Naturally Occurring Vegetation Connectivity Facilitates Ant-Mediated Coffee Berry Borer Removal
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Experiment
2.3. Sampling
2.3.1. Ant Activity
2.3.2. Pest Removal
2.3.3. Resource Recruitment
2.3.4. Establishing Foraging Pathways over Time
2.4. Data Analysis
3. Results
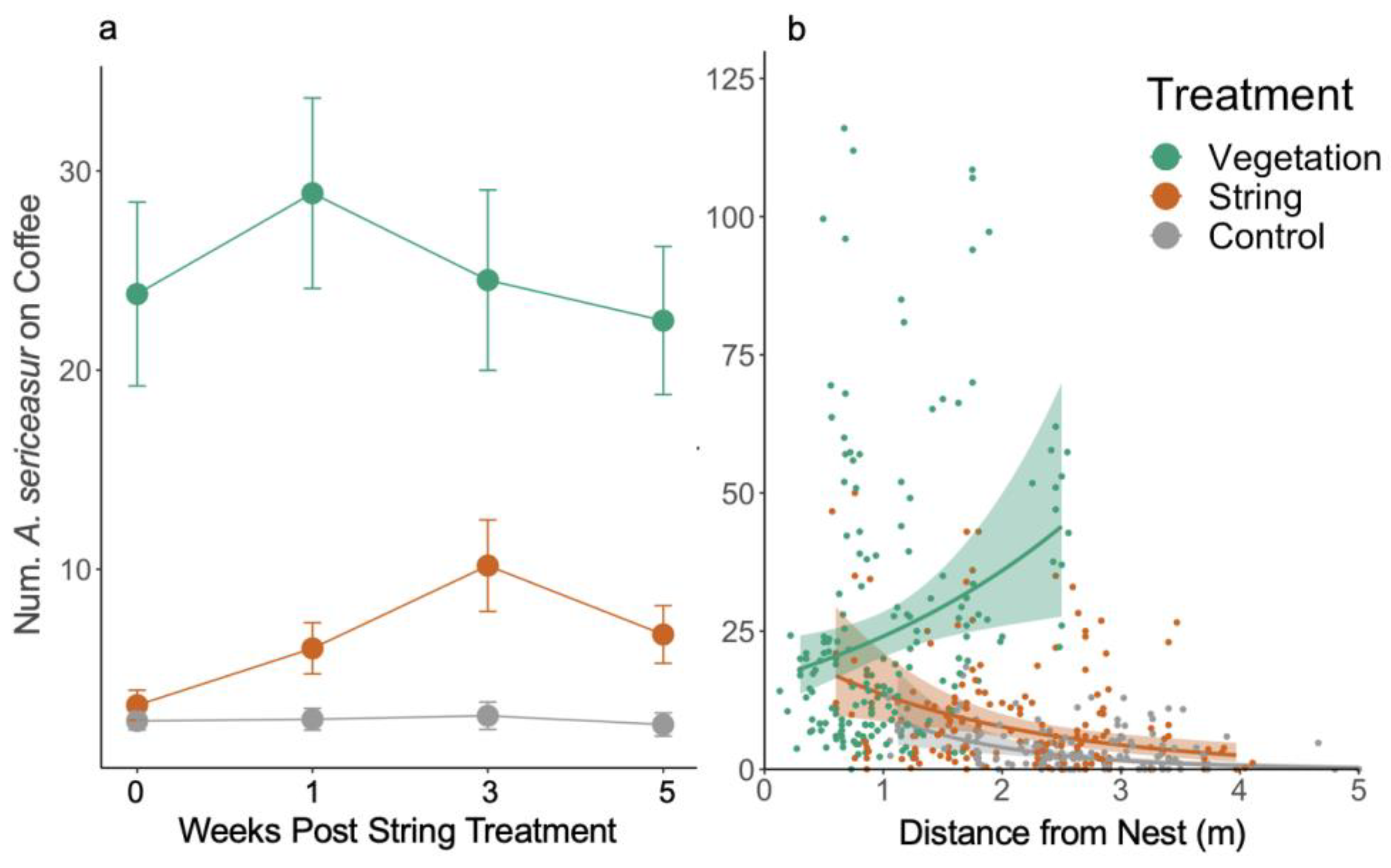
3.1. Ant Activity
3.2. Resource Recruitment
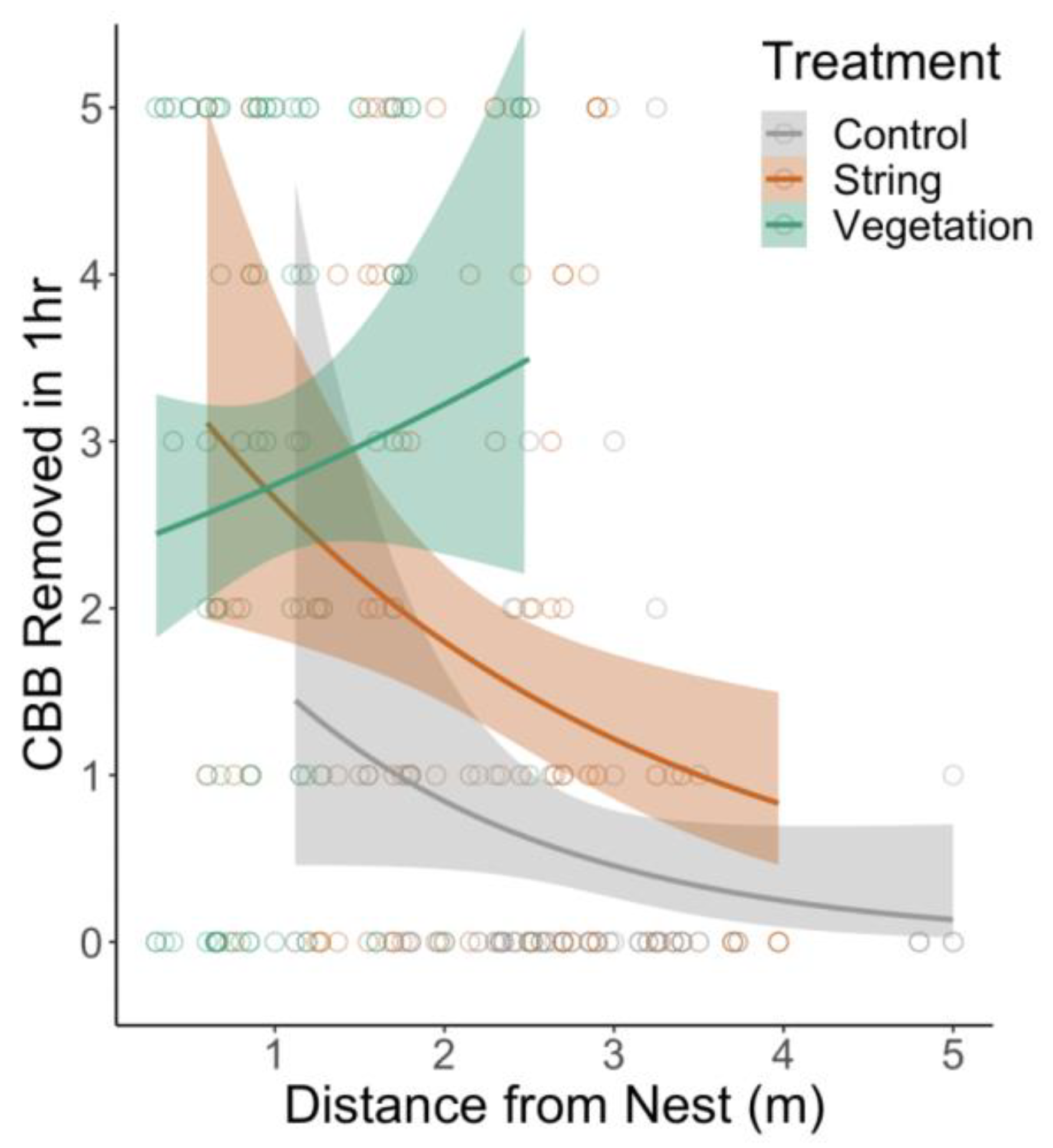
3.3. Pest Removal
4. Discussion
4.1. Ant Activity
4.2. Resource Recruitment
4.3. Pest Removal
4.4. Management Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swift, M.J.; Vandermeer, J.; Ramakrishnan, P.S.; Anderson, J.M.; Ong, C.K.; Hawkins, B.A. Biodiversity and Agroecosystem Function. In Functional Roles of Biodiversity: A Global Perspective; Mooney, H.A., Cushman, J.H., Medina, E., Sala, O.E., Schulze, E.D., Eds.; Wiley: New York, NY, USA, 1996; pp. 261–297. [Google Scholar]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape Perspectives on Agricultural Intensification and Biodiversity—Ecosystem Service Management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural Intensification and Ecosystem Properties; Kluwer Academic: Norwell, MA, USA, 1997; Volume 379. [Google Scholar]
- Lassau, S.A.; Hochuli, D.F. Effects of Habitat Complexity on Ant Assemblages. Ecography 2004, 27, 157–164. [Google Scholar] [CrossRef]
- Damptey, F.G.; Opuni-Frimpong, E.; Nsor, C.A.; Addai, J.; Debrah, D.K.; Schnerch, B.; Bentsi-Enchill, F.; Korjus, H. Taxonomic and Community Composition of Epigeal Arthropods in Monoculture and Mixed Tree Species Plantations in a Deciduous Forest of Ghana. J. For. Res. 2023, 34, 641–653. [Google Scholar] [CrossRef]
- Uetz, G. The Influence of Variation in Litter Habitats on Spider Communities. Oecologia 1979, 40, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Armbrecht, I.; Rivera, L.; Perfecto, I. Reduced Diversity and Complexity in the Leaf-Litter Ant Assemblage of Colombian Coffee Plantations. Conserv. Biol. 2005, 19, 897–907. [Google Scholar] [CrossRef]
- Gardner, S.M.; Cabido, M.R.; Valladares, G.R.; Diaz, S. The Influence of Habitat Structure on Arthropod Diversity in Argentine Semi-Arid Chaco Forest. J. Veg. Sci. 1995, 6, 349–356. [Google Scholar] [CrossRef]
- Hansen, R.A. Effects of Habitat Complexity and Composition on a Diverse Litter Microarthropod Assemblage. Ecology 2000, 81, 1120–1132. [Google Scholar] [CrossRef]
- Wilby, A.; Thomas, M.B. Natural Enemy Diversity and Pest Control: Patterns of Pest Emergence with Agricultural Intensification. Ecol. Lett. 2002, 5, 353–360. [Google Scholar] [CrossRef]
