Demography and Behaviour of Teinopodagrion oscillans (Odonata: Megapodagrionidae) in a Protected Area of the Colombian Andean Region
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species
2.3. Data Collection
2.4. Behavioural Study
2.5. Statistical Analyses
3. Results
3.1. Biometry and Population Structure
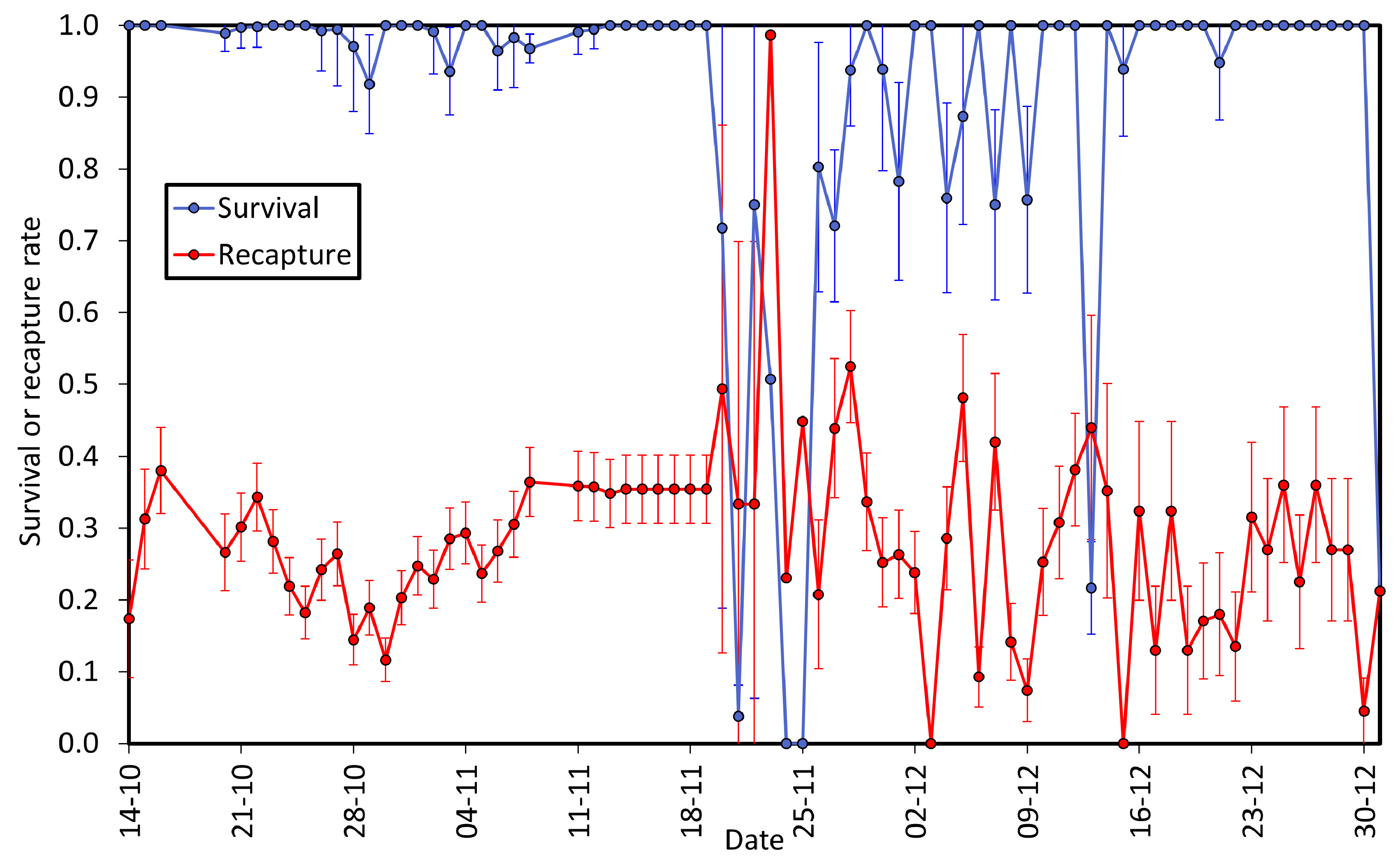
3.2. Survival and Recapture Rates
3.3. Behaviour
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, R.C. Handbook of Genetics: Volume 4 Vertebrates of Genetic Interest; Springer: Greer, SC, USA, 1975. [Google Scholar] [CrossRef]
- Morin, J.G.; Hastings, J.W. Biochemistry of the bioluminescence of colonial hydroids and other coelenterates. J. Cell. Physiol. 1971, 77, 305–312. [Google Scholar] [CrossRef]
- Naranjo, S.E. Influence of Two Mass-Marking Techniques on Survival and Flight Behavior of Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1990, 83, 1360–1364. [Google Scholar] [CrossRef]
- Davenport, D.; Nicol, J.A.C. Luminescence in Hydromedusae. Proc. R. Soc. Lond. Ser. B 1955, 144, 399–411. [Google Scholar]
- Bartlett, A.C. Isozyme Polymorphism in Populations of the Pink Bollworm12. Ann. Entomol. Soc. Am. 1981, 74, 9–13. [Google Scholar] [CrossRef]
- Fernandes, O.A.; Wright, R.J.; Baumgarten, K.H.; Mayo, Z.B. Use of Rubidium to Label Lysiphlebus testaceipes (Hymenoptera: Braconidae), a Parasitoid of Greenbugs (Homoptera: Aphididae), for Dispersal Studies. Environ. Entomol. 1997, 26, 1167–1172. [Google Scholar] [CrossRef]
- Kapp, R.O. How to Know Pollen and Spores; W. C. Brown Company: Dubuque, IA, USA, 1969. [Google Scholar]
- Carey, J.R. Insect biodemography. Annu. Rev. Entomol. 2001, 46, 79–110. [Google Scholar] [CrossRef]
- Kéry, M.; Schaub, M. Bayesian Population Analysis Using WinBUGS: A Hierarchical Perspective; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Lebreton, J.-D.; Burnham, K.P.; Clobert, J.; Anderson, D.R. Modeling Survival and Testing Biological Hypotheses Using Marked Animals: A Unified Approach with Case Studies. Ecol. Monogr. 1992, 62, 67–118. [Google Scholar] [CrossRef]
- Sanmartín-Villar, I.; Cordero-Rivera, A. Odonata survival: Insights from mark-recapture experiments. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Oxford Academic: Oxford, UK, 2022; pp. 129–140. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A. Adult Survival and Movement in Males of the Damselfly Hetaerina cruentata (Odonata: Calopterygidae). Fla. Entomol. 1994, 77, 256. [Google Scholar] [CrossRef]
- Altamiranda-Saavedra, M.; Ortega, O. Estructura poblacional de Polythore gigantea (Odonata: Polythoridae) en sistemas lóticos con diferentes estados de conservación en Antioquia, Colombia. Rev. Biol. Trop. 2012, 60, 1205–1216. [Google Scholar]
