Pierid Butterflies, Legume Hostplants, and Parasitoids in Urban Areas of Southern Florida
Abstract
Simple Summary
Abstract
1. Introduction
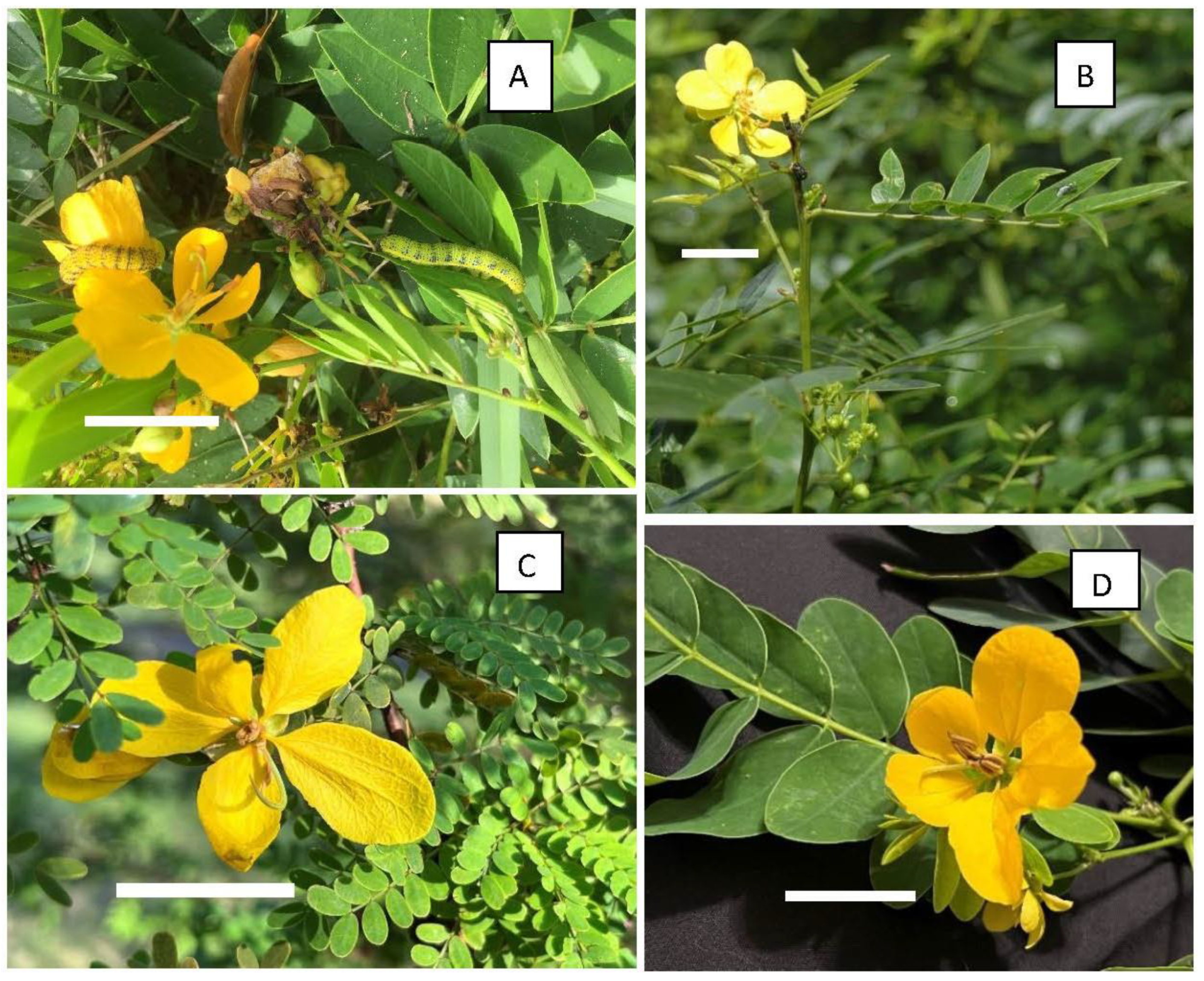
2. Materials and Methods
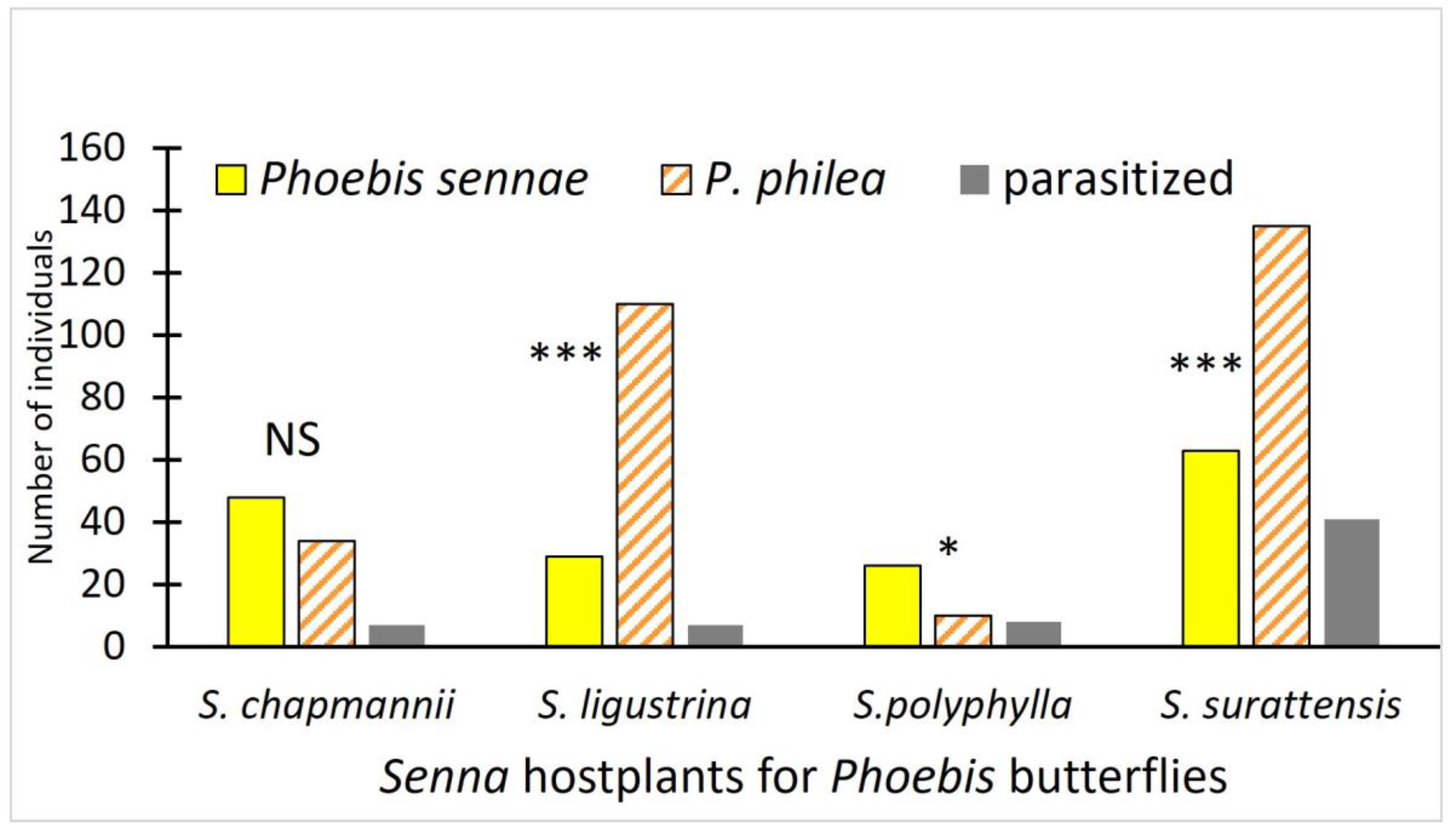
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrlich, P.R.; Raven, P.H. Butterflies and Plants: A Study in Coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Minno, M.C.; Minno, M. Florida Butterfly Gardening. A Complete Guide to Attracting, Identifying, and Enjoying Butterflies of the Lower South; University Press of Florida: Gainesville, FL, USA, 1999; 210p. [Google Scholar]
- Wagner, D.L. Caterpillars of Eastern North America: A Guide to Identification and Natural History; Princeton Field Guides; Princeton University Press: Princeton, NJ, USA, 2005; 512p. [Google Scholar]
- Braga, M.P.; Janz, N.; Nylin, S.; Ronquist, F.; Landis, M.J. Phylogenetic reconstruction of ancestral ecological networks through time for pierid butterflies and their host plants. Ecol. Lett. 2021, 24, 2134–2145. [Google Scholar] [CrossRef]
- Dethier, V.G. Evolution of feeding preferences in phytophagous insects. Evolution 1954, 8, 33–54. [Google Scholar] [CrossRef]
- Brower, L.P. Bird predation and food plant specificity in closely related procryptic insects. Am. Nat. 1958, 92, 183–187. [Google Scholar] [CrossRef]