- Anjos, D.; Tena, A.; Viana-Junior, A.; Carvalho, R.; Torezan-Silingard, H.; Del-Claro, K.; Perfecto, I. The Effects of Ants on Pest Control: A Meta-Analysis. Proc. R. Soc. B Biol. Sci. 2022, 289, 20221316. [Google Scholar] [CrossRef]
- Staver, C.; Guharay, F.; Monterroso, D.; Muschler, R.G. Designing Pest-Suppressive Multistrata Perennial Crop Systems: Shade-Grown Coffee in Central America. Agrofor. Syst. 2001, 53, 151–170. [Google Scholar] [CrossRef]
- Pumariño, L.; Sileshi, G.W.; Gripenberg, S.; Kaartinen, R.; Barrios, E.; Muchane, M.N.; Midega, C.; Jonsson, M. Effects of Agroforestry on Pest, Disease and Weed Control: A Meta-Analysis. Basic Appl. Ecol. 2015, 16, 573–582. [Google Scholar] [CrossRef]
- Swift, M.J.; Izac, A.M.N.; Van Noordwijk, M. Biodiversity and Ecosystem Services in Agricultural Landscapes—Are We Asking the Right Questions? Proc. Agric. Ecosyst. Environ. 2004, 104, 113–134. [Google Scholar] [CrossRef]
- Gordon, C.; Manson, R.; Sundberg, J.; Cruz-Angón, A. Biodiversity, Profitability, and Vegetation Structure in a Mexican Coffee Agroecosystem. Agric. Ecosyst. Environ. 2007, 118, 256–266. [Google Scholar] [CrossRef]
- Perfecto, I.; Rice, R.A.; Greenberg, R.; Van Der Voort, M.E. Shade Coffee: A Disappearing Refuge for Biodiversity. BioScience 1996, 46, 598–608. [Google Scholar] [CrossRef]
- Mas, A.H.; Dietsch, T.V. An Index of Management Intensity for Coffee Agroecosystems to Evaluate Butterfly Species Richness. Ecol. Appl. 2003, 13, 1491–1501. [Google Scholar] [CrossRef]
- Moguel, P.; Toledo, V.M. Biodiversity Conservation in Traditional Coffee Systems of Mexico. Conserv. Biol. 1999, 13, 11–21. [Google Scholar] [CrossRef]
- Hernández-Martínez, G.; Manson, R.H.; Hernández, A.C. Quantitative Classification of Coffee Agroecosystems Spanning a Range of Production Intensities in Central Veracruz, Mexico. Agric. Ecosyst. Environ. 2009, 134, 89–98. [Google Scholar] [CrossRef]
- Greenberg, R.; Bichier, P.; Angon, A.C.; Reitsma, R. Bird Populations in Shade and Sun Coffee Plantations in Central Guatemala. Conserv. Biol. 1997, 11, 448–459. [Google Scholar] [CrossRef]
- Jimenez-Soto, E.; Morris, J.R.; Letourneau, D.K.; Philpott, S.M. Vegetation Connectivity Increases Ant Activity and Potential for Ant-Provided Biocontrol Services in a Tropical Agroforest. Biotropica 2019, 51, 50–61. [Google Scholar] [CrossRef]
- Powell, S.; Costa, A.N.; Lopes, C.T.; Vasconcelos, H.L. Canopy Connectivity and the Availability of Diverse Nesting Resources Affect Species Coexistence in Arboreal Ants. J. Anim. Ecol. 2011, 80, 352–360. [Google Scholar] [CrossRef]
