Effect of Microclimate on the Mass Emergence of Hypothenemus hampei in Coffee Grown under Shade of Trees and in Full Sun Exposure
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Plots
2.3. CBB Trapping
2.4. Microclimate Variables
2.5. Data Analysis
2.5.1. Comparison of CBB Caught in Sun and Shade
2.5.2. Effect of Microclimate on the Mass Emergence of CBB
3. Results
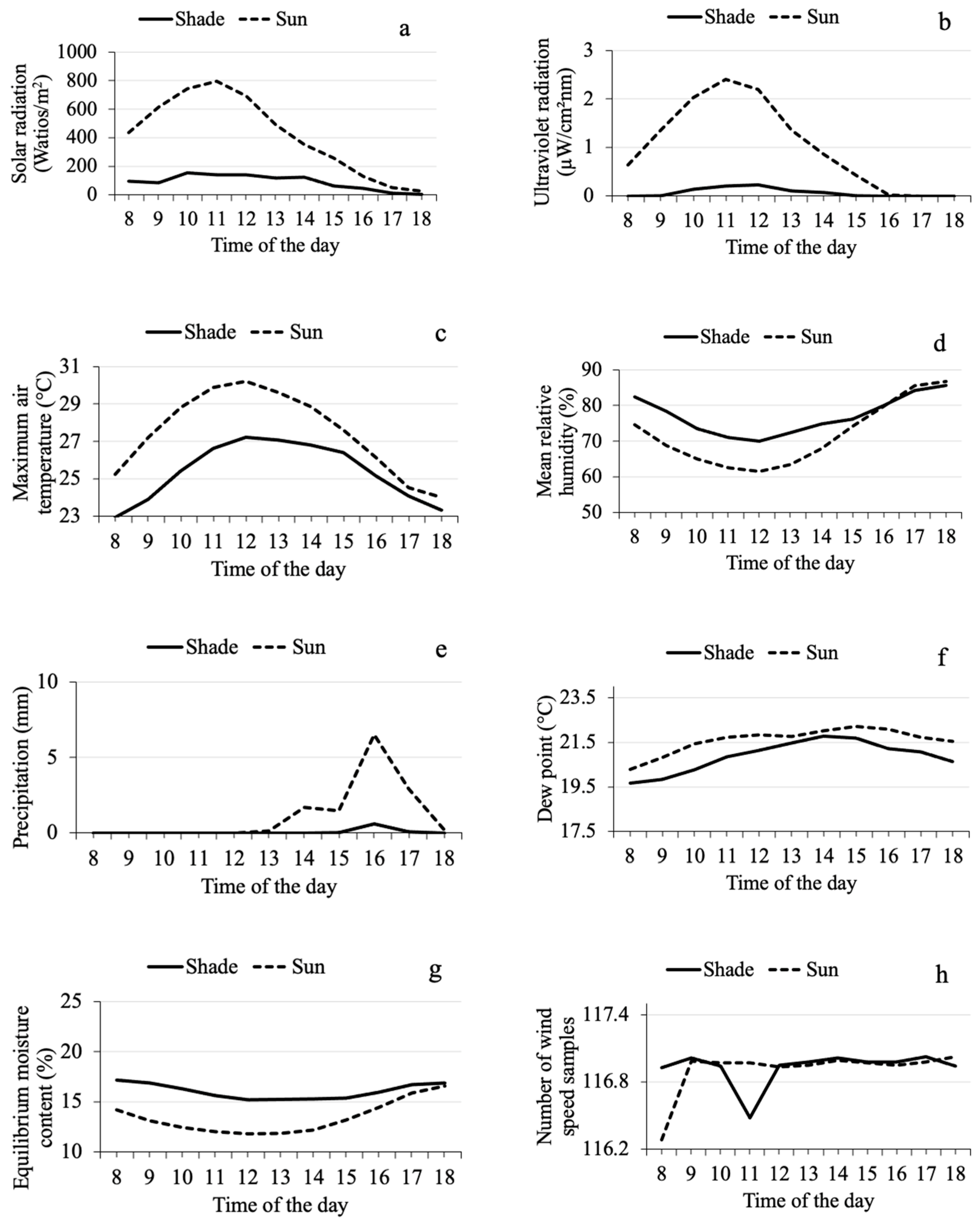
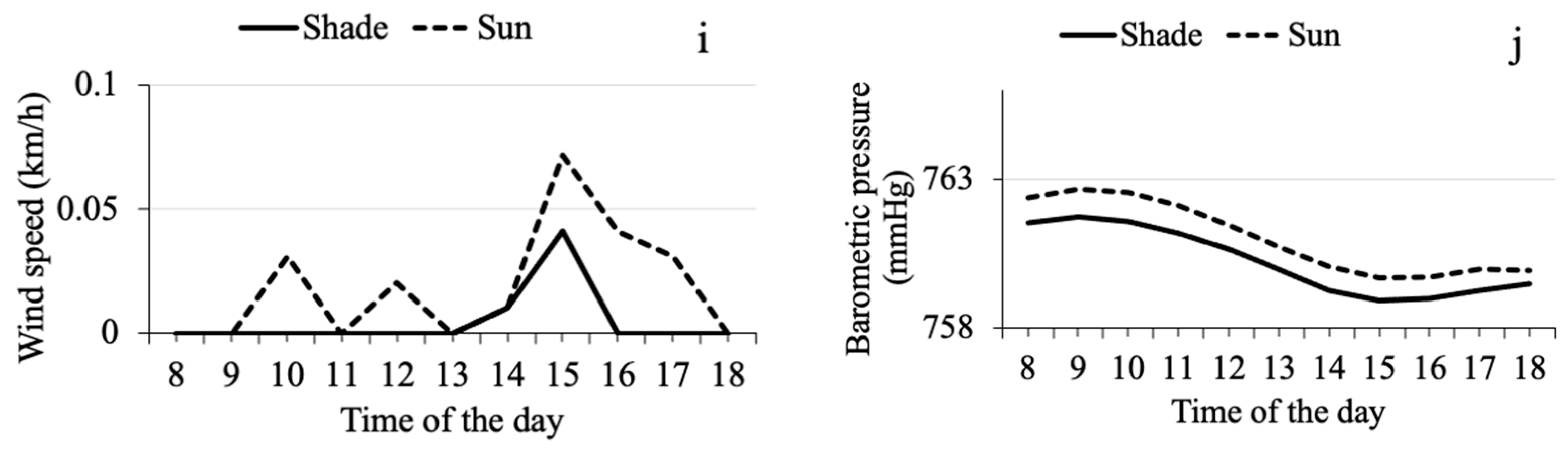
3.1. Seasonal and Daily Fluctuation in Microclimate Variables in Shade and Sun
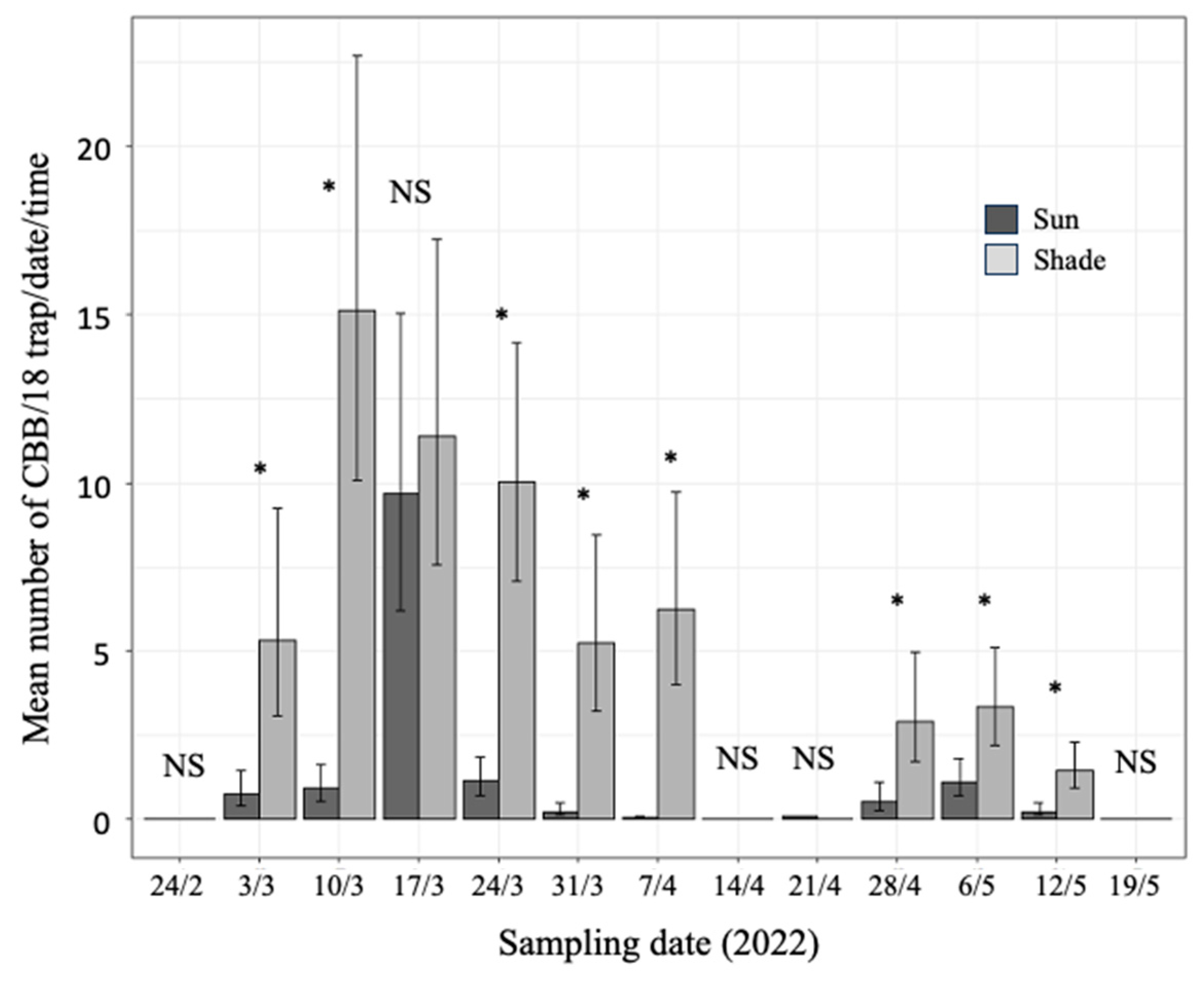
3.2. Comparison of CBB Captured in Shade and Sun
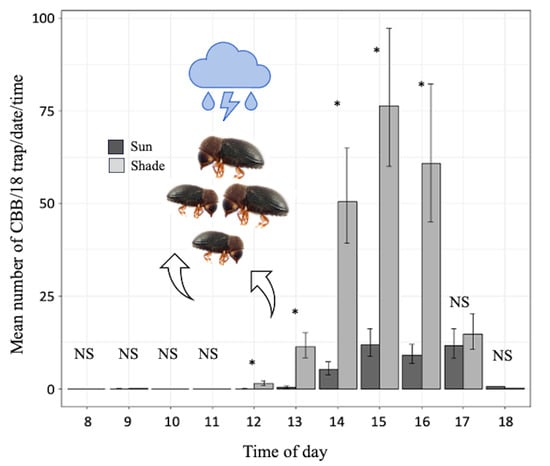
