Resilin Distribution and Abundance in Apis mellifera across Biological Age Classes and Castes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
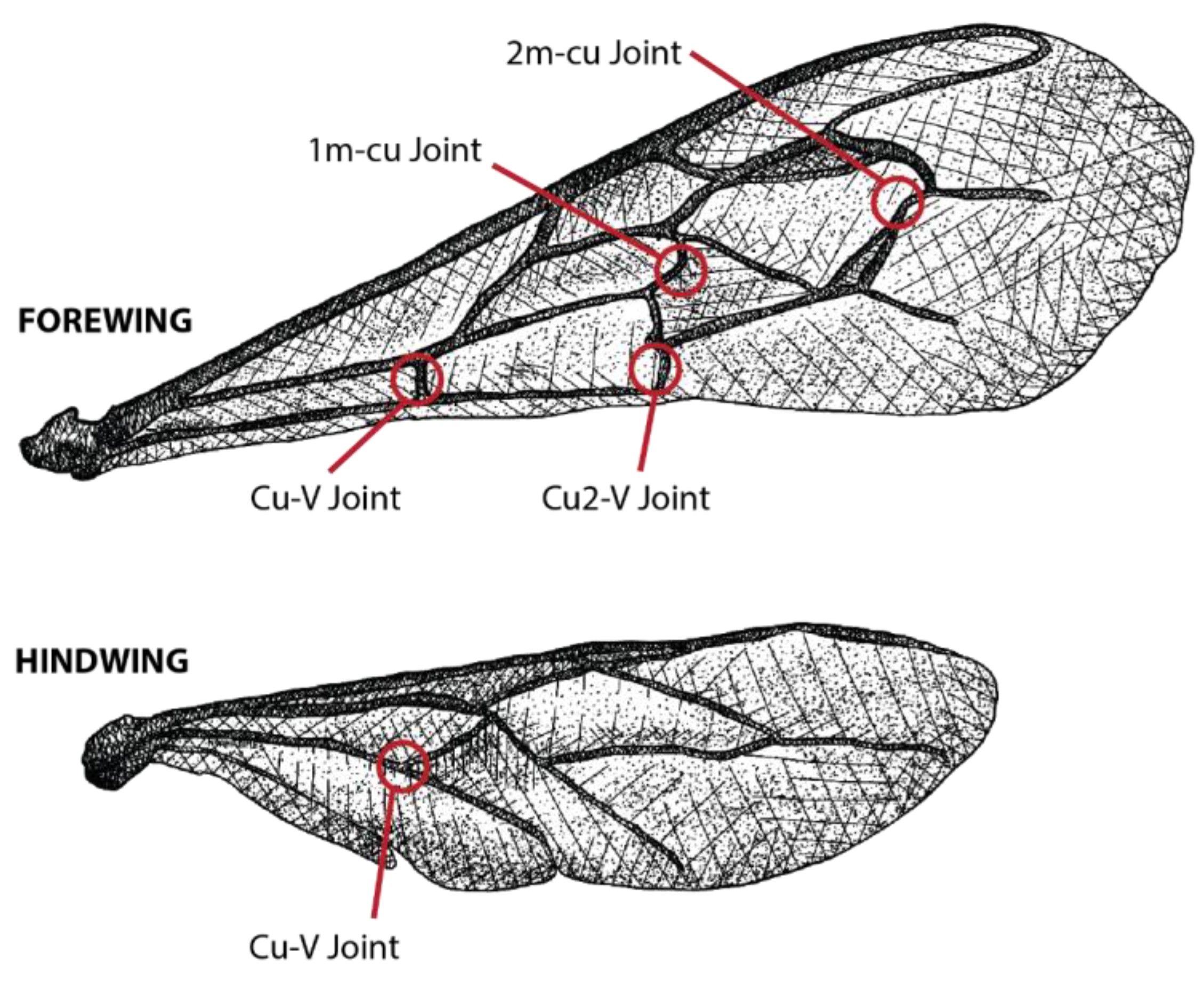
2.2. Resilin Autofluorescence
2.2.1. Dissection and Slide Preparation
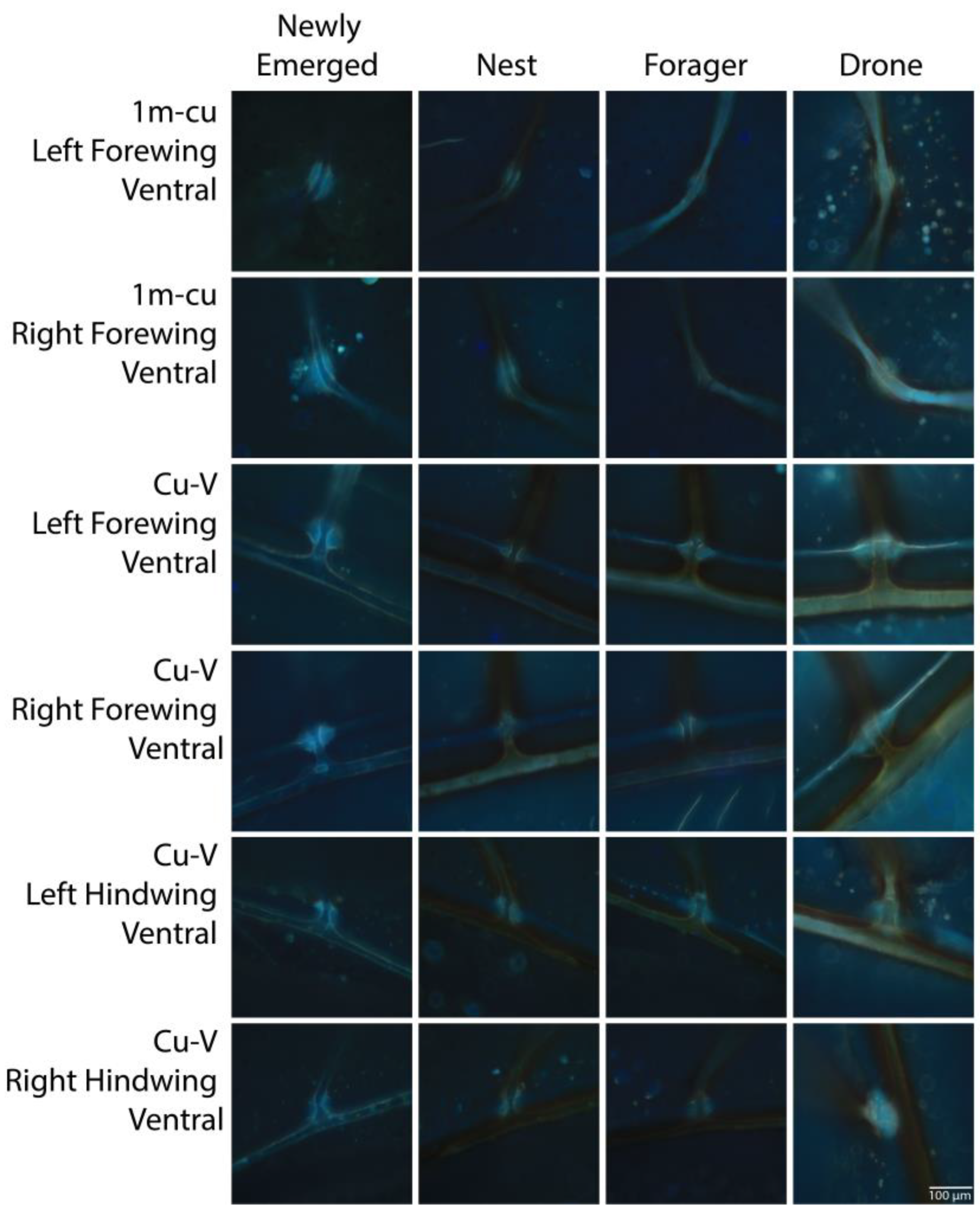
2.2.2. Microscopy
2.2.3. Image Analysis
2.3. Resilin Gene Expression
2.3.1. RNA Extraction
2.3.2. Reverse Transcription
2.3.3. ddPCR
2.4. Statistical Analyses
2.4.1. Resilin Gene Expression by Age Class
2.4.2. Resilin Autofluorescence by Age Class
3. Results
3.1. Resilin Gene Expression
3.2. Resilin Autofluorescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The Worldwide Importance of Honey Bees as Pollinators in Natural Habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.R. Organization of Work in the Honeybee: A Compromise between Division of Labour and Behavioural Flexibility. Proc. R. Soc. B Biol. Sci. 2003, 270, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.R. Limited Flexibility in the Temporal Caste System of the Honey Bee. Behav. Ecol. Sociobiol. 2005, 58, 219–226. [Google Scholar] [CrossRef]
- Johnson, B.R. Division of Labor in Honeybees: Form, Function, and Proximate Mechanisms. Behav. Ecol. Sociobiol. 2010, 64, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Beshers, S.N.; Huang, Z.Y.; Oono, Y.; Robinson, G.E. Social Inhibition and the Regulation of Temporal Polyethism in Honey Bees. J. Theor. Biol. 2001, 213, 461–479. [Google Scholar] [CrossRef]
- Robinson, G.E. Regulation of Division of Labor in Insect Societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef]
- Alaux, C.; Soubeyrand, S.; Prado, A.; Peruzzi, M.; Maisonnasse, A.; Vallon, J.; Hernandez, J.; Jourdan, P.; Le Conte, Y. Measuring Biological Age to Assess Colony Demographics in Honeybees. PLoS ONE 2018, 13, e0209192. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Donoughe, S.; Crall, J.D.; Merz, R.A.; Combes, S.A. Resilin in Dragonfly and Damselfly Wings and Its Implications for Wing Flexibility. J. Morphol. 2011, 272, 1409–1421. [Google Scholar] [CrossRef]
- Iwamoto, H. Structure, Function and Evolution of Insect Flight Muscle. Seibutsu Butsuri 2010, 50, 21–28. [Google Scholar] [CrossRef]
- Wootton, R.J. Functional Morphology of Insect Wings. Annu. Rev. Entomol. 1992, 37, 113–140. [Google Scholar] [CrossRef]
