Evaluation of the Potential Effect of Postbiotics Obtained from Honey Bees against Varroa destructor and Their Combination with Other Organic Products
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Isolation
2.2. Postbiotic Elaboration
2.3. LAB Bioassays
2.4. Combined Bioassays
2.5. Data Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D.L.; Trueman, J.W.H. Varroa jacobsoni (Acari: Varroidae) Is More than One Species. Exp. Appl. Acarol. 2020, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, B.P. Coevolution While You Wait: Varroa jacobsoni, a New Parasite of Western Honeybees. Trends Ecol. Evol. 1999, 14, 312–315. [Google Scholar] [CrossRef]
- Peck, D.T. The Parasitic Mite Varroa destructor: History, Biology, Monitoring and Management. In Honey Bee Medicine for the Veterinary Practitioners, 1st ed.; Kane, D.S., Faux, C.M., Eds.; John Wiley & Sons, Wiley Blackwells: Hoboken, NJ, USA, 2021. [Google Scholar]
- Reams, T.; Rangel, J. Understanding the Enemy: A Review of the Genetics, Behavior and Chemical Ecology of Varroa destructor, the Parasitic Mite of Apis mellifera. J. Insect Sci. 2022, 22, 18. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.S.; Mondet, F.; de Miranda, J.R.; Techer, M.; Kowallik, V.; Oddie, M.A.Y.; Chantawannakul, P.; McAfee, A. Varroa destructor: A Complex Parasite, Crippling Honey Bees Worldwide. Trends Parasitol. 2020, 36, 592–606. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor Feeds Primarily on Honey Bee Fat Body Tissue and Not Hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Donzé, G.; Patrick, M. Guerin Behavioral Attributes and Parental Care of Varroa Mites Parasitizing Honeybee Brood. Behav. Ecol. Sociobiol. 1994, 34, 305–319. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. The Energy and Nutritional Demand of the Parasitic Life of the Mite Varroa destructor. Apidologie 2004, 35, 419–430. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Giacobino, A.; Molineri, A.; Bulacio Cagnolo, N.; Merke, J.; Orellano, E.; Bertozzi, E.; Masciangelo, G.; Pietronave, H.; Pacini, A.; Salto, C.; et al. Key Management Practices to Prevent High Infestation Levels of Varroa destructor in Honey Bee Colonies at the Beginning of the Honey Yield Season. Prev. Vet. Med. 2016, 131, 95–102. [Google Scholar] [CrossRef]