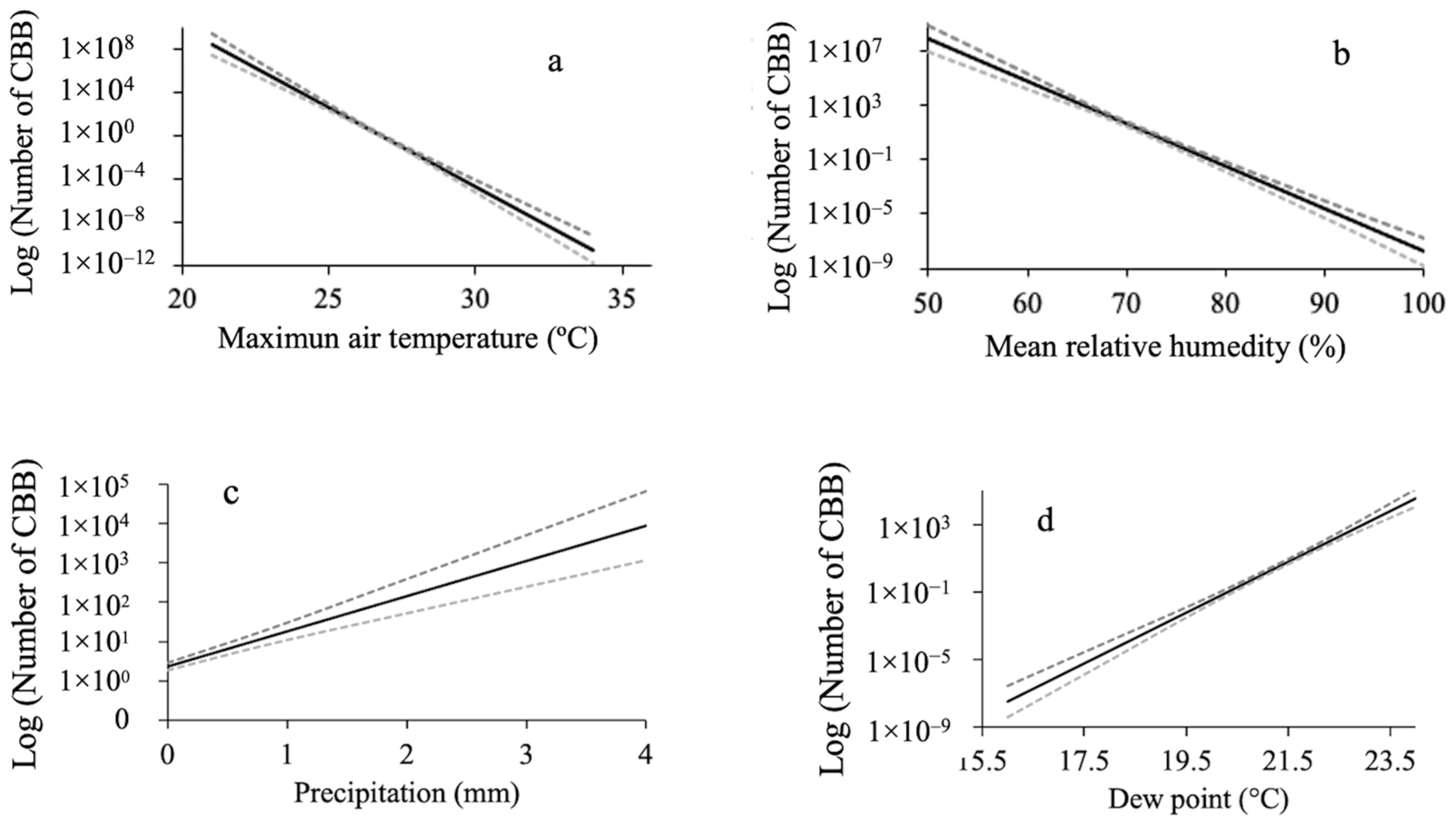
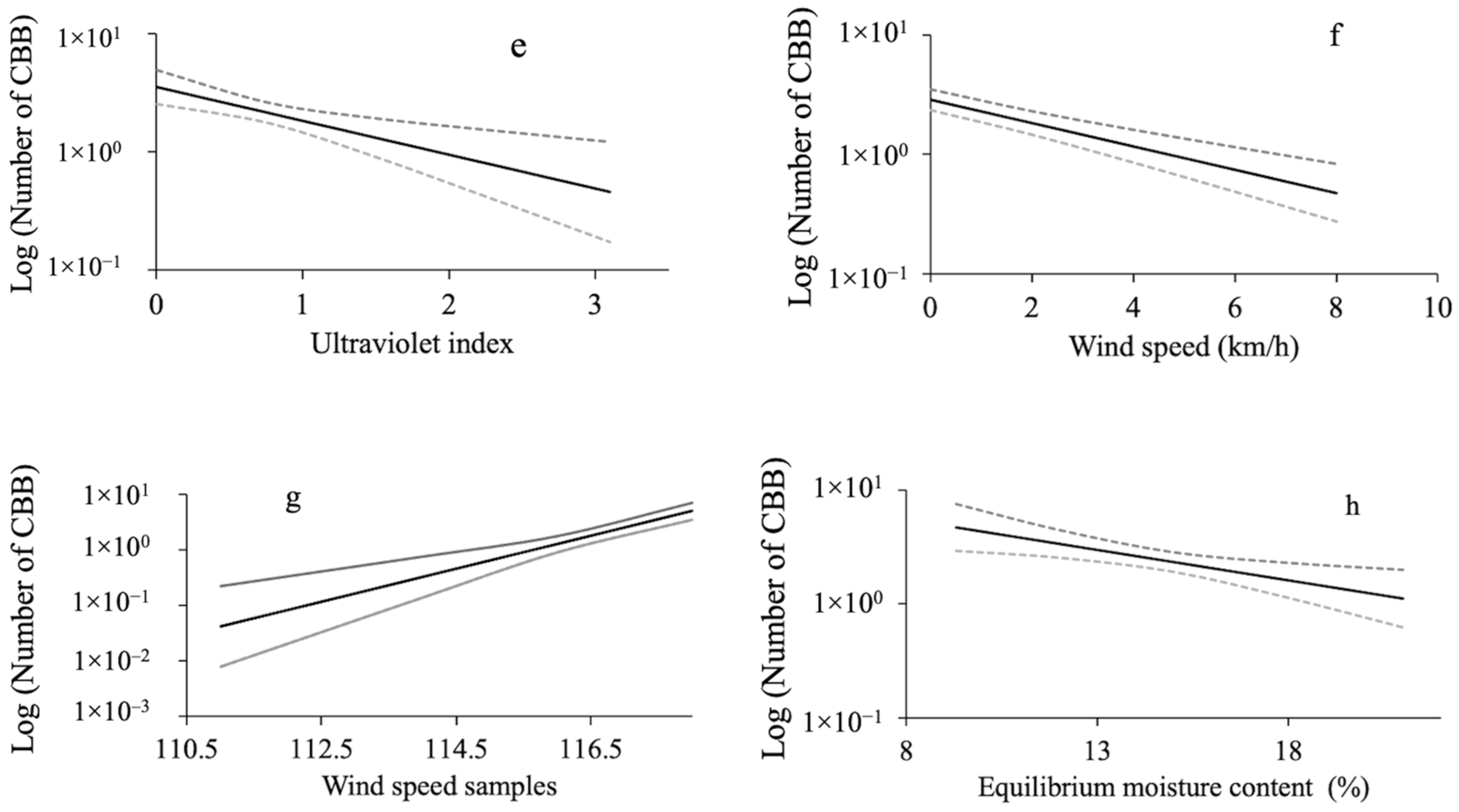
3.3. Relationship between Microclimate Variables and the Mass Emergence of CBB
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Coffee Organization. Crop Year Production by Country. Data as at May 2021. Available online: https://www.ico.org/prices/po-production.pdf (accessed on 8 August 2023). (In Spanish).
- Servicio de Información Agroalimentaria y Pesquera. Avance de Siembras y Cosechas. Resumen Nacional por Estado. Situación al 31 de Diciembre de 2020. Available online: http://infosiap.siap.gob.mx:8080/agricola_siap_gobmx/ResumenProducto.do (accessed on 8 August 2023). (In Spanish).
