Sperm Competition and Paternity in the Endangered Firefly Pyrocoelia pectoralis (Coleoptera: Lampyridae: Lampyrinae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Feeding Scheme
2.2. Anatomy of the Reproductive System
2.3. Target SSRs and Design of Multiplex Primers for Genotyping
2.4. Paternity Assignment
3. Results
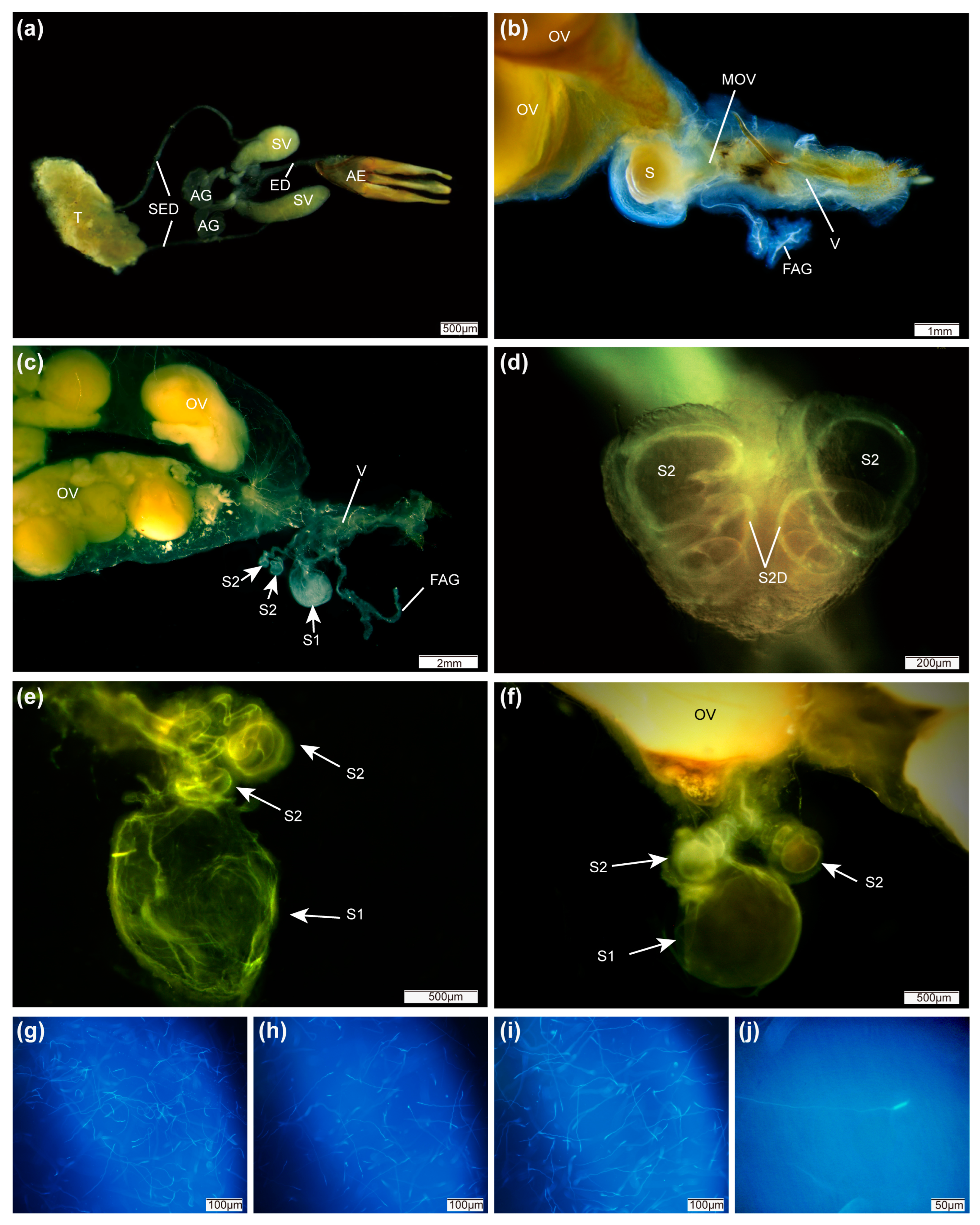
3.1. Anatomy of Reproductive System
3.2. Identification and Characterization of SSR Motifs
3.3. SSR Marker Development
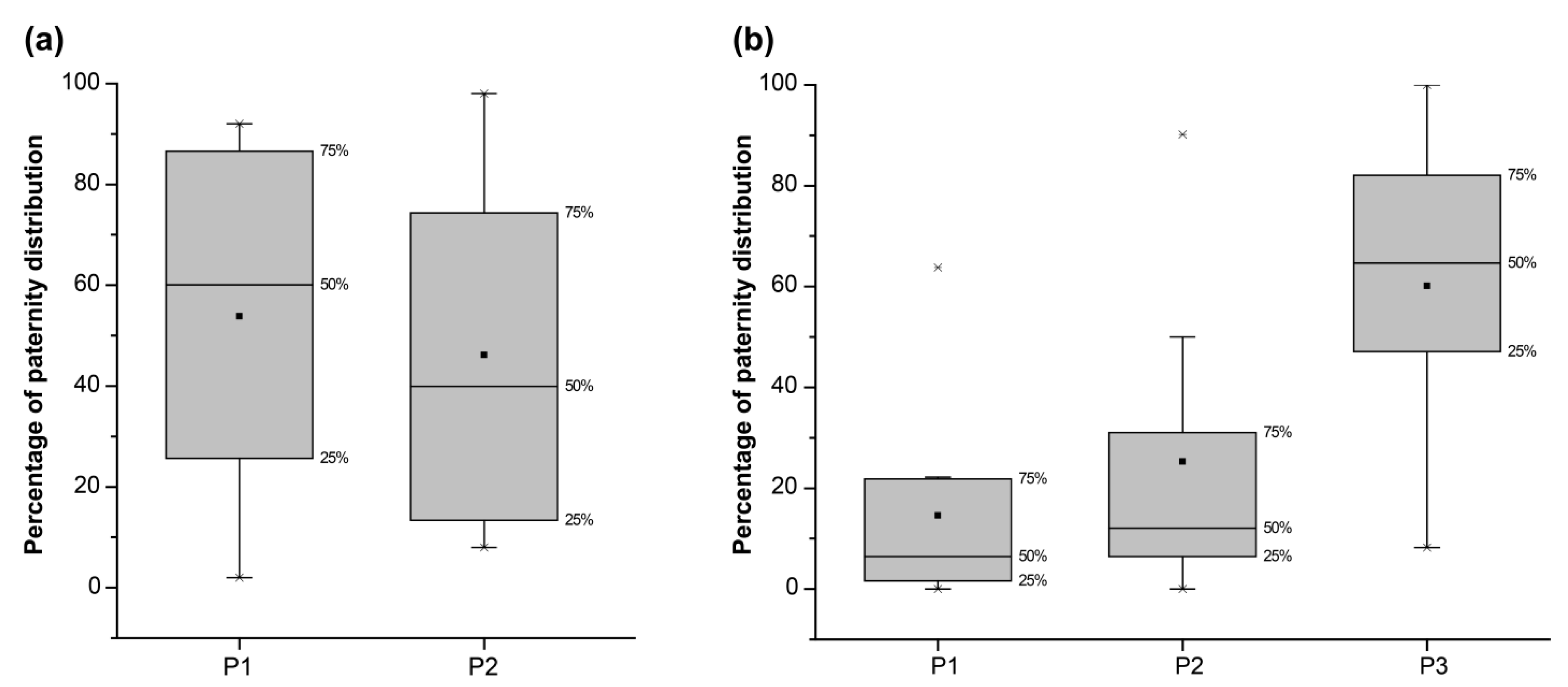
3.4. Paternity Assignment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, S.M.; Cratsley, C.K. Flash signal evolution, mate choice, and predation in fireflies. Annu. Rev. Entomol. 2008, 53, 293–321. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Hastings, J.W. Fireflies and other beetles luciferase-dependent bioluminescence color and rhythmic displays. In Bioluminescence: Living Lights, Lights for Living; Harvard University Press: Cambridge, MI, USA; London, UK, 2013; pp. 31–43. [Google Scholar]
- Owens, A.C.S.; Meyer-Rochow, V.B.; Yang, E.C. Short and mid-wavelength artificial light influences the flash signals of Aquatica ficta fireflies (Coleoptera: Lampyridae). PLoS ONE 2018, 13, e0191576. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, L.; Elgert, C.; Lehtonen, T.K.; Candolin, U. The color of artificial light affects mate attraction in the common glow-worm. Sci. Total Environ. 2023, 857, 159451. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.; Van den Broeck, M.; De Cock, R.; Lewis, S.M. Behavioral responses of bioluminescent fireflies to artificial light at night. Front. Ecol. Evol. 2022, 10, 946640. [Google Scholar] [CrossRef]
- Owens, A.C.; Lewis, S.M. Artificial light impacts the mate success of female fireflies. R. Soc. Open Sci. 2022, 9, 220468. [Google Scholar] [CrossRef]
- Owens, A.C.; Dressler, C.T.; Lewis, S.M. Costs and benefits of “insect friendly” artificial lights are taxon specific. Oecologia 2022, 199, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Khattar, G.; Vaz, S.; Braga, P.H.P.; Macedo, M.; Silveira, L.F.L.d. Life history traits modulate the influence of environmental stressors on biodiversity: The case of fireflies, climate and artificial light at night. Divers. Distrib. 2022, 28, 1820–1831. [Google Scholar] [CrossRef]