- Boecking, O.; Genersch, E. Varroosis—The Ongoing Crisis in Bee Keeping. J. Verbraucherschutz Leb. 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Chen, Y.; Siede, R. Honey Bee Viruses. Adv. Virus Res. 2007, 70, 33–80. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Pennacchio, F.; Caprio, E.; Boncristiani, H.F.; Evans, J.D.; Chen, Y. Varroa destructor Is an Effective Vector of Israeli Acute Paralysis Virus in the Honeybee, Apis mellifera. J. Gen. Virol. 2011, 92, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Genersch, E. Direct Evidence for Infection of Varroa destructor Mites with the Bee-Pathogenic Deformed Wing Virus Variant B, but Not Variant A, via Fluorescence In Situ Hybridization Analysis. J. Virol. 2021, 95, e01786-20. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Dead or Alive: Deformed Wing Virus and Varroa destructor Reduce the Life Span of Winter Honeybees. Appl. Environ. Microbiol. 2012, 78, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Mondet, F.; de Miranda, J.R.; Kretzschmar, A.; Le Conte, Y.; Mercer, A.R. On the Front Line: Quantitative Virus Dynamics in Honeybee (Apis mellifera L.) Colonies along a New Expansion Front of the Parasite Varroa destructor. PLoS Pathog. 2014, 10, e1004323. [Google Scholar] [CrossRef]
- González-Cabrera, J.; Rodríguez-Vargas, S.; Davies, T.G.E.; Field, L.M.; Schmehl, D.; Ellis, J.D.; Krieger, K.; Williamson, M.S. Novel Mutations in the Voltage-Gated Sodium Channel of Pyrethroid-Resistant Varroa destructor Populations from the Southeastern USA. PLoS ONE 2016, 11, e0155332. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, C.S.; Marín, Ó.; Calatayud, F.; Mahiques, M.J.; Mompó, A.; Segura, I.; Simó, E.; González-Cabrera, J. Large-Scale Monitoring of Resistance to Coumaphos, Amitraz, and Pyrethroids in Varroa destructor. Insects 2021, 12, 27. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Hernández-Rodríguez, C.S.; González-Cabrera, J. Assessing the Resistance to Acaricides in Varroa destructor from Several Spanish Locations. Parasitol. Res. 2020, 119, 3595–3601. [Google Scholar] [CrossRef]
- Mitton, G.A.; Szawarski, N.; Ramos, F.; Fuselli, S.; Meroi Arcerito, F.R.; Eguaras, M.J.; Ruffinengo, S.R.; Maggi, M.D. Varroa destructor: When Reversion to Coumaphos Resistance Does Not Happen. J. Apic. Res. 2018, 57, 536–540. [Google Scholar] [CrossRef]
- Rinkevich, F.D. Detection of Amitraz Resistance and Reduced Treatment Efficacy in the Varroa Mite, Varroa destructor, within Commercial Beekeeping Operations. PLoS ONE 2020, 15, e0227264. [Google Scholar] [CrossRef]
- Rinkevich, F.D.; Moreno-Martí, S.; Hernández-Rodríguez, C.S.; González-Cabrera, J. Confirmation of the Y215H Mutation in the B2-Octopamine Receptor in Varroa destructor Is Associated with Contemporary Cases of Amitraz Resistance in the United States. Pest Manag. Sci. 2023, 79, 2840–2845. [Google Scholar] [CrossRef]