- Sánchez-Herrera, M.; Realpe, E. Population structure of Polythore procera at a Colombian stream (Odonata: Polythoridae). Int. J. Odonatol. 2010, 13, 27–37. [Google Scholar] [CrossRef]
- Peixoto, P.E.; De Marco, P., Jr. No size or density effect on alternative mate-locating tactics in the tropical damselfly Hetaerina rosea males (Odonata: Calopterygidae). Rev. Biol. Trop. 2009, 57, 361–370. [Google Scholar] [CrossRef]
- Loiola, G.R.; De Marco, P. Behavioral ecology of Heteragrion consors Hagen (Odonata, Megapodagrionidae): A shade-seek Atlantic forest damselfly. Rev. Bras. Entomol. 2011, 55, 373–380. [Google Scholar] [CrossRef]
- Díaz-Flórez, B.; Pozo-García, M.; Altamiranda-Saavedra, M.; Martínez-Hernández, N. Population structure of Mecistogaster ornata (Odonata: Pseudostigmatidae) in two fragments of tropical dry forest, in the department of Atlantic, Colombia. Bol. Científico Cent. Mus. Mus. Hist. Nat. 2018, 22, 107–131. [Google Scholar]
- Resende, D.C.; De Marco, P., Jr. Residence and territorial characteristics of Libellulidae species in a neotropical assemblage (Anisoptera). Odonatologica 2008, 37, 213–220. [Google Scholar]
- Resende, B.O.; Ferreira, V.R.; Brasil, L.S.; Calvão, L.B.; Mendes, T.P.; de Carvalho, F.G.; Mendoza-Penagos, C.C.; Bastos, R.C.; Brito, J.S.; Oliveira-Junior, J.M.; et al. Impact of environmental changes on the behavioral diversity of the Odonata (Insecta) in the Amazon. Sci. Rep. 2021, 11, 9742. [Google Scholar] [CrossRef]
- Resende, D.C.; De Marco, P., Jr. First description of reproductive behavior of the Amazonian damselfly Chalcopteryx rutilans (Rambur) (Odonata, Polythoridae). Rev. Bras. Entomol. 2010, 54, 436–440. [Google Scholar] [CrossRef]
- Alves-Martins, F.; Del-Claro, K.; Jacobucci, G.B. Sexual size dimorphism, mating system and seasonality of a Neotropical damselfly, Telebasis carmesina (Coenagrionidae). Int. J. Odonatol. 2012, 15, 263–273. [Google Scholar] [CrossRef]
- Guillermo-Ferreira, R.; Del-Claro, K. Reproductive behavior of Acanthagrion truncatum Selys, 1876 (Odonata: Coenagrionidae). Int. J. Odonatol. 2012, 15, 299–304. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Borges, L.R.; Vilela, D.S.; Venâncio, H.; Santos, J.C. Odonate Communities of the Sucupira Reservoir, Rio Uberabinha, Minas Gerais, Brazil. Papéis Avulsos Zool. 2019, 59, e20195922. [Google Scholar] [CrossRef]
- Switzer, P.V. Territory Quality, Habitat Selection, and Competition in the Amberwing Dragonfly, Perithemis tenera (Say) (Odonata: Libellulidae): Population Patterns as a Consequence of Individual Behavior. J. Kans. Entomol. Soc. 2002, 75, 145–157. [Google Scholar]
- Fraser, A.M.; Herman, T.B. Territorial and reproductive behaviour in a sympatric species complex of the neotropical damselfly Cora Selys (Zygoptera: Polythoridae). Odonatologica 1993, 22, 411–429. [Google Scholar]