- Koptur, S.; Jones, I.M.; Pena, J.E. The Influence of Host Plant Extrafloral Nectaries on Multitrophic Interactions: An Experimental Investigation. PLoS ONE 2015, 10, e0138157. [Google Scholar] [CrossRef]
- Koptur, S.; Clayborn, J.; Harris, B.; Jones, I.M.; Pimienta, M.C.; Salas Primoli, A.; Oliveira, P.S. Caterpillar responses to ant protectors of plants. In Caterpillars in the Middle: Tritrophic Interactions in a Changing World; Marquis, R.J., Koptur, S., Eds.; Springer Nature: Cham, Switzerland, 2022; Chapter 10; pp. 297–317. [Google Scholar]
- Skelhorn, J.; Ruxton, G.D. Predators are less likely to misclassify masquerading prey when their models are present. Biol. Lett. 2010, 6, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, G.D.; Allen, W.L.; Sherratt, T.N.; Speed, M.P. Avoiding Attack: The Evolutionary Ecology of Crypsis, Aposematism, and Mimicry, 2nd ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Bernays, E.A.; Cornelius, M. L Generalist caterpillar prey are more palatable than specialists for the generalist predator Iridimyrmex humilis. Oecologia 1989, 79, 427–430. [Google Scholar] [CrossRef]
- Dyer, L.A. Effectiveness of caterpillar defenses against three species of invertebrate predators. J. Res. Lepid. 1997, 34, 48–68. [Google Scholar] [CrossRef]
- Henrique, A.; Portugal, A.; Trigo, J.R. Similarity of cuticular lipids between a caterpillar and its host plant: A way to make prey undetectable for predatory ants? J. Chem. Ecol. 2005, 31, 2551–2561. [Google Scholar] [CrossRef]