- Yanoviak, S.; Schnitzer, S. Functional Roles of Lianas for Forest Canopy Animals. In Treetops at Risk; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-7160-8. [Google Scholar]
- Yanoviak, S.P. Effects of Lianas on Canopy Arthropod Community Structure. In Ecology of Lianas; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 343–361. ISBN 9781118392409. [Google Scholar]
- Clay, N.A.; Bauer, M.; Solis, M.; Yanoviak, S.P. Arboreal Substrates Influence Foraging in Tropical Ants. Ecol. Entomol. 2010, 35, 417–423. [Google Scholar] [CrossRef]
- Adams, B.J.; Schnitzer, S.A.; Yanoviak, S.P. Trees as Islands: Canopy Ant Species Richness Increases with the Size of Liana-Free Trees in a Neotropical Forest. Ecography 2017, 40, 1067–1075. [Google Scholar] [CrossRef]
- Wildtruth, F.; Perfecto, I. Effects of Canopy Connectivity on the Arboreal Ant Community in Coffee Shade Trees. Biotropica 2023, 1–8. [Google Scholar] [CrossRef]
- Loreto, R.G.; Hart, A.G.; Pereira, T.M.; Freitas, M.L.R.; Hughes, D.P.; Elliot, S.L. Foraging Ants Trade off Further for Faster: Use of Natural Bridges and Trunk Trail Permanency in Carpenter Ants. Naturwissenschaften 2013, 100, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Gibb, H.; Parr, C.L. How Does Habitat Complexity Affect Ant Foraging Success? A Test Using Functional Measures on Three Continents. Oecologia 2010, 164, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Radnan, G.N.; Gibb, H.; Eldridge, D.J. Soil Surface Complexity Has a Larger Effect on Food Exploitation by Ants than a Change from Grassland to Shrubland. Ecol. Entomol. 2018, 43, 379–388. [Google Scholar] [CrossRef]
- Newson, J.; Vandermeer, J.; Perfecto, I. Differential Effects of Ants as Biological Control of the Coffee Berry Borer in Puerto Rico. Biol. Control 2021, 160, 104666. [Google Scholar] [CrossRef]
- Morris, J.R.; Jiménez-soto, E.; Philpott, S.M.; Perfecto, I. Ant-Mediated (Hymenoptera: Formicidae) Biological Control of the Coffee Berry Borer: Diversity, Ecological Complexity, and Conservation Biocontrol. Myrmecol. News 2018, 26, 1–17. [Google Scholar]
- Gonthier, D.J.; Ennis, K.K.; Philpott, S.M.; Vandermeer, J.; Perfecto, I. Ants Defend Coffee from Berry Borer Colonization. BioControl 2013, 58, 815–820. [Google Scholar] [CrossRef]
- Morris, J.R.; Perfecto, I. An Aggressive Nonconsumptive Effect Mediates Pest Control and Multipredator Interactions in a Coffee Agroecosystem. Ecol. Appl. 2022, 32, e2653. [Google Scholar] [CrossRef]