- García-Monsalve, M.; Altamiranda-Saavedra, M.; Palacino Rodríguez, F.; Cordero-Rivera, A. Demographic Traits and Behavior of Hetaerina cruentata (Odonata: Calopterygidae) in Ecosystems of the Andean Region of Colombia. Int. J. Odonatol. 2021, 24, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Ellenrieder, N. The larvae of Teinopodagrion decipiens De Marmels, 2001 and T. meridionale De Marmels, 2001 (Zygoptera: Megapodagrionidae). Odonatologica 2006, 35, 281–287. [Google Scholar]
- Palacino-Rodríguez, F.; Altamiranda-Saavedra, M.A.; Palacino-Penagos, D.A.; Penagos-Arévalo, A.C.; Ríos-Olaya, K.J. Factors influencing predation on Odonata by Argiope trifasciata (Forsskål, 1775). Int. J. Odonatol. 2023, 26, 36–43. [Google Scholar] [CrossRef]
- Alvarez, C.; Álvarez Covelli, M.; Palacino Rodríguez, F. Abdomen or wings? Comparing two body places for marking in Mesamphiagrion laterale (Odonata: Coenagrionidae). Odonatologica 2015, 44, 343–348. [Google Scholar]
- Plaistow, S.; Siva-Jothy, M.T. Energetic Constraints and Male Mate-Securing Tactics in the Damselfly Calopteryx splendens xanthostoma (Charpentier). Proc. Biol. Sci. 1996, 263, 1233–1239. [Google Scholar]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, S120–S139. [Google Scholar] [CrossRef]
- Ellenrieder, N.; Garrison, R. Libélulas de las Yungas (Odonata). Una Guía de Campo Para las Especies de Argentina/Dragonflies of the Yungas. A Field Guide to the Species from Argentina; Pensoft Series Faunistica: Sofia-Moscow, Russia, 2007. [Google Scholar]
- Ashton, K.G.; Feldman, C.R. Bergmann’s rule in nonavian reptiles: Turtles follow it, lizards and snakes reverse it. Evolution 2003, 57, 1151–1163. [Google Scholar]
- Gallesi, M.M.; Mobili, S.; Cigognini, R.; Hardersen, S.; Sacchi, R. Season matters: Differential variation of wing shape between sexes of Calopteryx splendens (Odonata: Calopterygidae). Zoomorphology 2016, 135, 313–322. [Google Scholar] [CrossRef]
- Johansson, F. Latitudinal Shifts in Body Size of Enallagma cyathigerum (Odonata). J. Biogeogr. 2003, 30, 29–34. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Temperature-size responses match latitudinal-size clines in arthropods, revealing critical differences between aquatic and terrestrial species. Ecol. Lett. 2015, 18, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Phillimore, A.B.; Hadfield, J.D.; Jones, O.R.; Smithers, R.J. Differences in spawning date between populations of common frog reveal local adaptation. Proc. Natl. Acad. Sci. USA 2010, 107, 8292–8297. [Google Scholar] [CrossRef]
- Muñoz, M.M.; Wegener, J.E.; Algar, A.C. Untangling intra-and interspecific effects on body size clines reveals divergent processes structuring convergent patterns in Anolis lizards. Am. Nat. 2014, 184, 5. [Google Scholar] [CrossRef]