- Gaitonde, N.; Joshi, J.; Kunte, K. Evolution of ontogenic change in color defenses of swallowtail butterflies. Ecol. Evol. 2018, 8, 9751–9763. [Google Scholar] [CrossRef]
- Bentley, B.L. Extrafloral nectaries and protection by pugnacious bodyguards. Annu. Rev. Ecol. Syst. 1977, 88, 407–427. [Google Scholar] [CrossRef]
- Koptur, S. Interactions between Insects and Plants Mediated by Extrafloral Nectaries. In Insect/Plant Interactions, Bernays, E., Ed.; CRC Press: Boca Raton, FL, USA, 1992; Volume 4, pp. 85–132. [Google Scholar]
- Rosumek, F.B.; Silveira, F.A.O.; Neves, F.d.S.; Barbosa, N.P.d.U.; Diniz, L.; Oki, Y.; Pezzini, F.; Fernandes, G.W.; Cornelissen, T. Ants on plants: A meta-analysis of the role of ants as plant biotic defenses. Oecologia 2009, 160, 537–549. [Google Scholar] [CrossRef]
- Wackers, F.L.; van Rijn, P.C.J. Food for protection: An introduction. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Wackers, F.L., van Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 1–14. [Google Scholar]
- Marazzi, B.; Gonzalez, A.M.; Delgado-Salinas, A.; Luckow, M.A.; Ringelberg, J.J.; Hughes, C.E. Extrafloral nectaries in Leguminosae: Phylogenetic distribution, morphological diversity and evolution. Aust. Syst. Bot. 2019, 32, 409–458. [Google Scholar] [CrossRef]
- Keeler, K.H.; Porturas, L.D.; Weber, M.G. World List of Plants with Extrafloral Nectaries. Available online: www.extrafloralnectaries.org (accessed on 29 November 2023).