- Figueroa-Hernández, E.; Pérez-Soto, F.; Godínez-Montoya, L. Importancia de la comercialización del café en México. In Ciencias Sociales: Economía y Humanidades; Pérez, F., Figueroa, E., Godínez, L., Eds.; Handbook T-I.-©ECORFAN: Texcoco de Mora, Mexico, 2015; pp. 64–82. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5365588 (accessed on 5 February 2024). (In Spanish)
- Higuera-Ciapara, I.; Rivera-Ramírez, J. Chiapas: Problemáticas del Sector Cafetalero; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. (CIATEJ): Guadalajara, Mexico, 2018; 61p, Available online: http://ciatej.repositorioinstitucional.mx/jspui/handle/1023/645 (accessed on 5 February 2024). (In Spanish)
- Centro de Estudios para el Desarrollo Rural Sustentable y la Soberanía Alimentaria. Comercio Internacional del Café, el Caso de México. Investigación Interna; Palacio Legislativo de San Lázaro: Mexico City, Mexico, 2019; 11p, Available online: https://portales.diputados.gob.mx/CEDRSSA/registro-solicitud?men=bb53bb03-1642-40d2-9a34-61c0a743c006&menu=Acerca%20de&lateral=false# (accessed on 5 February 2024). (In Spanish)
- Infante, F. Pest management strategies against the coffee berry borer (Coleoptera: Curculionidae: Scolytinae). J. Agric. Food Chem. 2018, 66, 5275–5280. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Manoukis, N.C. Influence of seasonal and climatic variables on coffee berry borer (Hypothenemus hampei Ferrari) flight activity in Hawaii. PLoS ONE 2021, 16, e0257861. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Diaz, C.P.; Verle-Rodrigues, J.C. Vertical trapping of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytinae), in coffee. Insects 2021, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, J.; Santos-Ovilla, A.; Valle-Mora, J.; Montoya-Gerardo, P.J. Determinación del establecimiento de parasitoides de la broca del café Hypothenemus hampei (Coleoptera: Curculionidae, Scolytinae) en cafetales del Soconusco, Chiapas, México. Entomotropica 2010, 25, 25–36. (In Spanish) [Google Scholar]
- Olvera-Vargas, L.A.; Contreras-Medina, D.I.; Aguilar-Rivera, N. Calculation of degrees days of Hypothenemus hampei through satellite images. Rev. Mexicana Cienc. Agríc. 2020, 11, 544–554. [Google Scholar] [CrossRef]
- Baker, P.S.; Ley, C.; Balbuena, R.; Barrera, J.F. Factors affecting the emergence of Hypothenemus hampei (Coleoptera: Scolytidae) from coffee berries. Bull. Entomol. Res. 1992, 82, 145–150. [Google Scholar] [CrossRef]
- Baker, P.S.; Rivas, A.; Balbuena, R.; Ley, C.; Barrera, J.F. Abiotic mortality factors of the coffee berry borer (Hypothenemus hampei). Entomol. Exp. Appl. 1994, 71, 201–209. [Google Scholar] [CrossRef]
- Baker, P.S. Some aspects of the behavior of the coffee berry borer in relation to its control in Southern Mexico (Coleoptera: Scolytidae). Folia Entomol. Mex. 1984, 61, 9–24. Available online: https://www.researchgate.net/publication/274390928_Some_aspects_of_the_behavior_of_the_coffee_berry_borer_in_relation_to_its_control_in_Southern_Mexico_Coleoptera_Scolytidae (accessed on 5 February 2024).
- Mansingh, A. Limitations of insecticides in the management of the coffee berry borer Hypothenemus hampei Ferrari. J. Coffee Res. 1991, 21, 67–98. [Google Scholar]
- Barrera, J.F.; Herrera, J.; Villacorta, A.; Garcia, H.; Cruz, L. Trampas de metanol-etanol para detección, monitoreo y control de la broca del café Hypothenemus hampei. In Simposio Sobre Trampas y Atrayentes en Detección, Monitoreo y Control de Plagas de Importancia Económica; Barrera, J.F., Montoya, P., Eds.; Sociedad Mexicana de Entomología y El Colegio de la Frontera Sur: Tapachula, Mexico, 2006; pp. 71–83. (In Spanish) [Google Scholar]
- Román-Ruiz, A.K.; Barrera, J.F.; Cruz-López, L.; Rojas, J.C.; Dufour, B.P. The singular insemination status of Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytinae) females during the inter-harvest season of a coffee crop. Bull. Entomol. Res. 2019, 109, 544–549. [Google Scholar] [CrossRef]
- Barrera, J.F.; Villacorta, A.; Herrera, J. Fluctuación estacional de las capturas de la “broca del café” (Hypothenemus hampei) con trampas de etanol—Metanol e implicaciones sobre el número de trampas. Entomol. Mex. 2004, 3, 540–544. (In Spanish) [Google Scholar]
- Gutiérrez-Martínez, A.; Ondarza, R.N. Kairomone effect of extracts from Coffea canephora over Hypothenemus hampei (Coleoptera: Scolytidae). Environ. Entomol. 1996, 25, 96–100. [Google Scholar] [CrossRef]
- Mathieu, F.; Brun, L.O.; Frérot, B.; Suckling, D.M.; Frampton, C. Progression in field infestation is linked with trapping of coffee berry borer, Hypothenemus hampei (Col., Scolytidae). J. Appl. Entomol. 1999, 123, 535–540. [Google Scholar] [CrossRef]