- Vlogiannitis, S.; Mavridis, K.; Dermauw, W.; Snoeck, S.; Katsavou, E.; Morou, E.; Harizanis, P.; Swevers, L.; Hemingway, J.; Feyereisen, R.; et al. Reduced Proinsecticide Activation by Cytochrome P450 Confers Coumaphos Resistance in the Major Bee Parasite Varroa destructor. Proc. Natl. Acad. Sci. USA 2021, 118, e2020380118. [Google Scholar] [CrossRef] [PubMed]
- El Agrebi, N.; Traynor, K.; Wilmart, O.; Tosi, S.; Leinartz, L.; Danneels, E.; de Graaf, D.C.; Saegerman, C. Pesticide and Veterinary Drug Residues in Belgian Beeswax: Occurrence, Toxicity, and Risk to Honey Bees. Sci. Total Environ. 2020, 745, 141036. [Google Scholar] [CrossRef]
- Korta, E.; Bakkali, A.; Berrueta, L.A.; Gallo, B.; Vicente, F.; Kilchenmann, V.; Bogdanov, S. Study of Acaricide Stability in Honey. Characterization of Amitraz Degradation Products in Honey and Beeswax. J. Agric. Food Chem. 2001, 49, 5835–5842. [Google Scholar] [CrossRef] [PubMed]
- Murcia Morales, M.; Gómez Ramos, M.J.; Parrilla Vázquez, P.; Díaz Galiano, F.J.; García Valverde, M.; Gámiz López, V.; Manuel Flores, J.; Fernández-Alba, A.R. Distribution of Chemical Residues in the Beehive Compartments and Their Transfer to the Honeybee Brood. Sci. Total Environ. 2020, 710, 136288. [Google Scholar] [CrossRef]
- Orantes-Bermejo, F.J.; Gómez-Pajuelo, A.; Megías, M.; Torres, C. Pesticide Residues in Beeswax and Beebread Samples Collected from Honey Bee Colonies (Apis mellifera L.) in Spain. Possible Implications for Bee Losses. J. Apic. Res. 2015, 49, 243–250. [Google Scholar] [CrossRef]
- Ravoet, J.; Reybroeck, W.; de Graaf, D.C. Pesticides for Apicultural and/or Agricultural Application Found in Belgian Honey Bee Wax Combs. Bull. Environ. Contam. Toxicol. 2015, 94, 543–548. [Google Scholar] [CrossRef]
- Tihelka, E. Effects of Synthetic and Organic Acaricides on Honey Bee Health: A Review. Slov. Vet. Res. 2018, 55, 119–140. [Google Scholar] [CrossRef]
- Bacandritsos, N.; Papanastasiou, I.; Saitanis, C.; Nanetti, A.; Roinioti, E. Efficacy of Repeated Trickle Applications of Oxalic Acid in Syrup for Varroosis Control in Apis mellifera: Influence of Meteorological Conditions and Presence of Brood. Vet. Parasitol. 2007, 148, 174–178. [Google Scholar] [CrossRef]
- Charriére, J.-D.; Imdorf, A. Oxalic Acid Treatment by Trickling against Varroa destructor: Recommendations for Use in Central Europe and under Temperate Climate Conditions. Bee World 2002, 83, 51–60. [Google Scholar] [CrossRef]
- Coffey, M.F.; Breen, J. Efficacy of Apilife Var® and Thymovar® against Varroa destructor as an Autumn Treatment in a Cool Climate. J. Apic. Res. 2013, 52, 210–218. [Google Scholar] [CrossRef]
- Rademacher, E.; Harz, M. Oxalic Acid for the Control of Varroosis in Honey Bee Colonies—A Review. Apidologie 2006, 37, 98–120. [Google Scholar] [CrossRef]