- Boaratti, A.Z.; Da Silva, F.R. Relationships between environmental gradients and geographic variation in the intraspecific body size of three species of frogs (Anura). Austral Ecol. 2015, 40, 869–876. [Google Scholar] [CrossRef]
- Cordero Rivera, A.; Zhang, H. Ethological uniqueness of a damselfly with no near relatives: The relevance of behaviour as part of biodiversity. Anim. Biodivers. Conserv. 2018, 41, 161–174. [Google Scholar] [CrossRef]
- Stoks, R. Male-biased sex ratios in mature damselfly populations: Real or artefact? Ecol. Entomol. 2001, 26, 181–187. [Google Scholar] [CrossRef]
- Torres-Cambas, Y.; Fonseca-Rodríguez, R. Sex ratio, survival, and recapture rate in a Cuban population of the damselfly Hypolestes trinitatis (Odonata: Megapodagrionidae). Acta Ethologica 2011, 14, 69–76. [Google Scholar] [CrossRef]
- Munguía-Steyer, R.; Córdoba-Aguilar, A.; Romo-Beltrán, A. Do individuals in better condition survive for longer? Field survival estimates according to male alternative reproductive tactics and sex. J. Evol. Biol. 2010, 23, 175–184. [Google Scholar] [CrossRef]
- Cook, L.M.; Brower, L.P.; Croze, H.J. The Accuracy of a Population Estimation from Multiple Recapture Data. J. Anim. Ecol. 1967, 36, 57–60. [Google Scholar] [CrossRef]
- Cordero-Rivera, A. Estructura de tres comunidades de Calopteryx (Odonata: Calopterygidae) con diferente composición específica. Limnética 1989, 5, 83–91. [Google Scholar] [CrossRef]
- Corbet, P. Biology of Odonata. Annu. Rev. Entomol. 2003, 25, 189–217. [Google Scholar] [CrossRef]
- Fincke, O.M. Consequences of Larval Ecology for Territoriality and Reproductive Success of a Neotropical Damselfly. Ecology 1992, 73, 449–462. [Google Scholar] [CrossRef]
- Sanmartín-Villar, I.; Cordero-Rivera, A. Female Colour Polymorphism and Unique Reproductive Behaviour in Polythore Damselflies (Zygoptera: Polythoridae). Neotrop. Entomol. 2016, 45, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Garrison, R.; Gonzalez-Soriano, E. Population dynamics of two sibling species of neotropical damselflies, Palaemnema desiderata Selys and P. paulitoyaca Calvert (Odonata: Platystictidae). Folia Entomológica Mex. 1988, 76, 5–24. [Google Scholar]
- Altamiranda-Saavedra, M.; Palacino-Rodríguez, F.; Lobo-Hernández, M. Daily abundance at the breeding site and reproductive behavior of Polythore gigantea (Odonata: Polythoridae). Odonatologica 2015, 43, 69–182. [Google Scholar]
- Corbet, P.; Suhling, F.; Söndgerath, D. Voltinism of Odonata: A review. Int. J. Odonatol. 2005, 9, 1–44. [Google Scholar] [CrossRef]
- Mahdjoub, H.; Bouslama, Z.; Khelifa, R.; Zebsa, R.; Houhamdi, M. Bivoltinism in Coenagrion mercuriale (Zygoptera: Odonata) in the southern margin of its distribution range: Emergence pattern and larval growth. Afr. Entomol. 2015, 23, 59–67. [Google Scholar] [CrossRef]