- David Morgan, E. Trail Pheromones of Ants. Physiol. Entomol. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Schmitt, L.; Aponte-Rolón, B.; Perfecto, I. Evaluating Community Effects of a Keystone Ant, Azteca sericeasur, on Inga Micheliana Leaf Litter Decomposition in a Shaded Coffee Agro-Ecosystem. Biotropica 2020, 52, 1253–1261. [Google Scholar] [CrossRef]
- Philpott, S.M.; Perfecto, I.; Vandermeer, J. Effects of Management Intensity and Season on Arboreal Ant Diversity and Abundance in Coffee Agroecosystems. Biodivers. Conserv. 2006, 15, 139–155. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Zurich, E.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Philpott, S.M.; Perfecto, I.; Vandermeer, J.; Uno, S. Spatial Scale and Density Dependence in a Host Parasitoid System: An Arboreal Ant, Azteca Instabilis, and Its Pseudacteon Phorid Parasitoid. Environ. Entomol. 2009, 38, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M. The Dynamics of Foraging Trails in the Tropical Arboreal Ant Cephalotes Goniodontus. PLoS ONE 2012, 7, e0050472. [Google Scholar] [CrossRef] [PubMed]
- Yanoviak, S.P.; Silveri, C.; Stark, A.Y.; Van Stan, J.T.; Levia, D.F. Surface Roughness Affects the Running Speed of Tropical Canopy Ants. Biotropica 2017, 49, 92–100. [Google Scholar] [CrossRef]
- Perfecto, I.; Vandermeer, J. The Effect of an Ant-Hemipteran Mutualism on the Coffee Berry Borer (Hypothenemus hampei) in Southern Mexico. Agric. Ecosyst. Environ. 2006, 117, 218–221. [Google Scholar] [CrossRef]
- Livingston, G.F.; White, A.M.; Kratz, C.J. Indirect Interactions Between Ant-Tended Hemipterans, a Dominant Ant Azteca Instabilis (Hymenoptera: Formicidae), and Shade Trees in a Tropical Agroecosystem. Environ. Entomol. 2008, 37, 734–740. [Google Scholar] [CrossRef]
- Jha, S.; Allen, D.; Liere, H.; Perfecto, I.; Vandermeer, J. Mutualisms and Population Regulation: Mechanism Matters. PLoS ONE 2012, 7, e0043510. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Liere, H.; Soto, E.J.; Perfecto, I. Cascading Trait-Mediated Interactions Induced by Ant Pheromones. Ecol. Evol. 2012, 2, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Farji-Brener, A.G.; Barrantes, G.; Laverde, O.; Fierro-Calderón, K.; Bascopé, F.; López, A. Fallen Branches as Part of Leaf-Cutting Ant Trails: Their Role in Resource Discovery and Leaf Transport Rates in Atta Cephalotes. Biotropica 2007, 39, 211–215. [Google Scholar] [CrossRef]
- Denny, A.J.; Wright, J.; Grief, B. Foraging Efficiency in the Wood Ant, Formica Rufa: Is Time of the Essence in Trail following? Anim. Behav. 2001, 62, 139–146. [Google Scholar] [CrossRef][Green Version]