- Bergou, A.J.; Xu, S.; Wang, Z.J. Passive Wing Pitch Reversal in Insect Flight. J. Fluid. Mech. 2007, 591, 321–337. [Google Scholar] [CrossRef]
- Combes, S.A.; Daniel, T.L. Flexural Stiffness in Insect Wings I. Scaling and the Influence of Wing Venation. J. Exp. Biol. 2003, 206, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- Combes, S.A.; Daniel, T.L. Flexural Stiffness in Insect Wings. II. Spatial Distribution and Dynamic Wing Bending. J. Exp. Biol. 2003, 206, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Mistick, E.A.; Mountcastle, A.M.; Combes, S.A. Wing Flexibility Improves Bumblebee Flight Stability. J. Exp. Biol. 2016, 219, 3384–3390. [Google Scholar] [CrossRef]
- Mountcastle, A.M.; Daniel, T.L. Aerodynamic and Functional Consequences of Wing Compliance. Exp. Fluids 2009, 46, 873–882. [Google Scholar] [CrossRef]
- Mountcastle, A.M.; Combes, S.A. Wing Flexibility Enhances Load-Lifting Capacity in Bumblebees. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130531. [Google Scholar] [CrossRef]
- Appel, E.; Heepe, L.; Lin, C.P.; Gorb, S.N. Ultrastructure of Dragonfly Wing Veins: Composite Structure of Fibrous Material Supplemented by Resilin. J. Anat. 2015, 227, 561–582. [Google Scholar] [CrossRef]
- Guillermo-Ferreira, R.; Appel, E.; Urban, P.; Bispo, P.C.; Gorb, S.N. The Unusual Tracheal System within the Wing Membrane of a Dragonfly. Biol. Lett. 2017, 13, 20160960. [Google Scholar] [CrossRef]
- Pass, G. Beyond Aerodynamics: The Critical Roles of the Circulatory and Tracheal Systems in Maintaining Insect Wing Functionality. Arthropod Struct. Dev. 2018, 47, 391–407. [Google Scholar] [CrossRef]
- Elvin, C.M.; Carr, A.G.; Huson, M.G.; Maxwell, J.M.; Pearson, R.D.; Vuocolo, T.; Liyou, N.E.; Wong, D.C.C.; Merritt, D.J.; Dixon, N.E. Synthesis and Properties of Crosslinked Recombinant Pro-Resilin. Nature 2005, 437, 999–1002. [Google Scholar] [CrossRef]
- Li, L.; Teller, S.; Clifton, R.J.; Jia, X.; Kiick, K.L. Tunable Mechanical Stability and Deformation Response of a Resilin-Based Elastomer. Biomacromolecules 2011, 12, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.E.; Wong, D.C.C.; Kim, M.; Lekieffre, N.; Huson, M.G.; Vuocolo, T.; Merritt, D.J.; Nairn, K.M.; Dudek, D.M.; Colgrave, M.L.; et al. Molecular and Functional Characterisation of Resilin across Three Insect Orders. Insect