- Wackers, F.L. Suitability of (extra)floral nectar, pollen, and honeydew as insect foods. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Wackers, F.L., van Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 17–74. [Google Scholar]
- Hawkins, B.A.; Cornell, H.V.; Hochberg, M.E. Predators, Parasitoids, and Pathogens as Mortality Agents in Phytophagous Insect Populations. Ecology 1997, 78, 2145–2152. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Gouinguene, S.; Degen, T.; Fritzsche-Hoballah, M.E. The chemical ecology of plant-caterpillar-parasitoid interactions. In Multitrophic Level Interactions; Tscharntke, T., Hawkins, B.A., Eds.; Cambridge University Press: Cambridge, UK, 2002; Chapter 7; pp. 148–173. [Google Scholar]
- Stireman, J.O., III; Shaw, S.R. Natural history and ecology of caterpillar parasitoids. In Caterpillars in the Middle: Tritrophic Interactions in a Changing World; Springer: New York, NY, USA, 2022; Chapter 11; pp. 225–272. [Google Scholar]
- Turlings, T.C.J.; Erb, M. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Stireman, J.O., III; Singer, M.S. Determinants of parasitoid-host associations: Insights from a natural tachinid-lepidopteran community. Ecology 2003, 84, 296–310. [Google Scholar] [CrossRef]
- Hrcek, J.; Miller, S.E.; Whitfield, J.B.; Shima, H.; Novotny, V. Parasitism rate, parasitoid community composition and host specificity on exposed and semi-concealed caterpillars from a tropical rainforest. Oecologia 2013, 173, 521–532. [Google Scholar] [CrossRef]
- Ruberson, J.R.; Whitfield, J.B. Facultative egg-larval parasitism of the beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae) by Cotesia marginiventris (Hymenoptera: Braconidae). Fla. Entomol. 1996, 79, 296–302. [Google Scholar] [CrossRef]
- Gibson, G.A.P. The species of Eupelmus (Eupelmus) Dalman and Eupelmus (Episolindelia) Girault (Hymenoptera: Eupelmidae) in North America north of Mexico. Zootaxa 2011, 2951, 1–97. [Google Scholar] [CrossRef]
- Wheeler, G.S.; Dyer, K.; Wright, S.A. Seasonal abundance of the adventive Chinese tallowtree herbivore Caloptilia triadicae (Lepidoptera: Gracillariidae) and its parasitoids. Fla. Entomol. 2017, 100, 52–56. [Google Scholar] [CrossRef]
- Bach, C.E. Effects of plant density and diversity on the population dynamics of a specialist herbivore, the striped cucumber beetle, Acalymma vittata (Fab.). Ecology 1980, 611750, 1515–1531. [Google Scholar] [CrossRef]
- Rausher, M.D.; Feeny, P. Herbivory, plant density, and plant reproductive success: The effect of Battus philenor on Aristolochia reticulata. Ecology 1980, 61, 905–917. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; De Frenne, P.; Galmán, A.; Gaytán, Á.; Jaatinen, R.; Lago-Núñez, B.; Meeussen, C.; Pulkkinen, P.; Rasmussen, P.U.; et al. Effects of latitude and conspecific plant density on insect leaf herbivory in oak saplings and seedlings. Am. J. Bot. 2021, 108, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Lill, J.T.; Marquis, R.J.; Ricklefs, R.E. Host plants influence parasitism of forest caterpillars. Nature 2002, 417, 170–173. [Google Scholar] [CrossRef]
