Mol. Biol. Evol. 2016, 33, 1042–1052. [Google Scholar] [CrossRef]
- Gilbert, S.F.; McDonald, E.; Boyle, N.; Buttino, N.; Gyi, L.; Mai, M.; Prakash, N.; Robinson, J. Symbiosis as a source of selectable epigenetic variation: Taking the heat for the big guy. Philos. Trans. R. Soc. B-Biol. Sci. 2010, 365, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, R.; Mukherjee, K.; Uckan, F.; Vilcinskas, A. Reprograming of epigenetic mechanisms controlling host insect immunity and development in response to egg-laying by a parasitoid wasp. Proc. Biol. Sci. 2020, 287, 20200704. [Google Scholar]
- Kim, G.W.; Yang, X.J. Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Biochem. Sci. 2011, 36, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.A.; Cossart, P. Histone modifications and chromatin remodeling during bacterial infections. Cell Host Microbe 2008, 4, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.N. Modification-specific proteomics: Characterization of post-translational modifications by mass spectrometry. Curr. Opin. Chem. Biol. 2004, 8, 33–41. [Google Scholar] [CrossRef]
- Mann, M.; Jensen, O.N. Proteomic analysis of post-translational modifications. Nat. Biotechnol. 2003, 21, 255–261. [Google Scholar] [CrossRef]
- Cho, Y.; Griswold, A.; Campbell, C.; Min, K.T. Individual histone deacetylases in Drosophila modulate transcription of distinct genes. Genomics 2005, 86, 606–617. [Google Scholar] [CrossRef]
- Mukherjee, K.; Fischer, R.; Vilcinskas, A. Histone acetylation mediates epigenetic regulation of transcriptional reprogramming in insects during metamorphosis, wounding and infection. Front. Zool. 2012, 9, 25. [Google Scholar] [CrossRef]
- Spange, S.; Wagner, T.; Heinzel, T.; Kramer, O.H. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int. J. Biochem. Cell Biol. 2009, 41, 185–198. [Google Scholar] [CrossRef]
- Takakura, M.; Nakagawa, R.; Ota, T.; Kimura, Y.; Ng, M.Y.; Alia, A.G.; Okuno, H.; Hirano, Y. Rpd3/CoRest-mediated activity-dependent transcription regulates the flexibility in memory updating in Drosophila. Nat. Commun. 2021, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Lin, Y.P.; Li, H.Y.; Wang, R.; Fu, L.; Jia, Q.C.; Hou, Y.M.; Tang, B.Z. Variation in parasitoid virulence of Tetrastichus brontispae during the targeting of two host beetles. Int. J. Mol. Sci. 2021, 22, 3581. [Google Scholar] [CrossRef]
- Meng, E.; Qiao, T.; Tang, B.Z.; Hou, Y.M.; Yu, W.Z.; Chen, Z.M. Effects of ovarian fluid, venom and egg surface characteristics of Tetrastichus brontispae (Hymenoptera: Eulophidae) on the immune response of Octodonta nipae (Coleoptera: Chrysomelidae). J. Insect Physiol. 2018, 109, 125–137. [Google Scholar] [CrossRef]
- Tang, B.; Xu, L.; Hou, Y. Effects of rearing conditions on the parasitism of Tetrastichus brontispae on its pupal host Octodonta nipae. Biocontrol 2014, 59, 647–657. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, Z.; Xu, C.; Tang, C.; Lu, B.; Jin, Q.; Wen, H.; Wan, F. Biological assessment of Tetrastichus brontispae, a pupal parasitoid of coconut leaf beetle Brontispa longissima. Biocontrol Sci. Technol. 2010, 20, 283–295. [Google Scholar] [CrossRef]
- Tang, B.Z.; Chen, J.; Hou, Y.M.; Meng, E. Transcriptome immune analysis of the invasive beetle Octodonta nipae (Maulik) (Coleoptera: Chrysomelidae) parasitized by Tetrastichus brontispae Ferrière (Hymenoptera: Eulophidae). PLoS ONE 2014, 9, e91482. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Zhang, X.; Hou, Y.M.; Tang, B.Z. Effects of multiple mating on the fecundity of an invasive pest (Octodonta nipae): The existence of an intermediate optimal female mating rate. Physiol. Entomol. 2014, 39, 348–354. [Google Scholar] [CrossRef]
- Koressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: Integrating masking of template sequence with primer design software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, H.J.; Liu, M.; Liu, B.; Zhang, X.F.; Ma, C.J.; Fu, T.T.; Hou, Y.M.; Tang, B.Z. Cloning and immunosuppressive properties of an acyl-activating enzyme from the venom apparatus of Tetrastichus brontispae (Hymenoptera: Eulophidae). Toxins 2019, 11, 672. [Google Scholar] [CrossRef]
- Horn, T.; Boutros, M. E-RNAi: A web application for the multi-species design of RNAi reagents—2010 update. Nucleic Acids Res. 2010, 38, 332–339. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, B.; Lin, Y.; Chen, Z.; Zhang, X.; Ji, T.; Zhang, X.; Hou, Y. Identification of three prophenoloxidase-activating factors (PPAFs) from an invasive beetle Octodonta nipae Maulik (Coleoptera: Chrysomelidae) and their roles in the prophenoloxidase activation. Arch. Insect Biochem. Physiol. 2017, 96, e21425. [Google Scholar] [CrossRef]