- Underwood, R.M.; Currie, R.W. The Effects of Temperature and Dose of Formic Acid on Treatment Efficacy against Varroa destructor (Acari: Varroidae), a Parasite of Apis mellifera (Hymenoptera: Apidae). Exp. Appl. Acarol. 2003, 29, 303–313. [Google Scholar] [CrossRef]
- Castagna, F.; Bava, R.; Piras, C.; Carresi, C.; Musolino, V.; Lupia, C.; Marrelli, M.; Conforti, F.; Palma, E.; Britti, D.; et al. Green Veterinary Pharmacology for Honey Bee Welfare and Health: Origanum heracleoticum L. (Lamiaceae) Essential Oil for the Control of the Apis mellifera Varroatosis. Vet. Sci. 2022, 9, 124. [Google Scholar] [CrossRef]
- Arredondo, D.; Castelli, L.; Porrini, M.P.; Garrido, P.M.; Eguaras, M.J.; Zunino, P.; Antúnez, K. Lactobacillus kunkeei Strains Decreased the Infection by Honey Bee Pathogens Paenibacillus larvae and Nosema ceranae. Benef. Microbes 2018, 9, 279–290. [Google Scholar] [CrossRef]
- Carvajal, R.I.; Silva-Mieres, F.; Ilabaca, A.; Rocha, J.; Arellano-Arriagada, L.; Zuniga Arbalti, F.A.; García-Cancino, A. Isolation and Characterization of Lactobacillus casei A14.2, a Strain with Immunomodulating Activity on Apis mellifera. Saudi J. Biol. Sci. 2023, 30, 103612. [Google Scholar] [CrossRef]
- García-Vicente, E.J.; Martín, M.; Rey-Casero, I.; Pérez, A.; Martínez, R.; Bravo, M.; Alonso, J.M.; Risco, D. Effect of Feed Supplementation with Probiotics and Postbiotics on Strength and Health Status of Honey Bee (Apis mellifera) Hives during Late Spring. Res. Vet. Sci. 2023, 159, 237–243. [Google Scholar] [CrossRef]
- Tejerina, M.R.; Benítez-Ahrendts, M.R.; Audisio, M.C. Lactobacillus salivarius A3iob Reduces the Incidence of Varroa destructor and Nosema spp. in Commercial Apiaries Located in the Northwest of Argentina. Probiotics Antimicrob. Proteins 2020, 12, 1360–1369. [Google Scholar] [CrossRef]
- Sanders, M.E. Probiotics and Microbiota Composition. BMC Med. 2016, 14, 82. [Google Scholar] [CrossRef]
- De Piano, F.G.; Maggi, M.; Pellegrini, M.C.; Cugnata, N.M.; Szawarski, N.; Buffa, F.; Negri, P.; Fuselli, S.R.; Audisio, C.M.; Ruffinengo, S.R. Effects of Lactobacillus johnsonii AJ5 Metabolites on Nutrition, Nosema Ceranae Development and Performance of Apis mellifera L. J. Apic. Sci. 2017, 61, 93–104. [Google Scholar] [CrossRef][Green Version]
- Saccà, M.L.; Lodesani, M. Isolation of Bacterial Microbiota Associated to Honey Bees and Evaluation of Potential Biocontrol Agents of Varroa destructor. Benef. Microbes 2020, 11, 641–654. [Google Scholar] [CrossRef] [PubMed]