- Villarreyna-Acuña, R.A. Efecto de la Sombra Sobre las Plagas y Enfermedades, a Través del Microclima, Fenología y Estado Fisiológico del Cafeto; Informe Proyecto CASCADA; CATIE-CIRAD-Conservation International: Turrialba, Costa Rica, 2016; 33p, Available online: https://agritrop.cirad.fr/581152/1/Reporte%201_Sombra%20y%20Plagas%20y%20E_RV_%20JA_BV_RV.pdf (accessed on 5 February 2024). (In Spanish)
- Pereira, E.; Gontijo, P.; Fantine, A.; Tinoco, R.; Ellersieck, M.; Carvalho, G.; Zanuncio, J.; Vilela, E. Emergence and infestation level of Hypothenemus hampei (Coleoptera: Curculionidae) on coffee berries on the plant or on the ground during the post-harvest period in Brazil. J. Insect Sci. 2021, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Barrera, J.F.; Covarrubias, M. Efecto de diferentes condiciones de sombra del cafetal sobre la intensidad del ataque de la broca del grano de café Hypothenemus hampei (Ferr.) (Coleoptera: Scolytidae) en el Soconusco, Chiapas, México. In Memorias del II Congr. Nac. Manejo Integr. Plagas; AGMIP: Ciudad Guatemala, Guatemala, 1984; pp. 208–218. (In Spanish) [Google Scholar]
- Muñoz, R.I.; Andino, A.; Zelaya, R.R. Fluctuación poblacional de la broca del fruto del cafeto (Hypothenemus hampei Ferr.) en la zona del Lago de Yojoa. In Memoria II Taller de Trabajo Internacional Sobre Manejo Integrado de la Broca del Grano de Café (Hypothenemus hampei, Ferr.); Urbina, N.E., Decazy, B., Eds.; Publicación Miscelánea A1/GT-87-005: Tapachula, Mexico, 1987; pp. 75–99. Available online: https://repositorio.iica.int/bitstream/handle/11324/12066/BVE20098367e.pdf?sequence=1&isAllowed=y (accessed on 12 November 2023). (In Spanish)
- Mariño, Y.A.; Pérez, M.-E.; Gallardo, F.; Trifilio, M.; Cruz, M.; Bayman, P. Sun vs. shade affects infestation, total population and sex ratio of the coffee berry borer (Hypothenemus hampei) in Puerto Rico. Agric. Ecosyst. Environ. 2016, 222, 258–266. [Google Scholar] [CrossRef]
- Constantino, L.M.; Rendón, J.R.; Cuesta, G.; Medina-Rivera, R.; Benavides-Machado, P. Dinámica poblacional, dispersión y colonización de la broca del café Hypothenemus hampei en Colombia. Rev. Cenicafé 2021, 72, e72102. (In Spanish) [Google Scholar] [CrossRef]
- Jaramillo, J.; Setamou, M.; Muchugu, E.; Chabi-Olaye, A.; Jaramillo, A.; Mukabana, J.; Maina, J.; Gathara, S.; Borgemeister, C. Climate change or urbanization? Impacts on a traditional coffee production system in East Africa over the last 80 years. PLoS ONE 2013, 8, e51815. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.L.; Ángeles, H. Diagnóstico de la Dinámica Económica, Social y Demográfica, con Énfasis en la Movilidad Humana en la Región del Soconusco, Chiapas (México), y en los Municipios Estrictamente Fronterizos; LC/MEX/TS.2023/7; Comisión Económica para América Latina y el Caribe (CEPAL): Ciudad de México, Mexico, 2023; 174p, Available online: https://www.cepal.org/es/publicaciones/48822-diagnostico-la-dinamica-economica-social-demografica-enfasis-la-movilidad-humana (accessed on 12 November 2023). (In Spanish)
- Instituto Nacional de Estadística y Geografía. Censo Agropecuario 2007; VIII Censo Agrícola, Ganadero y Forestal: Aguascalientes, Mexico, 2009; Available online: https://www.inegi.org.mx/contenidos/programas/cagf/2007/tabulados/Tabulado_Mpio_VIII_CAGyF_10_07.pdf (accessed on 12 November 2023). (In Spanish)
- Da Silva, F.C.; Ventura, M.U.; Morales, l. Capture of Hypothenemus hampei Ferrari (Coleoptera: Scolytidae) in response to trap characteristics. Sci. Agric. 2006, 63, 567–571. Available online: https://www.scielo.br/j/sa/a/TM9VBLrxBSTSK8r6HWhRFPR/?lang=en# (accessed on 5 February 2024). [CrossRef]
- Moreno Rodríguez, D.; Álvarez Núñez, A.; Vázquez Moreno, L.L.; Alfonso Simonetti, J. Evaluación de atrayentes para la captura de hembras adultas de broca del café Hypothenemus hampei (Ferrari) con trampas artesanales. Fitosanidad 2010, 14, 177–180. Available online: https://www.researchgate.net/publication/262438125_Evaluacion_de_atrayentes_para_la_captura_de_hembras_adultas_de_broca_del_cafe_hypothenemus_hampei_ferrari_con_trampas_artesanales (accessed on 5 February 2024).
- Quispe-Condori, R.; Loza-Murguia, M.; Marza-Mamani, F.; Gutiérrez Rolando, R.C.; Aliaga, F.; Fernández, C. Trampas artesanales con atrayentes alcohólicos en el control de la broca del café, Hypothenemus hampei (Ferrari 1867) en la Colonia Bolinda, Caranavi. J. Selva Andin. Biosph. 2015, 3, 2–14. Available online: http://www.scielo.org.bo/pdf/jsab/v3n1/v3n1_a02.pdf (accessed on 5 February 2024). [CrossRef]
- Villacorta, A.; Possagnolo, A.F.; Silva, R.Z.; Rodriguez, P.S. Um modelo de armadilha com semioquímicos para o manejo integrado da broca do café Hypothenemus hampei (Ferrari) no Paraná. In Anais do II Simposio de Pesquisa dos Cafés do Brasil, 24–27 de Septiembre, 2001; Consórcio Pesquisa Café: Vitória, Brasil, 2001; pp. 2093–2098. Available online: http://www.sapc.embrapa.br/arquivos/consorcio/spcb_anais/simposio2/pragas28.pdf (accessed on 5 February 2024).