- Philpott, S.M.; Maldonado, J.; Vandermeer, J.; Perfecto, I. Taking Trophic Cascades up a Level: Behaviorally-Modified Effects of Phorid Flies on Ants and Ant Prey in Coffee Agroecosystems. Oikos 2004, 105, 141–147. [Google Scholar] [CrossRef]
- Pardee, G.L.; Philpott, S.M. Cascading Indirect Effects in a Coffee Agroecosystem: Effects of Parasitic Phorid Flies on Ants and the Coffee Berry Borer in a High-Shade and Low-Shade Habitat. Environ. Entomol. 2011, 40, 581–588. [Google Scholar] [CrossRef] [PubMed]
- KAREIVA, P.; PERRY, R. Leaf Overlap and the Ability of Ladybird Beetles to Search among Plants. Ecol. Entomol. 1989, 14, 127–129. [Google Scholar] [CrossRef]
- Skirvin, D.; Fenlon, J. Of Mites and Movement: The Effects of Plant Connectedness and Temperature on Movement of Phytoseiulus Persimilis. Biol. Control 2003, 27, 242–250. [Google Scholar] [CrossRef]
- Armbrecht, I.; Gallego, M.C. Testing Ant Predation on the Coffee Berry Borer in Shaded and Sun Coffee Plantations in Colombia. Entomol. Exp. Appl. 2007, 124, 261–267. [Google Scholar] [CrossRef]
- Gingras, D.; Boivin, G. Effect Of Plant Structure, Host Density And Foraging Duration On Host Finding By Trichogramma Evanescens (Hymenoptera: Trichogrammatidae). Community Ecosyst. Ecol. 2002, 31, 1153–1157. [Google Scholar]
- Cloyd, R.A.; Sadof, C.S. Effects of Plant Architecture on the Attack Rate of Leptomastix Dactylopii (Hymenoptera: Encyrtidae), a Parasitoid of the Citrus Mealybug (Homoptera: Pseudococcidae). Environ. Entomol. 2000, 29, 535–541. [Google Scholar] [CrossRef]
- Jha, S.; Vandermeer, J.H.; Perfecto, I. Population Dynamics of Coccus Viridis, a Ubiquitous Ant-Tended Agricultural Pest, Assessed by a New Photographic Method. Bull. Insectology 2009, 62, 183–189. [Google Scholar]
- Vandermeer, J.; Perfecto, I.; Liere, H. Evidence for Hyperparasitism of Coffee Rust (Hemileia vastatrix) by the Entomogenous Fungus, Lecanicillium lecanii, through a Complex Ecological Web. Plant Pathol. 2009, 58, 636–641. [Google Scholar] [CrossRef]
- Alarcón, R.; Carrión, G. Uso de Verticillium Lecanii En Cafetales Como Control Biológico de La Roya Del Cafeto. Fitopatología 1994, 29, 82–85. [Google Scholar]
- Jackson, D.; Skillman, J.; Vandermeer, J. Indirect Biological Control of the Coffee Leaf Rust, Hemileia vastatrix, by the Entomogenous Fungus Lecanicillium lecanii in a Complex Coffee Agroecosystem. Biol. Control 2012, 61, 89–97. [Google Scholar] [CrossRef]
- Jackson, D.; Zemenick, K.; Huerta, G. Occurrence in the Soil and dispersal of Lecanicillium lecanii, a Fungal Pathogen of the Green Coffee Scale (Coccus Viridis) and Coffee Rust (Hemileia vastatrix). Trop. Subtrop. 2012, 15, 389–401. [Google Scholar]