- Vaz, S.; Manes, S.; Gama-Maia, D.; Silveira, L.; Mattos, G.; Paiva, P.C.; Figueiredo, M.; Lorini, M.L. Light pollution is the fastest growing potential threat to firefly conservation in the Atlantic Forest hotspot. Insect Conserv. Divers. 2021, 14, 211–224. [Google Scholar] [CrossRef]
- Fallon, C.E.; Walker, A.C.; Lewis, S.; Cicero, J.; Faust, L.; Heckscher, C.M.; Pérez-Hernández, C.X.; Pfeiffer, B.; Jepsen, S. Evaluating firefly extinction risk: Initial red list assessments for North America. PLoS ONE 2021, 16, e0259379. [Google Scholar] [CrossRef]
- Chatragadda, R. Decline of luminous firefly Abscondita chinensis population in Barrankula, Andhra Pradesh, India. Int. J. Trop. Insect Sci. 2020, 40, 461–465. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; Lei, C.; Jeng, M.L.; Nobuyoshi, O. Biological characteristics of the terrestrial firefly Pyrocoelia pectoralis (Cleoptera: Lampyridae). Coleopt. Bull. 2007, 61, 85–93. [Google Scholar] [CrossRef]
- Fu, X.; South, A.; Lewis, S.M. Sexual dimorphism, mating systems, and nuptial gifts in two Asian fireflies (Coleoptera: Lampyridae). J. Insect Physiol. 2012, 58, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Oppelt, A.; Heinze, J. Dynamics of sperm transfer in the ant Leptothorax gredleri. Naturwissenschaften 2007, 94, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Pascini, T.V.; Martins, G.F. The insect spermatheca: An overview. Zoology 2017, 121, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Pitnick, S.; Marrow, T.; Spicer, G.S. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution 1999, 53, 1804–1822. [Google Scholar]
- Krzemińska, E.; Gorzka, D. Ventral receptacle in the genus Trichocera: A case of parallel evolution between remote lineages of the Diptera (Diptera: Nematocera: Trichoceridae). Zool. Anz. 2016, 263, 6–15. [Google Scholar] [CrossRef]
- Hayashi, F.; Suzuki, H. Fireflies with or without prespermatophores: Evolutionary origins and life-history consequences. Entomol. Sci. 2003, 6, 3–10. [Google Scholar] [CrossRef]
- South, A.; Stanger-Hall, K.; Jeng, M.L.; Lewis, S.M. Correlated evolution of female neoteny and flightlessness with male spermatophore production in fireflies (Coleoptera: Lampyridae). Evolution 2011, 65, 1099–1113. [Google Scholar] [CrossRef]
- Fu, X.H.; Ballantyne, L. Reproductive Systems, Transfer and digestion of spermatophores in two Asian Luciolinae fireflies (Coleoptera: Lampyridae). Insects 2021, 12, 365. [Google Scholar] [CrossRef]
- Eberhard, W.G. Postcopulatory sexual selection: Darwin’s omission and its consequences. Proc. Natl. Acad. Sci. USA 2009, 106, 10025–10032. [Google Scholar] [CrossRef]