- Hamilton, L.D.; Montgomerie, R.D. Population demography and sex ratio in a Neotropical damselfly (Odonata: Coenagrionidae) in Costa Rica. J. Trop. Ecol. 1989, 5, 159–171. [Google Scholar] [CrossRef]
- Cordero-Rivera, A.; Abad, J.A.A. Lifetime Mating Success, Survivorship and Synchronized Reproduction in the Damselfly Ischnura pumilio (Odonata: Coenagrionidae). Int. J. Odonatol. 1999, 2, 105–114. [Google Scholar] [CrossRef]
- Kormondy, E.J.; Gower, J.L. Life History Variations in An Association of Odonata. Ecology 1965, 46, 882–886. [Google Scholar] [CrossRef]
- Cothran, M.L.; Thorp, J.H. Emergence Patterns and Size Variation of Odonata in a Thermal Reservoir. Freshw. Invertebr. Biol. 1982, 1, 30–39. [Google Scholar] [CrossRef]
- Ward, J.V.; Stanford, J.A. Thermal Responses in the Evolutionary Ecology of Aquatic Insects. Annu. Rev. Entomol. 1982, 27, 97–117. [Google Scholar] [CrossRef]
- Hassall, C.; Thompson, D.J. The effects of environmental warming on Odonata: A review. Int. J. Odonatol. 2008, 11, 131–153. [Google Scholar] [CrossRef]
- Hassall, C. Odonata as candidate macroecological barometers for global climate change. Freshw. Sci. 2015, 34, 1040–1049. [Google Scholar] [CrossRef]
- Instituto de Hidrología, Meteorología y Estudios Ambientales—IDEAM. Variabilidad Diaria de Temperatura; 2021. Available online: http://www.ideam.gov.co/web/tiempo-y-clima/atlas#_48_INSTANCE_xoDpvO7rhD5O_%3Dhttp%253A%252F%252Fwww.ideam.gov.co%252FAtlasWeb%252Findex.html%253F (accessed on 13 December 2022).
- DuBois, R.B. Odonata drift: A reassessment. Int. J. Odonatol. 2020, 23, 381–396. [Google Scholar] [CrossRef]
- Kopp, M.; Jeschke, J.M.; Gabriel, W. Exact compensation of stream drift as an evolutionarily stable strategy. Oikos 2001, 92, 522–530. [Google Scholar] [CrossRef]
- Anholt, B.R. Density Dependence Resolves the Stream Drift Paradox. Ecology 1995, 76, 2235–2239. [Google Scholar] [CrossRef]
- Beukema, J.J. Survival rates, site fidelity and homing ability in territorial Calopteryx haemorrhoidalis (Vander Linden) (Zygoptera: Calopterygidae). Odonatologica 2002, 31, 9–22. [Google Scholar]
- Kietzka, G.J.; Pryke, J.S.; Samways, M.J. Comparative effects of urban and agricultural land transformation on Odonata assemblages in a biodiversity hotspot. Basic Appl. Ecol. 2018, 33, 89–98. [Google Scholar] [CrossRef]
- Amaya-Vallejo, V.; Bota-Sierra, C.A.; Cano-Cobos, Y.; Montes-Fontalvo, J.M.; Perez, L.; Realpe, E.; Saade, E. Teinopodagrion oscillans. The IUCN Red List of Threatened Species 2020: E.T49254537A49256381. 2020. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T49254537A49256381.en (accessed on 16 January 2024).