- Rivera-Salinas, I.S.; Hajian-Forooshani, Z.; Jiménez-Soto, E.; Cruz-Rodríguez, J.A.; Philpott, S.M. High Intermediary Mutualist Density Provides Consistent Biological Control in a Tripartite Mutualism. Biol. Control 2018, 118, 26–31. [Google Scholar] [CrossRef]
- Offenberg, J. Ants as Tools in Sustainable Agriculture. J. Appl. Ecol. 2015, 52, 1197–1205. [Google Scholar] [CrossRef]
- Wielgoss, A.; Tscharntke, T.; Rumede, A.; Fiala, B.; Seidel, H.; Shahabuddin, S.; Clough, Y. Interaction Complexity Matters: Disentangling Services and Disservices of Ant Communities Driving Yield in Tropical Agroecosystems. Proc. R. Soc. B Biol. Sci. 2013, 281, 20132144. [Google Scholar] [CrossRef]
- Vandermeer, J.; Perfecto, I.; Philpott, S. Ecological Complexity and Pest Control in Organic Coffee Production: Uncovering an Autonomous Ecosystem Service. Bioscience 2010, 60, 527–537. [Google Scholar] [CrossRef]
- Klimes, P.; Fibich, P.; Idigel, C.; Rimandai, M. Disentangling the Diversity of Arboreal Ant Communities in Tropical Forest Trees. PLoS ONE 2015, 10, e0117853. [Google Scholar] [CrossRef]
- Jaramillo, J.; Muchugu, E.; Vega, F.E.; Davis, A.; Borgemeister, C.; Chabi-Olaye, A. Some like It Hot: The Influence and Implications of Climate Change on Coffee Berry Borer (Hypothenemus hampei) and Coffee Production in East Africa. PLoS ONE 2011, 6, e0024528. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Ruiz-Diaz, C.P.; Manoukis, N.C.; Rodrigues, J.C.V. Coffee Berry Borer (Hypothenemus hampei), a Global Pest of Coffee: Perspectives from Historical and Recent Invasions, and Future Priorities. Insects 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]


| Fixed Effects | Estimate | Std Error | Z Value | Pr(>|z|) |
|---|---|---|---|---|
| Reference: Treatment (Control) | ||||
| (Intercept) | 1.030 | 0.300 | 3.435 | <0.001 |
| TreatmentVegetation | 2.138 | 0.440 | 4.862 | <0.001 |
| TreatmentString | 0.250 | 0.312 | 0.800 | 0.424 |
| Time | −0.062 | 0.082 | −0.758 | 0.449 |
| Distance | −1.291 | 0.307 | −4.204 | <0.001 |
| TreatmentVegetation:Time | 0.013 | 0.098 | 0.136 | 0.891 |
| TreatmentString:Time | 0.372 | 0.109 | 3.423 | <0.001 |
| TreatmentVegetation:Distance | 1.648 | 0.435 | 3.787 | <0.001 |
| TreatmentString:Distance | 0.265 | 0.375 | 0.707 | 0.480 |
| Reference: Treatment (String) | ||||
| (Intercept) | 1.279 | 0.230 | 5.572 | <0.001 |
| TreatmentControl | −0.250 | 0.312 | −0.800 | 0.424 |
| TreatmentVegetation | 1.888 | 0.388 | 4.867 | <0.001 |
| Time | 0.310 | 0.072 | 4.316 | <0.001 |