- Demary, K.C.; Lewis, S.M. Male courtship attractiveness and paternity success in Photinus greeni fireflies. Evolution 2007, 61, 431–439. [Google Scholar] [CrossRef]
- Zhang, D.X.; Hewitt, G.M. Nuclear DNA analyses in genetic studies of populations: Practice, problems and prospects. Mol. Ecol. 2003, 12, 563–584. [Google Scholar] [CrossRef] [PubMed]
- Pasini, M.E.; Redi, C.; Caviglia, O.; Perotti, M. Ultrastructural and cytochemical analysis of sperm dimorphism in Drosophila subobscura. Tissue Cell 1996, 28, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L. GMATA: An integrated software package for genome-scale SSR mining, marker development and viewing. Front. Plant Sci. 2016, 7, 1350. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Rangasamy, M.; Tan, S.Y.; Wang, H.; Siegfried, B.D. Evaluation of five methods for total DNA extraction from western corn rootworm beetles. PLoS ONE 2010, 5, e11963. [Google Scholar] [CrossRef]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- GA, P. Sperm competition and the evolution of ejaculates: Towards a theory base. In Sperm Competition and Sexual Selection; Elsevier: Amsterdam, The Netherlands, 1998; pp. 3–54. [Google Scholar]
- Boorman, E.; Parker, G. Sperm (ejaculate) competition in Drosophila melanogaster, and the reproductive value of females to males in relation to female age and mating status. Ecol. Entomol. 1976, 1, 145–155. [Google Scholar] [CrossRef]
- Pan, Y.B. Highly polymorphic microsatellite DNA markers for sugarcane germplasm evaluation and variety identity testing. Sugar Tech 2006, 8, 246–256. [Google Scholar] [CrossRef]
- Rodriguez, S.; Gaunt, T.R.; Day, I.N. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am. J. Epidemiol. 2009, 169, 505–514. [Google Scholar] [CrossRef]
- Reijden, E.V.D.; Monchamp, J.D.; Lewis, S.M. The formation, transfer, and fate of spermatophores in Photinus fireflies (Coleoptera: Lampyridae). Can. J. Zool. 1997, 75, 1202–1207. [Google Scholar] [CrossRef]
- Boggs, C.L. Male nuptial gifts: Phenotypic consequences and evolutionary implications. In Insect Reproduction; CRC Press: Boca Raton, FL, USA, 2018; pp. 215–242. [Google Scholar]
- Vahed, K. The function of nuptial feeding in insects: A review of empirical studies. Biol. Rev. 1998, 73, 43–78. [Google Scholar] [CrossRef]