- De Piano, F.G.; Maggi, M.D.; Meroi Arceitto, F.R.; Audisio, M.C.; Eguaras, M.; Ruffinengo, S.R. Effects of Bacterial Cell-Free Supernatant on Nutritional Parameters of Apis mellifera and Their Toxicity Against Varroa destructor. J. Apic. Sci. 2020, 64, 55–66. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- MAPA Caracterización Agroclimática de La Provincia de Cáceres, 2nd ed.; Ministerio de Agricultura Pesca y Alimentación, Dirección General de La Producción Agraria: Madrid, Spain, 1991.
- Lau, S.K.P.; Woo, P.C.Y.; Li, N.K.H.; Teng, J.L.L.; Leung, K.-W.; Ng, K.H.L.; Que, T.L. Yuen Globicatella Bacteraemia Identified by 16S Ribosomal RNA Gene Sequencing. J. Clin. Pathol. 2006, 59, 303–307. [Google Scholar] [CrossRef]
- Men, A.E.; Wilson, P.; Siemering, K.; Forrest, S. Sanger DNA Sequencing. In Next Generation Genome Sequencing; Wiley: Hoboken, NJ, USA, 2008; pp. 1–11. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Shah, A.H.; Aurongzeb, M.; Kori, J.; Azim, M.K.; Ansari, M.J.; Bin, L. Characterization of Gut Bacterial Flora of Apis mellifera from North-West Pakistan. Saudi J. Biol. Sci. 2018, 25, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Gasper, J.; Brindza, J.; Schubertová, Z.; Ivanišová, E. Bacteria of Apis mellifera Gastrointestinal Tract: Counts, Identification And Their Antibiotic Resistance. In Agrobiodiversity for Improving Nutrition, Health and Life Quality; Klymenko, S., Ed.; Slovak University of Agriculture: Nitra, Slovakia, 2017; pp. 210–215. ISBN 978-80-552-1726-0. [Google Scholar]
- Xiong, Z.R.; Cobo, M.; Whittal, R.M.; Snyder, A.B.; Worobo, R.W. Purification and Characterization of Antifungal Lipopeptide Produced by Bacillus velezensis Isolated from Raw Honey. PLoS ONE 2022, 17, e0266470. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; İspirli, H.; Taylan, O.; Taşdemir, V.; Sagdic, O.; Dertli, E. Characterisation and Functional Roles of a Highly Branched Dextran Produced by a Bee Pollen Isolate Leuconostoc mesenteroides BI-20. Food Biosci. 2022, 45, 101330. [Google Scholar] [CrossRef]
- Zendo, T.; Ohashi, C.; Maeno, S.; Piao, X.; Salminen, S.; Sonomoto, K.; Endo, A. Kunkecin A, a New Nisin Variant Bacteriocin Produced by the Fructophilic Lactic Acid Bacterium, Apilactobacillus kunkeei FF30-6 Isolated from Honey Bees. Front. Microbiol. 2020, 11, 571903. [Google Scholar] [CrossRef]
- Lambe, D.W., Jr.; Ferguson, K.P.; Keplinger, J.L.; Gemmell, C.G.; Kalbfleisch, J.H. Pathogenicity of Staphylococcus lugdunensis, Staphylococcus schleiferi, and Three Other Coagulase-Negative Staphylococci in a Mouse Model and Possible Virulence Factors. Can. J. Microbiol. 