| Distance | −1.026 | 0.250 | −4.101 | <0.001 |
| TreatmentControl:Time | −0.372 | 0.109 | −3.423 | <0.001 |
| TreatmentVegetation:Time | −0.359 | 0.090 | −3.974 | <0.001 |
| TreatmentControl:Distance | −0.265 | 0.375 | −0.707 | 0.480 |
| TreatmentVegetation:Distance | 1.384 | 0.402 | 3.438 | <0.001 |
| Reference: Treatment (Vegetation) | ||||
| (Intercept) | 3.167 | 0.373 | 8.481 | <0.001 |
| TreatmentString | −1.888 | 0.388 | −4.867 | <0.001 |
| TreatmentControl | −2.138 | 0.440 | −4.862 | <0.001 |
| Time | −0.048 | 0.055 | −0.887 | 0.375 |
| Distance | 0.357 | 0.322 | 1.108 | 0.268 |
| TreatmentString:Time | 0.359 | 0.090 | 3.974 | <0.001 |
| TreatmentControl:Time | −0.013 | 0.098 | −0.136 | 0.891 |
| TreatmentString:Distance | −1.384 | 0.402 | −3.438 | <0.001 |
| TreatmentControl:Distance | −1.648 | 0.435 | −3.787 | <0.001 |
| Fixed Effects | Estimate | Std Error | Z Value | Pr(>|z|) |
|---|---|---|---|---|
| Reference: Treatment (Control) | ||||
| (Intercept) | 0.509 | 0.471 | 1.082 | 0.279 |
| TreatmentVegetation | 2.528 | 0.654 | 3.867 | <0.001 |
| TreatmentString | 0.961 | 0.467 | 2.059 | 0.039 |
| Time | −0.493 | 0.191 | −2.576 | 0.010 |
| Distance | −1.198 | 0.426 | −2.815 | 0.005 |
| TreatmentVegetation:Time | 0.247 | 0.228 | 1.086 | 0.278 |
| TreatmentString:Time | 0.612 | 0.240 | 2.549 | 0.011 |
| TreatmentVegetation:Distance | 1.187 | 0.606 | 1.959 | 0.050 |
| TreatmentString:Distance | −0.106 | 0.519 | −0.205 | 0.838 |
| Reference: Treatment (String) | ||||
| (Intercept) | 1.470 | 0.357 | 4.121 | <0.001 |
| TreatmentControl | −0.961 | 0.467 | −2.060 | 0.039 |
| TreatmentVegetation | 1.567 | 0.558 | 2.809 | 0.005 |
| Time | 0.119 | 0.145 | 0.819 | 0.413 |
| Distance | −1.304 | 0.367 | −3.549 | <0.001 |
| TreatmentControl:Time | −0.612 | 0.240 | −2.549 | 0.011 |
| TreatmentVegetation:Time | −0.364 | 0.191 | −1.913 | 0.056 |
| TreatmentControl:Distance | 0.106 | 0.519 | 0.205 | 0.838 |
| TreatmentVegetation:Distance | 1.294 | 0.581 | 2.226 | 0.026 |
| Reference: Treatment (Vegetation) | ||||
| (Intercept) | 3.037 | 0.550 | 5.520 | <0.001 |
| TreatmentString | −1.567 | 0.558 | −2.809 | 0.005 |
| TreatmentControl | −2.528 | 0.654 | −3.867 | <0.001 |
| Time | −0.246 | 0.124 | −1.983 | 0.047 |
| Distance | −0.010 | 0.461 | −0.023 | 0.982 |
| TreatmentString:Time | 0.364 | 0.191 | 1.913 | 0.056 |
| TreatmentControl:Time | −0.247 | 0.228 | −1.086 | 0.278 |
| TreatmentString:Distance | −1.294 | 0.581 | −2.226 | 0.026 |
| TreatmentControl:Distance | −1.187 | 0.606 | −1.959 | 0.050 |
| Fixed Effects: | Estimate | Std. Error | Z Value | Pr(>|z|) |
|---|---|---|---|---|