- De Marmels, J. Revision of Megapodagrion Selys, 1886 (Insecta, Odonata: Megapodoagrionidae). Ph.D. Thesis, University of Zürich, Zürich, Switzerland, 2001. [Google Scholar]


| Age | Teneral | Sexually Immature | Sexually Mature |
|---|---|---|---|
| PS | Yellow-Light Brown | Light blue | Light blue |
| MSP | 1 longitudinal dark brown stripe and 1 longitudinal yellow stripe | Light blue (80%) and Light brown (20%) | 1 longitudinal black stripe and 1 longitudinal light blue stripe |
| MES | 1 longitudinal brown stripe | Light blue (60%) and Light brown (40%) | Light blue (80%) and Light brown (20%) |
| MET | 1 longitudinal yellow stripe | Light blue (90%) and Light brown (10%) | Light blue |
| MTP | Yellow-Light brown | Light blue (80%) and Light brown (20%) | Light blue |
| S1–3 | Yellow-Light brown | Light blue (50%) and Light brown (50%) | Light blue (80%) and Light brown (20%) |
| W | Yellow | Hyaline | Hyaline |
| Pt | Yellow-Light brown | Light blue | Light blue |
| Recaptures | Lifespan | Maturation | Body Length | Wing Length | Distance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | A | Mk | R | Mean | SE | Mean | SE | Mean | SE (N) | Mean | SE | Mean | SE | Mean | SE |
| Male | T | 28 | 25 (89.3) | 5.18 | 0.55 | 12.75 | 1.56 | 9.43 | 0.57 (21) | 41.86 | 0.31 | 30.69 | 0.30 | 2.82 | 0.15 |
| I | 70 | 53 (75.7) | 4.64 | 0.41 | 12.87 | 1.28 | 8.16 | 0.47 (49) | 41.50 | 0.23 | 30.51 | 0.16 | 2.64 | 0.08 | |
| M | 69 | 48 (70.0) | 5.70 | 0.48 | 16.12 | 1.38 | - | - | 42.01 | 0.25 | 30.40 | 0.17 | 2.71 | 0.08 | |
| Female | T | 26 | 24 (92.3) | 4.31 | 0.51 | 10.46 | 1.26 | 8.95 | 0.52 (29) | 40.89 | 0.29 | 30.34 | 0.28 | 2.77 | 0.13 |
| I | 65 | 51 (78.5) | 5.80 | 0.46 | 16.08 | 1.29 | 9.22 | 0.48 (55) | 40.79 | 0.23 | 30.14 | 0.16 | 2.69 | 0.10 | |
| M | 66 | 46 (70.0) | 4.77 | 0.43 | 13.39 | 1.43 | - | - | 41.68 | 0.22 | 30.92 | 0.16 | 2.71 | 0.10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacino-Rodríguez, F.; Palacino, D.A.; Penagos Arevalo, A.; Cordero-Rivera, A. Demography and Behaviour of Teinopodagrion oscillans (Odonata: Megapodagrionidae) in a Protected Area of the Colombian Andean Region. Insects 2024, 15, 125. https://doi.org/10.3390/insects15020125
Palacino-Rodríguez F, Palacino DA, Penagos Arevalo A, Cordero-Rivera A. Demography and Behaviour of Teinopodagrion oscillans (Odonata: Megapodagrionidae) in a Protected Area of the Colombian Andean Region. Insects. 2024; 15(2):125. https://doi.org/10.3390/insects15020125
Chicago/Turabian StylePalacino-Rodríguez, Fredy, Diego Andres Palacino, Andrea Penagos Arevalo, and Adolfo Cordero-Rivera. 2024. "Demography and Behaviour of Teinopodagrion oscillans (Odonata: Megapodagrionidae) in a Protected Area of the Colombian Andean Region" Insects 15, no. 2: 125. https://doi.org/10.3390/insects15020125
APA StylePalacino-Rodríguez, F., Palacino, D. A., Penagos Arevalo, A., & Cordero-Rivera, A. (2024). Demography and Behaviour of Teinopodagrion oscillans (Odonata: Megapodagrionidae) in a Protected Area of the Colombian Andean Region. Insects, 15(2), 125. https://doi.org/10.3390/insects15020125