Biochem. Mol. Biol. 2011, 41, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O. Characterization of a New Type of Cross-Linkage in Resilin, a Rubber-like Protein. BBA Biochim. Biophys. Acta 1963, 69, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O. The Cross-Links in Resilin Identified as Dityrosine and Trityrosine. BBA General. Subj. 1964, 93, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Gorb, S.N. Serial Elastic Elements in the Damselfly Wing: Mobile Vein Joints Contain Resilin. Naturwissenschaften 1999, 86, 552–555. [Google Scholar] [CrossRef]
- Ma, Y.; Ning, J.G.; Ren, H.L.; Zhang, P.F.; Zhao, H.Y. The Function of Resilin in Honeybee Wings. J. Exp. Biol. 2015, 218, 2136–2142. [Google Scholar] [CrossRef]
- Weis-Fogh, T. A Rubber-Like Protein in Insect Cuticle. J. Exp. Biol. 1960, 37, 889–907. [Google Scholar] [CrossRef]
- Michels, J.; Appel, E.; Gorb, S.N. Functional Diversity of Resilin in Arthropoda. Beilstein J. Nanotechnol. 2016, 7, 1241–1259. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghoroubi, N.; Darvizeh, A.; Appel, E.; Gorb, S.N. Effects of Multiple Vein Microjoints on the Mechanical Behaviour of Dragonfly Wings: Numerical Modelling. R. Soc. Open Sci. 2016, 3, 150610. [Google Scholar] [CrossRef]
- Appel, E.; Gorb, S.N. Resilin-Bearing Wing Vein Joints in the Dragonfly Epiophlebia Superstes. Bioinspir Biomim. 2011, 6, 046006. [Google Scholar] [CrossRef]
- Mamat-Noorhidayah; Yazawa, K.; Numata, K.; Norma-Rashid, Y. Morphological and Mechanical Properties of Flexible Resilin Joints on Damselfly Wings (Rhinocypha spp.). PLoS ONE 2018, 13, e0193147. [Google Scholar] [CrossRef]
- Amdam, G.V.; Page, R.E. The Developmental Genetics and Physiology of Honeybee Societies. Anim. Behav. 2010, 79, 973–980. [Google Scholar] [CrossRef]
- Amdam, G.V. Social Context, Stress, and Plasticity of Aging. Aging Cell 2011, 10, 18–27. [Google Scholar] [CrossRef]
- Ament, S.A.; Wang, Y.; Robinson, G.E. Nutritional Regulation of Division of Labor in Honey Bees: Toward a Systems Biology Perspective. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Münch, D.; Amdam, G.V. The Curious Case of Aging Plasticity in Honey Bees. FEBS Lett. 2010, 584, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- VanEngelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.; Saegerman, C.; Cox-Foster, D.L. Colony Collapse Disorder (CCD) and Bee Age Impact Honey Bee Pathophysiology. PLoS ONE 2017, 12, e0179535. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee, 1st ed.; Harvard University Press: Cambridge, UK, 1991. [Google Scholar]