- Hassell, M.P. Host-parasitoid population dynamics. J. Anim. Ecol. 2000, 69, 543–566. [Google Scholar] [CrossRef]
- Braga, M.P.; Janz, N. Host repertoires and changing insect-plant interactions. Ecol. Entomol. 2021, 46, 1241–1253. [Google Scholar] [CrossRef]
- Strand, M.R.; Obrycky, J.J. Host specificity of insect parasitoids and predators. Bioscience 1996, 46, 422–429. [Google Scholar] [CrossRef]
- Stireman, J.O.; Dyer, L.A.; Greeney, H.F.; Didham, R.; Broad, G. Specialised generalists? Food web structure of a tropical tachinid-caterpillar community. Insect Conserv. Divers. 2017, 10, 367–384. [Google Scholar] [CrossRef]
- Smith, M.A.; Wood, D.M.; Janzen, D.H.; Hallwachs, W.; Hebert, P.D.N. DNA Barcodes Affirm That 16 Species of Apparently Generalist Tropical Parasitoid Flies (Diptera, Tachinidae) Are Not All Generalists. Proc. Natl. Acad. Sci. USA 2007, 104, 4967–4972. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Buckley, Y.M.; Memmott, J. Diet breadth influences how the impact of invasive plants is propagated through food webs. Ecology 2010, 91, 1063–1074. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Harvey, J.A.; Cronin, J.T. Response of native insect communities to invasive plants. Annu. Rev. Entomol. 2014, 59, 119–141. [Google Scholar] [CrossRef] [PubMed]
- López-Núñez, F.A.; Heleno, R.H.; Ribeiro, S.; Marchante, H.; Marchante, E. Four-trophic level food webs reveal the cascading impacts of an invasive plant targeted for biocontrol. Ecology 2017, 98, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Zemenick, A.T.; Kula, R.R.; Russo, L.; Tooker, J. A network approach reveals parasitoid wasps to be generalized nectar foragers. Arthropod.-Plant Interact. 2019, 13, 239–251. [Google Scholar] [CrossRef]
- Egerer, M.H.; Arel, C.; Otoshi, M.D.; Quistberg, R.D.; Bichier, P.; Philpott, S.M. Urban arthropods respond variably to changes in landscape context and spatial scale. J. Urban Ecol. 2017, 3, jux001. [Google Scholar] [CrossRef]
- Burks, J.M.; Philpott, S.M. Local and Landscape Drivers of Parasitoid Abundance, Richness, and Composition in Urban Gardens. Environ. Entomol. 2017, 46, 201–209. [Google Scholar] [CrossRef]
- Corcos, D.; Cerretti, P.; Caruso, V.; Mei, M.; Falco, M.; Marini, L. Impact of urbanization on predator and parasitoid insects at multiple spatial scales. PLoS ONE 2019, 14, e0214068. [Google Scholar] [CrossRef]
- Hochmair, H.H.; Benjamin, A.; Gann, D.; Juhasz, L.; Olivas, P.C.; Fu, J. Miami-Dade County Urban Tree Canopy Analysis; GIS Center: 2021. Florida International University: Miami, FL, USA. Available online: https://digitalcommons.fiu.edu/gis/89 (accessed on 21 January 2024).