- Serrano-Domínguez, A.; Barrera-Gaytán, J.F.; Valle-Mora, F.J.; Rojas-León, J.C.; Cruz-López, L.; Albores-Flores, V.J. Dosis óptima del atrayente para la captura de la broca del café Hypothenemus hampei (Coleoptera: Curculionidae). Entomol. Mex. 2012, 11, 614–619. [Google Scholar]
- Hilbe, J. Negative Binomial Regression, 2nd ed.; Cambridge University Press: Cambridge, UK, 2011; 553p. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 5 February 2024).
- Demidenko, E. Mixed Models: Theory and Applications with R, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013; 768p. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/index.html (accessed on 5 February 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Mustafa, A. Understanding Random Effects and Fixed Effects in Statistical Analysis. Medium. July 2023. Available online: https://medium.com/@akif.iips/understanding-random-effect-and-fixed-effect-in-statistical-analysis-db4983cdf8b1 (accessed on 5 February 2024).
- Stalliviere-Corrêa, I.C. Topografia Aplicada à Engenharia Civil, 20th ed.; Departamento de Geodésia, Instituto de Geociências, Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2021; 166p, Available online: https://lume.ufrgs.br/handle/10183/217687 (accessed on 10 November 2023). (In Portuguese)
- Dufour, B.P.; Franco-Franco, F.; Hernández, A. Evaluación del trampeo en el marco del manejo integrado de la broca del café. In La Broca del Café en América Tropical: Hallazgos y Enfoques; Barrera, J.F., García, A., Domínguez, V., Luna, C., Eds.; Sociedad Mexicana de Entomología y El Colegio de la Frontera Sur: Acapulco, Mexico, 2007; pp. 89–99. [Google Scholar]
- Dufour, B.P.; Frérot, B. Optimization of coffee berry borer, Hypothenemus hampei Ferrari (Col., Scolytidae), mass trapping with an attractant mixture. J. Appl. Entomol. 2008, 132, 591–600. [Google Scholar] [CrossRef]
- Benavides, P.; Gil, Z.N.; Escobar, L.E.; Navarro-Escalante, L.; Follett, P.; Diaz-Soltero, H. Pilot testing of an area-wide biological control strategy against the coffee berry borer in Colombia using African parasitoids. Insects 2023, 14, 865. [Google Scholar] [CrossRef] [PubMed]
- Benavides, P.; Bustillo, A.; Góngora, C. IPM program to control coffee berry borer Hypothenemus hampei, with emphasis on highly pathogenic mixed strains of Beauveria bassiana, to overcome insecticide resistance in Colombia. In Insecticides Advances in Integrated Pest Management; Perveen, F., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 512–540. [Google Scholar]
- Metellus, D.; Sampaio, M.V.; Celoto, F.J. Activity of insecticides on coffee berry borer, Hypothenemus hampei (Coleoptera: Curculionidae, Scolytinae). Biosci. J. 2020, 36, 1099–1115. [Google Scholar] [CrossRef]
- Bustillo, A.E.; Cárdenas, R.; Villalba, D.A.; Benavides, P.; Orozco, J.; Posada, F.J. Manejo Integrado de la Broca del Café: Hypothenemus hampei (Ferrari) en Colombia; Cenicafé: Manizales, Colombia, 1998; ISBN 978-958-96554-0-5. [Google Scholar]
- Aristizábal, L.F.; Johnson, M.A.; Mariño, Y.A.; Bayman, P.; Wright, M.G. Establishing an Integrated Pest Management program for coffee berry borer (Hypothenemus hampei) in Hawaii and Puerto Rico coffee agroecosystems: Achievements and challenges. Insects 2023, 14, 603. [Google Scholar] [CrossRef] [PubMed]
- Corbett, G.H. Some preliminary observations on the coffee berry beetle borer, Stephanoderes (Cryphalus) hampei Ferr. Malay. Agric. J. 1933, 21, 8–22. [Google Scholar]
- Féliz, D.; Guharay, F.; Beer, J. Incidencia de la broca (Hypothenemus hampei) en plantas de café a pleno sol y bajo sombra de Eugenia jambos y Gliricidia sepium en San Marcos, Nicaragua. Agroforestería En Las Américas 2004, 41–42, 56–61. (In Spanish) [Google Scholar]
- Leefmans, S. De kaffiebessenboeboek (Stephanoderes hampeï Ferrari = coffeae Hagedorn). I. Levenswijze En Oecologie. Meded. Inst. Voor Plantenziekten 1923, 57, 1–94, (Summary). [Google Scholar]
- Morallo-Rejesus, B.; Baldos, E. The biology of coffee berry borer Hypothenemus hampei (Ferr.) (Scolytidae, Coleoptera) and its incidence in the southern Tagalop Provinces. Phillip. Ent. 1980, 4, 303–316. [Google Scholar]
- De Souza, M.S.; Medeiros Costa, J.N.; Curitiba Espindula, M.; de Almeida e Silva, A. Performance of baited traps for integrated management of Hypothenemus hampei Ferrari (Coleoptera: Scolytinae) in a conilon coffee crop in Rondônia State, Brazil. EntomoBrasilis 2020, 13, e913. [Google Scholar] [CrossRef]
- Barrera, J.F.; Herrera, J.; Valle, J. Efecto de la altura de la trampa en la captura de la broca del café: Implicaciones en dispersión y muestreo. Entomol. Mex. 2005, 4, 542–546. [Google Scholar]
- Organización Meteorológica Mundial. El Niño/La Niña Hoy-Febrero de 2022. 2022. Available online: https://public.wmo.int/es/media/noticias/el-niño-la-niña-hoy-febrero-de-2022 (accessed on 10 November 2023). (In Spanish).