- Schneeberg, K.; Bauernfeind, R.; Pohl, H. Comparison of Cleaning Methods for Delicate Insect Specimens for Scanning Electron Microscopy. Microsc. Res. Tech. 2017, 80, 1199–1204. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- McCloy, R.A.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial Inhibition of Cdk1 in G2 Phase Overrides the SAC and Decouples Mitotic Events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Ardell, D.H.; Andersen, S.O. Tentative Identification of a Resilin Gene in Drosophila Melanogaster. Insect Biochem. Mol. Biol. 2001, 31, 965–970. [Google Scholar] [CrossRef]
- Richards, E.H.; Jones, B.; Bowman, A. Salivary Secretions from the Honeybee Mite, Varroa Destructor: Effects on Insect Haemocytes and Preliminary Biochemical Characterization. Parasitology 2011, 138, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.J.; Aubert, A.; Grozinger, C.M. Modulation of Social Interactions by Immune Stimulation in Honey Bee, Apis mellifera, Workers. BMC Biol. 2008, 6, 50. [Google Scholar] [CrossRef]
- Jeon, J.H.; Moon, K.H.; Kim, Y.H.; Kim, Y.H. Reference Gene Selection for QRT-PCR Analysis of Season- and Tissue-Specific Gene Expression Profiles in the Honey Bee Apis mellifera. Sci. Rep. 2020, 10, 13935. [Google Scholar] [CrossRef] [PubMed]
- Tokach, R. An Evaluation of Honey Bee (Apis mellifera) Colony Behaviors and Aging When Exposed to a Pesticide-Contaminated Environment. J. Insect Sci. 2023, in press. [Google Scholar]
- Dickinson, M.H.; Lehmann, F.O.; Sane, S.P. Wing Rotation and the Aerodynamic Basis of Insect Right. Science 1999, 284, 1954–1960. [Google Scholar] [CrossRef]
- Ellington, C.P. The Aerodynamics of Hovering Insect Flight. IV. Aerodynamic Mechanisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 305, 79–113. [Google Scholar] [CrossRef]
- Sane, S.P. The Aerodynamics of Insect Flight. J. Exp. Biol. 2003, 206, 4191–4208. [Google Scholar] [CrossRef]
- Wootton, R. The Geometry and Mechanics of Insect Wing Deformations in Flight: A Modelling Approach. Insects 2020, 11, 446. [Google Scholar] [CrossRef]