- Zhang, X.F.; Cui, W.; Wang, M.J.; Zhou, Y.; Fu, T.T.; Jiang, K.; Hou, Y.M.; Tang, B.Z. Role of prophenoloxidase 1 from the beetle Octodonta nipae in melanized encapsulation of a wasp egg. Dev. Comp. Immunol. 2024, 150, 105082. [Google Scholar] [CrossRef]
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic hymenoptera. Annu. Rev. Entomol. 2006, 51, 233–258. [Google Scholar] [CrossRef]
- Poirie, M.; Carton, Y.; Dubuffet, A. Virulence strategies in parasitoid Hymenoptera as an example of adaptive diversity. C. R. Biol. 2009, 332, 311–320. [Google Scholar] [CrossRef]
- Glatz, R.V.; Asgari, S.; Schmidt, O. Evolution of polydnaviruses as insect immune suppressors. Trends Microbiol. 2004, 12, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Federici, B.A.; Bigot, Y. Origin and evolution of polydnaviruses by symbiogenesis of insect DNA viruses in endoparasitic wasps. J. Insect Physiol. 2003, 49, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Drezen, J.-M.; Chevignon, G.; Louis, F.; Huguet, E. Origin and evolution of symbiotic viruses associated with parasitoid wasps. Curr. Opin. Insect Sci. 2014, 6, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R.; Burke, G.R. Polydnaviruses: Evolution and function. Curr. Issues Mol. Biol. 2019, 34, 163–182. [Google Scholar] [PubMed]
- Talbert, P.B.; Armache, K.-J.; Henikoff, S. Viral histones: Pickpocket’s prize or primordial progenitor? Epigenet. Chromatin 2022, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Hepat, R.; Song, J.-J.; Lee, D.; Kim, Y. A viral histone H4 joins to eukaryotic nucleosomes and alters host gene expression. J. Virol. 2013, 87, 11223–11230. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Weinert, B.T.; Choudhary, C. Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Shvedunova, M.; Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2022, 23, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A. The impact of parasites on host insect epigenetics. Insect Epigenet. 2017, 53, 145–165. [Google Scholar]
- Chang, C.S.; Pillus, L. Collaboration between the essential Esa1 acetyltransferase and the Rpd3 deacetylase is mediated by H4K12 histone acetylation in Saccharomyces cerevisiae. Genetics 2009, 183, 149–160. [Google Scholar] [CrossRef]
- Foglietti, C.; Filocamo, G.; Cundari, E.; De Rinaldis, E.; Lahm, A.; Cortese, R.; Steinkuhler, C. Dissecting the biological functions of Drosophila histone deacetylases by RNA interference and transcriptional profiling. J. Biol. Chem. 2006, 281, 17968–17976. [Google Scholar] [CrossRef]
- Pile, L.A.; Schlag, E.M.; Wassarman, D.A. The SIN3/RPD3 deacetylase complex is essential for G(2) phase cell cycle progression and regulation of SMRTER corepressor levels. Mol. Cell Biol. 2002, 22, 4965–4976. [Google Scholar] [CrossRef]
- Rogina, B.; Helfand, S.L.; Frankel, S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science 2002, 298, 1745. [Google Scholar] [CrossRef]
- George, S.; Gaddelapati, S.C.; Palli, S.R. Histone deacetylase 1 suppresses Kruppel homolog 1 gene expression and influences juvenile hormone action in Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2019, 116, 17759–17764. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, N.T.; Fischer, M.L.; Waring, A.L.; Kr, P.; Kacsoh, B.Z.; Brantley, S.E.; Keebaugh, E.S.; Hill, J.; Lark, C.; Martin, J.; et al. Extracellular matrix protein N-glycosylation mediates immune self-tolerance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2021, 118, e2017460118. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.; Tang, B.Z.; Hou, Y.M.; Chen, X.X.; Chen, J.T.; Yu, X.Q. Altered immune function of Octodonta nipae (Maulik) to its pupal endoparasitoid, Tetrastichus brontispae Ferrière. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 198, 100–109. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, K.; Zhou, Y.; Cui, W.; Han, Y.-W.; Chen, P.; Liao, G.-M.; Hou, Y.-M.; Tang, B.-Z. Histone Acetylation Enhancing Host Melanization in Response to Parasitism by an Endoparasitoid Wasp. Insects 2024, 15, 161. https://doi.org/10.3390/insects15030161
Jiang K, Zhou Y, Cui W, Han Y-W, Chen P, Liao G-M, Hou Y-M, Tang B-Z. Histone Acetylation Enhancing Host Melanization in Response to Parasitism by an Endoparasitoid Wasp. Insects. 2024; 15(3):161. https://doi.org/10.3390/insects15030161
Chicago/Turabian StyleJiang, Kun, Yan Zhou, Wen Cui, Yan-Wei Han, Pei Chen, Gui-Ming Liao, You-Ming Hou, and Bao-Zhen Tang. 2024. "Histone Acetylation Enhancing Host Melanization in Response to Parasitism by an Endoparasitoid Wasp" Insects 15, no. 3: 161. https://doi.org/10.3390/insects15030161
APA StyleJiang, K., Zhou, Y., Cui, W., Han, Y.-W., Chen, P., Liao, G.-M., Hou, Y.-M., & Tang, B.-Z. (2024). Histone Acetylation Enhancing Host Melanization in Response to Parasitism by an Endoparasitoid Wasp. Insects, 15(3), 161. https://doi.org/10.3390/insects15030161