- Lin, T.; Vrieling, K.; Laplanche, D.; Klinkhamer, P.cG.L.; Lou, Y.; Bekooy, L.; Degen, T.; Bustos-Segura, C.; Turlings, T.C.J.; Desurmont, G.A. Evolutionary changes in an invasive plant support the defensive role of plant volatiles. Curr. Biol. 2021, 31, 3450–3456.e5. [Google Scholar] [CrossRef]
| Sites | Pinecrest | South Miami | Westchester | All Sites Combined $Plant Species Totals | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant Species | Found | Counted | Parasitized | Found | Counted | Parasitized | Found | Counted | Parasitized | Found | Counted | Parasitized |
| S. chapmanii | 16 | 13 | 1 (8%) | 67 | 55 | 6 (11%) | 26 | 21 | 0 (0%) | 109 | 89 | 7 (8%) |
| S. ligustrina | 21 | 17 | 2 (12%) | 1 | 1 | 0 (0%) | 174 | 128 | 7 (5%) | 196 | 146 | 7 (5%) |
| S. polyphylla * | 5 | 5 | 2 (40%) | 45 | 32 | 5 (16%) | 8 | 7 | 1 (14%) | 58 | 44 | 8 (18%) |
| S. surattensis * | 17 | 16 | 3 (19%) | 83 | 65 | 14 (22%) | 231 | 158 | 24 (15%) | 331 | 239 | 41 (17%) |
| Totals on native plants | 37 | 30 | 3 (10%) a | 68 | 56 | 6 (11%) a | 200 | 149 | 7 (5%) a | 305 | 235 | 17 (7%) a |
| Totals on non-native plants | 22 | 21 | 5 (24%) a | 128 | 97 | 19 (20%) a | 239 | 165 | 25 (15%) b | 389 | 283 | 49 (17%) b |
| Overall totals on all plants | 59 | 51 | 8 (16%) | 196 | 153 | 25 (16%) | 439 | 314 | 32 (10%) | 694 | 518 | 66 (13%) |
| Taxon | Family | Senna chapmanii | S. ligustrina | S. polyphylla * | S. surattensis * |
|---|---|---|---|---|---|
| Glyptapanteles cassianus (Riley 1881) | Braconidae Microgastrinae | x | x | x | x |
| Brasema sp. | Chalcidoidea, Eupelmidae, Eupelminae | x | x | x | x |
| Encrateola maculithorax Ashmead, 1895 | Ichneumonidae | x | x | ||
| Lespesia parviteres (Aldrich and Webber, 1924) | Tachinidae | x | |||
| Mesochorus sp. (hyperparasitoid) | Ichneumonidae | x | x | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koptur, S.; Primoli, A.S.; Paulino-Neto, H.F.; Whitfield, J. Pierid Butterflies, Legume Hostplants, and Parasitoids in Urban Areas of Southern Florida. Insects 2024, 15, 123. https://doi.org/10.3390/insects15020123
Koptur S, Primoli AS, Paulino-Neto HF, Whitfield J. Pierid Butterflies, Legume Hostplants, and Parasitoids in Urban Areas of Southern Florida. Insects. 2024; 15(2):123. https://doi.org/10.3390/insects15020123
Chicago/Turabian StyleKoptur, Suzanne, Andrea Salas Primoli, Hipólito Ferreira Paulino-Neto, and James Whitfield. 2024. "Pierid Butterflies, Legume Hostplants, and Parasitoids in Urban Areas of Southern Florida" Insects 15, no. 2: 123. https://doi.org/10.3390/insects15020123
APA StyleKoptur, S., Primoli, A. S., Paulino-Neto, H. F., & Whitfield, J. (2024). Pierid Butterflies, Legume Hostplants, and Parasitoids in Urban Areas of Southern Florida. Insects, 15(2), 123. https://doi.org/10.3390/insects15020123