| Reference: Treatment (Control) | ||||
| (Intercept) | −0.244 | 0.254 | −0.960 | 0.337 |
| TreatmentVegetation | 1.250 | 0.331 | 3.780 | <0.001 |
| TreatmentString | 0.621 | 0.258 | 2.408 | 0.016 |
| Time | −0.238 | 0.182 | −1.307 | 0.191 |
| Distance | −0.779 | 0.247 | −3.147 | 0.002 |
| TreatmentVegetation:Time | −0.081 | 0.204 | −0.397 | 0.691 |
| TreatmentString:Time | 0.209 | 0.212 | 0.985 | 0.324 |
| TreatmentVegetation:Distance | 0.841 | 0.326 | 2.580 | 0.010 |
| TreatmentString:Distance | 0.259 | 0.293 | 0.885 | 0.376 |
| Reference: Treatment (String) | ||||
| (Intercept) | 0.377 | 0.175 | 2.153 | 0.031 |
| TreatmentControl | −0.621 | 0.258 | −2.410 | 0.016 |
| TreatmentVegetation | 0.629 | 0.271 | 2.320 | 0.020 |
| Time | −0.030 | 0.107 | −0.276 | 0.782 |
| Distance | −0.520 | 0.182 | −2.854 | 0.004 |
| TreatmentControl:Time | −0.208 | 0.211 | −0.983 | 0.325 |
| TreatmentVegetation:Time | −0.289 | 0.140 | −2.063 | 0.039 |
| TreatmentControl:Distance | −0.259 | 0.293 | −0.885 | 0.376 |
| TreatmentVegetation:Distance | 0.582 | 0.282 | 2.067 | 0.039 |
| Reference: Treatment (Vegetation) | ||||
| (Intercept) | 1.007 | 0.263 | 3.822 | <0.001 |
| TreatmentString | −0.630 | 0.271 | −2.320 | 0.020 |
| TreatmentControl | −1.250 | 0.331 | −3.779 | <0.001 |
| Time | −0.319 | 0.091 | −3.526 | <0.001 |
| Distance | 0.063 | 0.223 | 0.281 | 0.778 |
| TreatmentString:Time | 0.290 | 0.141 | 2.058 | 0.040 |
| TreatmentControl:Time | 0.080 | 0.204 | 0.395 | 0.693 |
| TreatmentString:Distance | −0.582 | 0.282 | −2.067 | 0.039 |
| TreatmentControl:Distance | −0.841 | 0.326 | −2.580 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cowal, S.; Morris, J.R.; Jiménez-Soto, E.; Philpott, S.M. Naturally Occurring Vegetation Connectivity Facilitates Ant-Mediated Coffee Berry Borer Removal. Insects 2023, 14, 869. https://doi.org/10.3390/insects14110869
Cowal S, Morris JR, Jiménez-Soto E, Philpott SM. Naturally Occurring Vegetation Connectivity Facilitates Ant-Mediated Coffee Berry Borer Removal. Insects. 2023; 14(11):869. https://doi.org/10.3390/insects14110869
Chicago/Turabian StyleCowal, Sanya, Jonathan R. Morris, Estelí Jiménez-Soto, and Stacy M. Philpott. 2023. "Naturally Occurring Vegetation Connectivity Facilitates Ant-Mediated Coffee Berry Borer Removal" Insects 14, no. 11: 869. https://doi.org/10.3390/insects14110869
APA StyleCowal, S., Morris, J. R., Jiménez-Soto, E., & Philpott, S. M. (2023). Naturally Occurring Vegetation Connectivity Facilitates Ant-Mediated Coffee Berry Borer Removal. Insects, 14(11), 869. https://doi.org/10.3390/insects14110869