- Andersen, S.O. Studies on Resilin-like Gene Products in Insects. Insect Biochem. Mol. Biol. 2010, 40, 541–551. [Google Scholar] [CrossRef]
- Salcedo, M.K.; Jun, B.H.; Socha, J.J.; Pierce, N.E.; Vlachos, P.P.; Combes, S.A. Complex Hemolymph Circulation Patterns in Grasshopper Wings. Commun. Biol. 2023, 6, 313. [Google Scholar] [CrossRef]
- Salcedo, M.K.; Socha, J.J. Circulation in Insect Wings. Integr. Comp. Biol. 2020, 60, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O.; Weis-Fogh, T. Resilin. A Rubberlike Protein in Arthropod Cuticle. Adv. Insect Phys. 1964, 2, 889–907. [Google Scholar] [CrossRef]
- Martin, S.J.; Brettell, L.E. Deformed Wing Virus in Honeybees and Other Insects. Annu. Rev. Virol. 2019, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef]
- Perry, C.J.; Søvik, E.; Myerscough, M.R.; Barron, A.B. Rapid Behavioral Maturation Accelerates Failure of Stressed Honey Bee Colonies. Proc. Natl. Acad. Sci. USA 2015, 112, 3427–3432. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]


| Locus | Amplicon Size (bp) | Forward Primer | Reverse Primer | Probe | Accession Number |
|---|---|---|---|---|---|
| Pro-resilin | 133 | CGGTAATGGAGGTTATGG | CTCCATCTCTGCTTTCC | TCCAGATTGCTCGTCTTTCACTTC | XM_006563102.3 |
| eIF3-S8 | 82 | CGTACTGATCGTAGGGAA | CTACTGCTGGTCCAAGA | TTGCAATCCATAGCAGACACTCAT | XM_006564593.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, A.; Keime, N.; Fong, C.; Kraemer, A.; Fassbinder-Orth, C. Resilin Distribution and Abundance in Apis mellifera across Biological Age Classes and Castes. Insects 2023, 14, 764. https://doi.org/10.3390/insects14090764
Anderson A, Keime N, Fong C, Kraemer A, Fassbinder-Orth C. Resilin Distribution and Abundance in Apis mellifera across Biological Age Classes and Castes. Insects. 2023; 14(9):764. https://doi.org/10.3390/insects14090764
Chicago/Turabian StyleAnderson, Audrey, Noah Keime, Chandler Fong, Andrew Kraemer, and Carol Fassbinder-Orth. 2023. "Resilin Distribution and Abundance in Apis mellifera across Biological Age Classes and Castes" Insects 14, no. 9: 764. https://doi.org/10.3390/insects14090764
APA StyleAnderson, A., Keime, N., Fong, C., Kraemer, A., & Fassbinder-Orth, C. (2023). Resilin Distribution and Abundance in Apis mellifera across Biological Age Classes and Castes. Insects, 14(9), 764. https://doi.org/10.3390/insects14090764