- Ballantyne, L.; Lambkin, C.; Ho, J.Z.; Jusoh, W.; Nada, B.; Nak-Eiam, S.; Thancharoen, A.; Wattanachaiyingcharoen, W.; Yiu, V. The Luciolinae of SE Asia and the Australopacific region: A revisionary checklist (Coleoptera: Lampyridae) including description of three new genera and 13 new species. Zootaxa 2019, 4687, 1–174. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, M.; De Cock, R.; Van Dongen, S.; Matthysen, E. Blinded by the light: Artificial light lowers mate attraction success in female glow-worms (Lampyris noctiluca L.). Insects 2021, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, M.; De Cock, R.; Van Dongen, S.; Matthysen, E. White LED light intensity, but not colour temperature, interferes with mate-finding by glow-worm (Lampyris noctiluca L.) males. J. Insect Conserv. 2021, 25, 339–347. [Google Scholar] [CrossRef]
- Chen, Y.R.; Wei, W.L.; Tzeng, D.T.; Owens, A.C.; Tang, H.C.; Wu, C.S.; Lin, S.S.; Zhong, S.; Yang, E.C. Effects of artificial light at night (ALAN) on gene expression of Aquatica ficta firefly larvae. Environ. Pollut. 2021, 281, 116944. [Google Scholar] [CrossRef]
- Elgert, C.; Hopkins, J.; Kaitala, A.; Candolin, U. Reproduction under light pollution: Maladaptive response to spatial variation in artificial light in a glow-worm. Proc. R. Soc. B 2020, 287, 20200806. [Google Scholar] [CrossRef]
- Parker, G.A.; Lehtonen, J. Gamete evolution and sperm numbers: Sperm competition versus sperm limitation. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Meyer-Rochow, V.B.; Ballantyne, L.; Zhu, X.; Zhang, Q. Sperm Competition and Paternity in the Endangered Firefly Pyrocoelia pectoralis (Coleoptera: Lampyridae: Lampyrinae). Insects 2024, 15, 66. https://doi.org/10.3390/insects15010066
Fu X, Meyer-Rochow VB, Ballantyne L, Zhu X, Zhang Q. Sperm Competition and Paternity in the Endangered Firefly Pyrocoelia pectoralis (Coleoptera: Lampyridae: Lampyrinae). Insects. 2024; 15(1):66. https://doi.org/10.3390/insects15010066
Chicago/Turabian StyleFu, Xinhua, Victor Benno Meyer-Rochow, Lesley Ballantyne, Xinlei Zhu, and Qiyulu Zhang. 2024. "Sperm Competition and Paternity in the Endangered Firefly Pyrocoelia pectoralis (Coleoptera: Lampyridae: Lampyrinae)" Insects 15, no. 1: 66. https://doi.org/10.3390/insects15010066
APA StyleFu, X., Meyer-Rochow, V. B., Ballantyne, L., Zhu, X., & Zhang, Q. (2024). Sperm Competition and Paternity in the Endangered Firefly Pyrocoelia pectoralis (Coleoptera: Lampyridae: Lampyrinae). Insects, 15(1), 66. https://doi.org/10.3390/insects15010066