- Constantino, L.M.; Gil, Z.N.; Montoya, E.C.; Benavides, P. Coffee berry borer (Hypothenemus hampei) emergence from ground fruits across varying altitudes and climate cycles, and the effect on coffee tree infestation. Neotrop. Entomol. 2021, 50, 374–387. [Google Scholar] [CrossRef]
- Gil Palacio, Z.N.; Constantino Chaure, L.M.; Benavides Machado, P. Dispersión de la broca del café. Avances Técnicos Cenicafé 2021, 531, 1–12. (In Spanish) [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Villacorta, A.; Cure, J.R.; Ellis, K. Tritrophic analysis of the coffee (Coffea arabica)-coffee berry borer [Hypothenemus hampei (Ferrari)]-parasitoid system. An. Soc. Entomol. Brasil 1998, 27, 357–385. [Google Scholar] [CrossRef]



| Variable | Key | Units |
|---|---|---|
| Solar radiation | Sol_rad | W/m2 |
| Wind speed | Win_sp | km/h |
| Precipitation | Rain | mm |
| Ultraviolet radiation index | Uv_in | µW/cm2nm |
| Mean relative humidity | Mean_RH | % |
| Maximum air temperature | Max_aT | °C |
| Dew point | Dew_p | °C |
| Equilibrium moisture content | EMC | % |
| Barometric pressure | Bar | mmHg |
| Wind speed samples | Samp_vi | Number |
| Source of Variation | Logistic Regression Model Chi-Square | Degree of Fredom | p |
|---|---|---|---|
| Sampling date (Sam_d) | 5062.5 | 12 | <0.0001 |
| Plot condition, sun/shade (Condic) | 1254.4 | 1 | <0.0001 |
| Time of day (Time) | 14,454.1 | 10 | <0.0001 |
| Sam_d: Condic | 916.7 | 12 | <0.0001 |
| Sam_d: Time | 2994.8 | 120 | <0.0001 |
| Condic: Time | 788.9 | 10 | <0.0001 |
| Sam_d: Condic: Time | 1535.8 | 109 | <0.0001 |
| Sources of Variation | Chi-Square | Degree of Freedom | p |
|---|---|---|---|
| Polynomial function of degree 3 (poly(Time, 3)) | 2102.3146 | 3 | <0.0001 |
| Plot condition, sun/shade (Condic) | 3.7927 | 1 | 0.0515 |
| Sampling date (Sam_d) | 1628.8696 | 12 | <0.0001 |
| Solar radiation (Sol_rad) | 1.4951 | 1 | 0.2214 |
| Wind speed (Win_sp) | 41.7858 | 1 | <0.0001 |
| Precipitation (Rain) | 60.9070 | 1 | <0.0001 |
| Ultraviolet radiation index (Uv_in) | 10.7842 | 1 | 0.0010 |
| Mean relative humidity (Mean_RH) | 238.1158 | 1 | <0.0001 |
| Maximum air temperature (Max_aT) | 268.9199 | 1 | <0.0001 |
| Dew point (Dew_p) | 270.6297 | 1 | <0.0001 |
| Equilibrium moisture content (EMC) | 8.0771 | 1 | 0.0045 |
| Wind speed samples (Samp_vi) | 23.1626 | 1 | <0.0001 |
| poly(Time, 3): Condic | 190.4641 | 3 | <0.0001 |
| Condic: Sam_d | 945.3627 | 12 | <0.0001 |
| Sam_d: Sol_rad | 321.9021 | 12 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Méndez, V.; González-Gómez, R.; Toledo, J.; Valle-Mora, J.F.; Barrera, J.F. Effect of Microclimate on the Mass Emergence of Hypothenemus hampei in Coffee Grown under Shade of Trees and in Full Sun Exposure. Insects 2024, 15, 124. https://doi.org/10.3390/insects15020124
García-Méndez V, González-Gómez R, Toledo J, Valle-Mora JF, Barrera JF. Effect of Microclimate on the Mass Emergence of Hypothenemus hampei in Coffee Grown under Shade of Trees and in Full Sun Exposure. Insects. 2024; 15(2):124. https://doi.org/10.3390/insects15020124
Chicago/Turabian StyleGarcía-Méndez, Valentina, Rebeca González-Gómez, Jorge Toledo, Javier Francisco Valle-Mora, and Juan F. Barrera. 2024. "Effect of Microclimate on the Mass Emergence of Hypothenemus hampei in Coffee Grown under Shade of Trees and in Full Sun Exposure" Insects 15, no. 2: 124. https://doi.org/10.3390/insects15020124
APA StyleGarcía-Méndez, V., González-Gómez, R., Toledo, J., Valle-Mora, J. F., & Barrera, J. F. (2024). Effect of Microclimate on the Mass Emergence of Hypothenemus hampei in Coffee Grown under Shade of Trees and in Full Sun Exposure. Insects, 15(2), 124. https://doi.org/10.3390/insects15020124