1990, 36, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Teixeira, L.; Iorio, N.; Bastos, C.; Fonseca, L.; Soutopadron, T.; Dossantos, K. Heterogeneous Resistance to Vancomycin in Staphylococcus epidermidis, Staphylococcus haemolyticus and Staphylococcus warneri Clinical Strains: Characterisation of Glycopeptide Susceptibility Profiles and Cell Wall Thickening. Int. J. Antimicrob. Agents 2006, 27, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Szczuka, E.; Krzymińska, S.; Kaznowski, A. Clonality, Virulence and the Occurrence of Genes Encoding Antibiotic Resistance among Staphylococcus warneri Isolates from Bloodstream Infections. J. Med. Microbiol. 2016, 65, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Akyol, E.; YeniNar, H. Use of Oxalic Acid to Control Varroa destructor in Honeybee (Apis mellifera L.) Colonies. Turk. J. Vet. Anim. Sci. 2009, 33, 285–288. [Google Scholar] [CrossRef]
- Kraus, B.; Berg, S. Effect of a Lactic Acid Treatment during Winter in Temperate Climate upon Varroa jacobsoni Oud. and the Bee (Apis mellifera L.) Colony. Exp. Appl. Acarol. 1994, 18, 459–468. [Google Scholar] [CrossRef]
- Milani, N. Activity of Oxalic and Citric Acidson the Mite Varroa destructor in Laboratory Assays. Apidologie 2001, 32, 127–138. [Google Scholar] [CrossRef]
| ID | Sample | Specie |
|---|---|---|
| 1 | Digestive tract | Leuconostoc mesenteroides |
| 2 | Adult bee exoskeleton | Leuconostoc mesenteroides |
| 3 | Brood exoskeleton | Staphylococcus epidermidis |
| 4 | Digestive tract | Lactobacillus helsingborgensis |
| 5 | Adult bee exoskeleton | Staphylococcus warneri |
| 6 | Brood exoskeleton | Bacillus velezensis |
| 7 | Brood exoskeleton | Bacillus velezensis |
| 8 | Brood exoskeleton | Bacillus velezensis |
| 9 | Digestive tract | Staphylococcus epidermidis |
| 10 | Digestive tract | Lactobacillus helsingborgensis |
| 11 | Adult bee exoskeleton | Staphylococcus epidermidis |
| 12 | Adult bee exoskeleton | Lactobacillus helsingborgensis |
| 13 | Adult bee exoskeleton | Staphylococcus epidermidis |
| 14 | Digestive tract | Lactobacillus helsingborgensis |
| 15 | Adult bee exoskeleton | Lactobacillus helsingborgensis |
| 16 | Digestive tract | Lactobacillus helsingborgensis |
| 17 | Digestive tract | Lactobacillus helsingborgensis |
| 18 | Adult bee exoskeleton | Lactobacillus helsingborgensis |
| 19 | Adult bee exoskeleton | Lactobacillus helsingborgensis |
| 20 | Digestive tract | Lactobacillus helsingborgensis |
| 21 | Digestive tract | Lactobacillus helsingborgensis |
| 22 | Adult bee exoskeleton | Apilactobacillus kunkeei |
| 23 | Adult bee exoskeleton | Lactobacillus helsingborgensis |
| 24 | Adult bee exoskeleton | Apilactobacillus kunkeei |
| 25 | Digestive tract | Apilactobacillus kunkeei |
| LAB Species | Mean Viability ± sd |
|---|---|
| Control | 1.82 ± 0.53 |
| L. mesenteroides (LM) | 1.48 ± 0.73 |
| A. kunkeei (AK) | 1.72 ± 0.6 |
| L. helsingborgensis (LH) | 1.54 ± 0.57 |
| B. velezensis (BV) | 1.52 ± 0.73 |
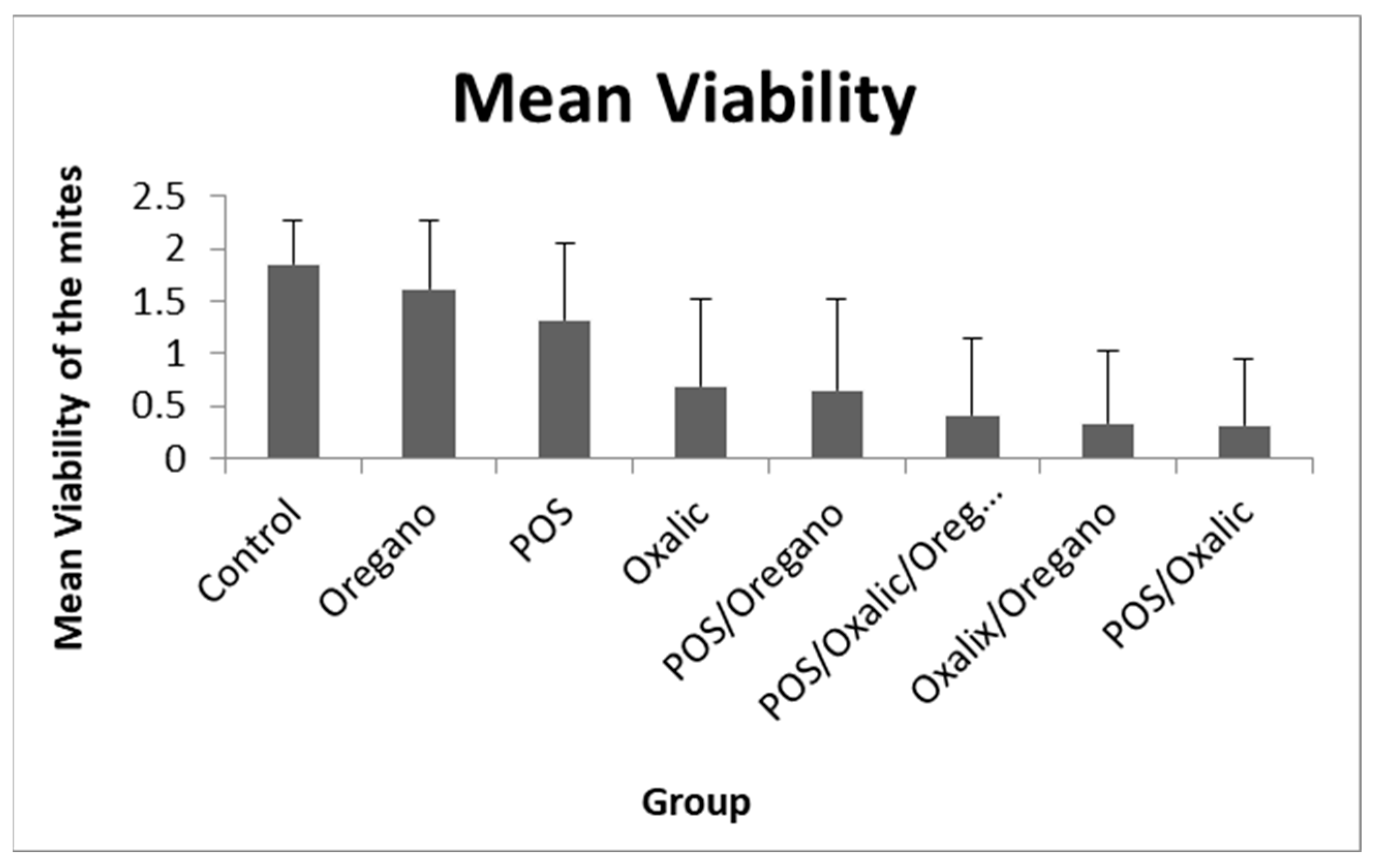
| Group | Mean Viability ± sd |
|---|---|
| Control | 1.86 ± 0.4 |
| POS | 1.31 ± 0.74 |
| Oxalic | 0.68 ± 0.83 |
| Oregano | 1.61 ± 0.65 |
| POS/Oxalic | 0.31 ± 0.63 |
| POS/Oregano | 0.65 ± 0.87 |
| Oxalic/Oregano | 0.33 ± 0.7 |
| POS/Oxalic/Oregano | 0.41 ± 0.74 |
| Control | Oregano | POS | Oxalic | POS/Oregano | POS/Oxalic/Oregano | Oxalic/Oregano | POS/Oxalic | |
|---|---|---|---|---|---|---|---|---|
| Control | - | 6.90 × 10−4 | 5.80 × 10−10 | <2 × 10−16 | <2 × 10−16 | <2 × 10−16 | <2 × 10−16 | <2 × 10−16 |
| Oregano | 6.90 × 10−4 | - | 0.013 | 2.00 × 10−9 | 1.40 × 10−10 | 8.00 × 10−16 | <2 × 10−16 | 6.30 × 10−16 |
| POS | 5.80 × 10−10 | 0.013 | - | 9.60 × 10−5 | 1.30 × 10−5 | 5.80 × 10−10 | 1.90 × 10−11 | 1.50 × 10−10 |
| Oxalic | <2 × 10−16 | 2.00 × 10−9 | 9.60 × 10−5 | - | 0.701 | 0.035 | 0.006 | 0.011 |
| POS/Oregano | <2 × 10−16 | 1.40 × 10−10 | 1.30 × 10−5 | 0.701 | - | 0.085 | 0.016 | 0.026 |
| POS/Oxalic/Oregano | <2 × 10−16 | 8.00 × 10−16 | 5.80 × 10−10 | 0.035 | 0.085 | - | 0.477 | 0.554 |
| Oxalic/Oregano | <2 × 10−16 | <2 × 10−16 | 1.90 × 10−11 | 0.006 | 0.016 | 0.477 | - | 0.914 |
| POS/Oxalic | <2 × 10−16 | 6.30 × 10−16 | 1.50 × 10−10 | 0.011 | 0.026 | 0.554 | 0.914 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Vicente, E.J.; Benito-Murcia, M.; Martín, M.; Rey-Casero, I.; Pérez, A.; González, M.; Alonso, J.M.; Risco, D. Evaluation of the Potential Effect of Postbiotics Obtained from Honey Bees against Varroa destructor and Their Combination with Other Organic Products. Insects 2024, 15, 67. https://doi.org/10.3390/insects15010067
García-Vicente EJ, Benito-Murcia M, Martín M, Rey-Casero I, Pérez A, González M, Alonso JM, Risco D. Evaluation of the Potential Effect of Postbiotics Obtained from Honey Bees against Varroa destructor and Their Combination with Other Organic Products. Insects. 2024; 15(1):67. https://doi.org/10.3390/insects15010067
Chicago/Turabian StyleGarcía-Vicente, Eduardo José, María Benito-Murcia, María Martín, Ismael Rey-Casero, Ana Pérez, María González, Juan Manuel Alonso, and David Risco. 2024. "Evaluation of the Potential Effect of Postbiotics Obtained from Honey Bees against Varroa destructor and Their Combination with Other Organic Products" Insects 15, no. 1: 67. https://doi.org/10.3390/insects15010067
APA StyleGarcía-Vicente, E. J., Benito-Murcia, M., Martín, M., Rey-Casero, I., Pérez, A., González, M., Alonso, J. M., & Risco, D. (2024). Evaluation of the Potential Effect of Postbiotics Obtained from Honey Bees against Varroa destructor and Their Combination with Other Organic Products. Insects, 15(1), 67. https://doi.org/10.3390/insects15010067







