Unraveling the Significance of Draglines: Female Sexual Signalization in the Nursery-Web Spider, Pisaura mirabilis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Organism
2.2. Collection and Maintenance
2.3. Female Pheromones/Gift Production Experiment
2.4. Sample Preparation
2.5. HPLC Analysis
2.6. Statistical Analyses
3. Results
3.1. Biometry of Fed and Hungry Females
3.2. Behavioral Evidence to Trigger Male Sexual Behavior by Chemical Signals
3.3. Male Investment in the Production of Nuptial Gifts
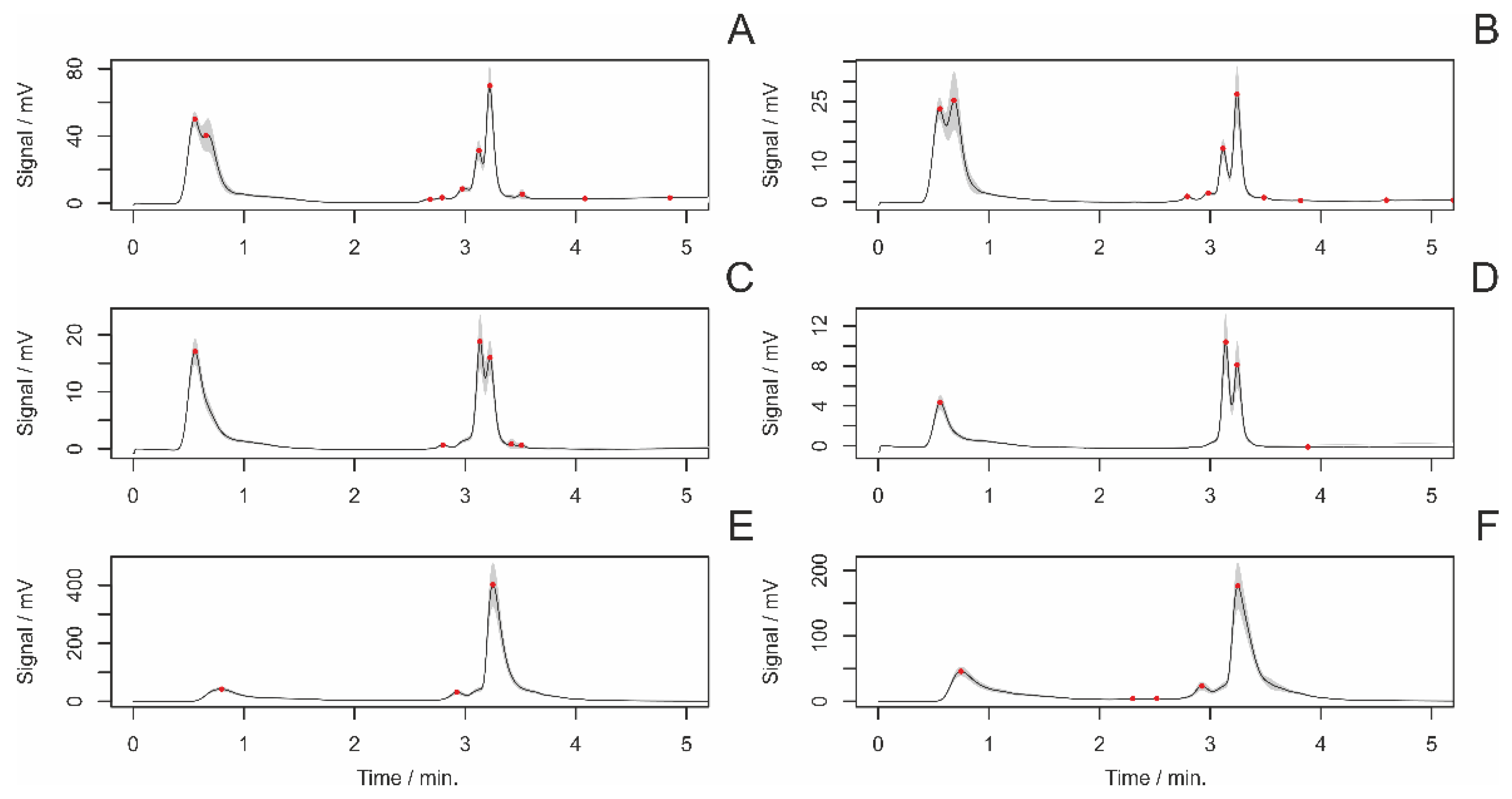
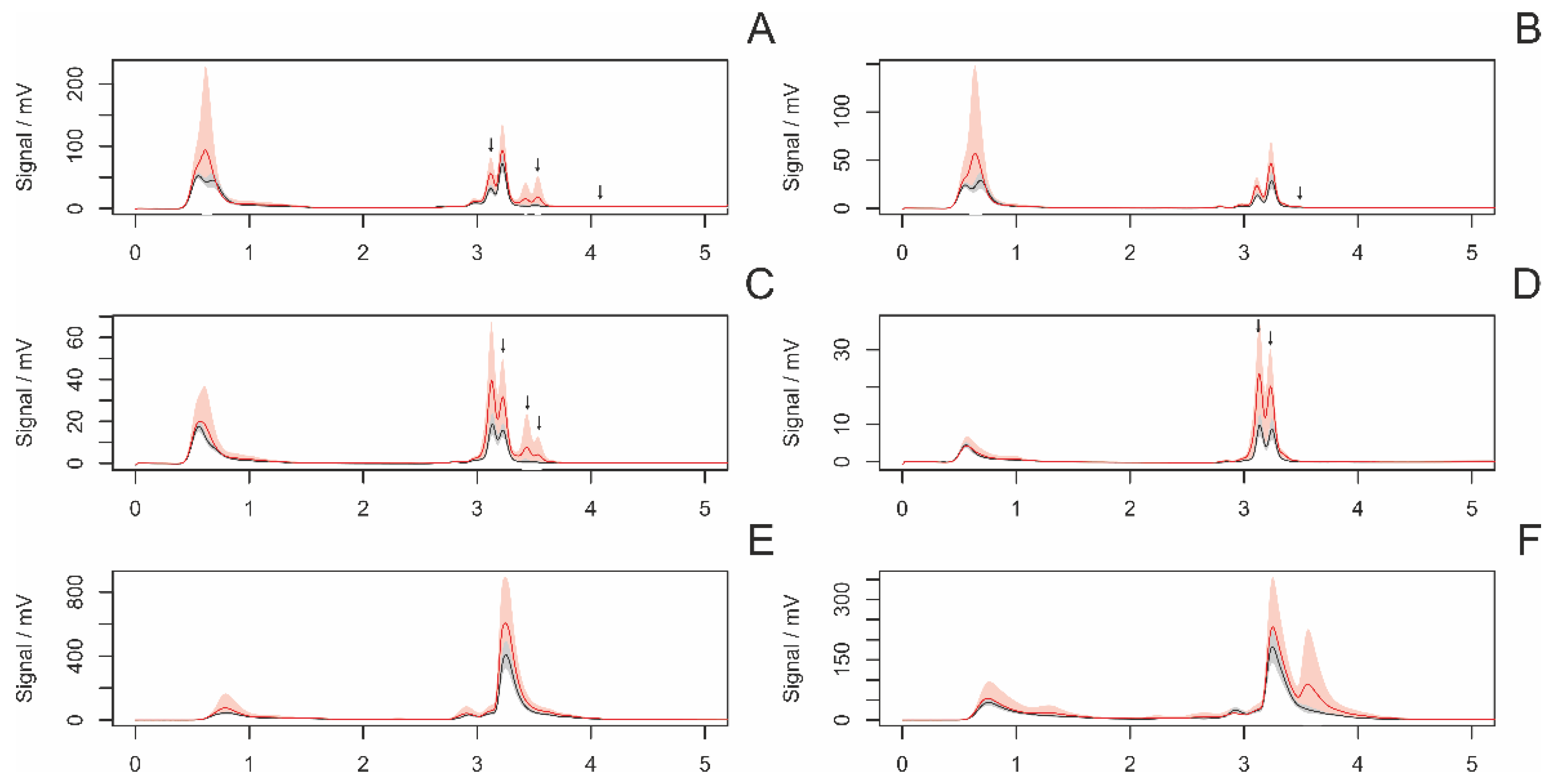
3.4. HPLC Analyses of Female Draglines
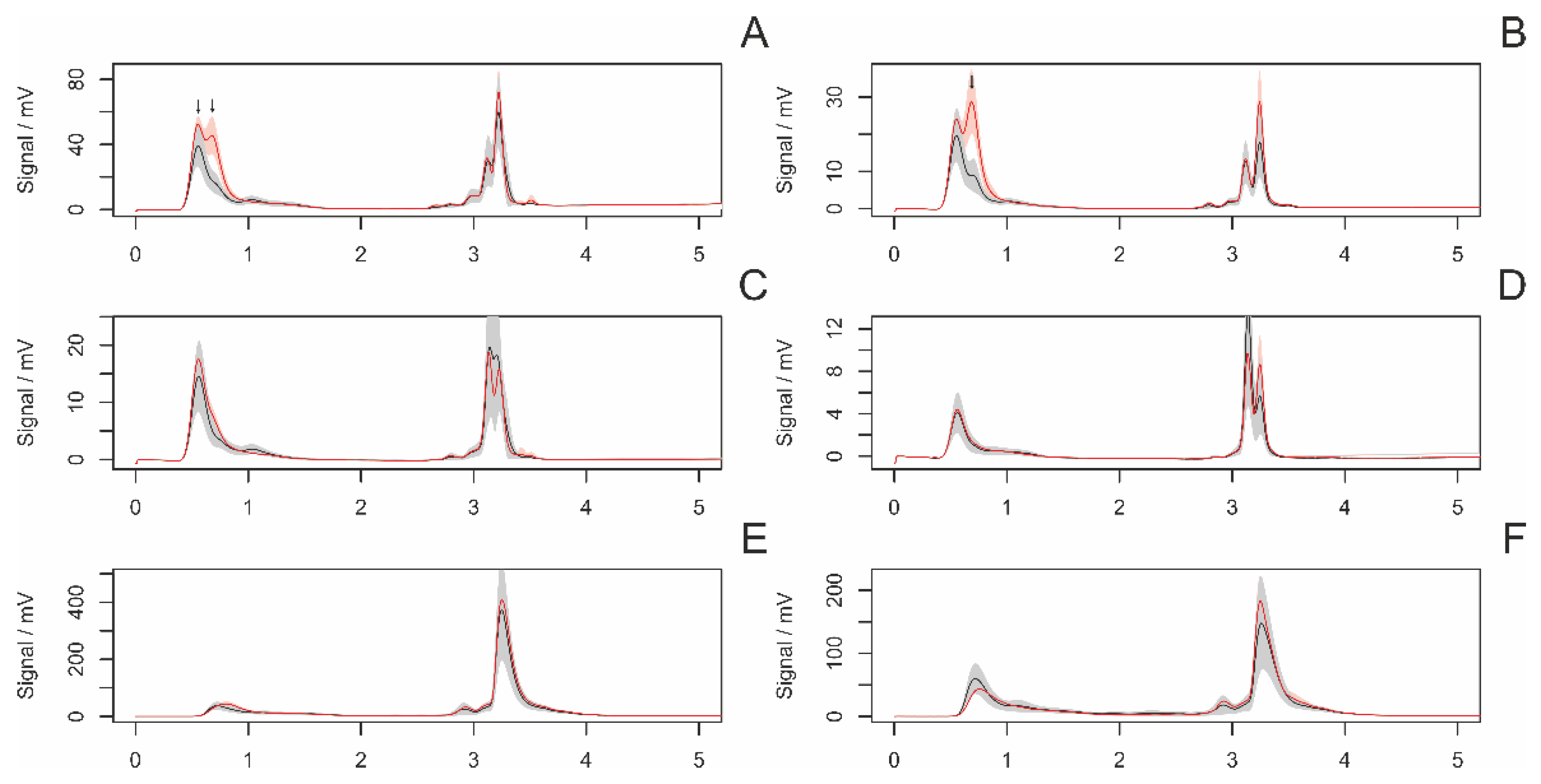
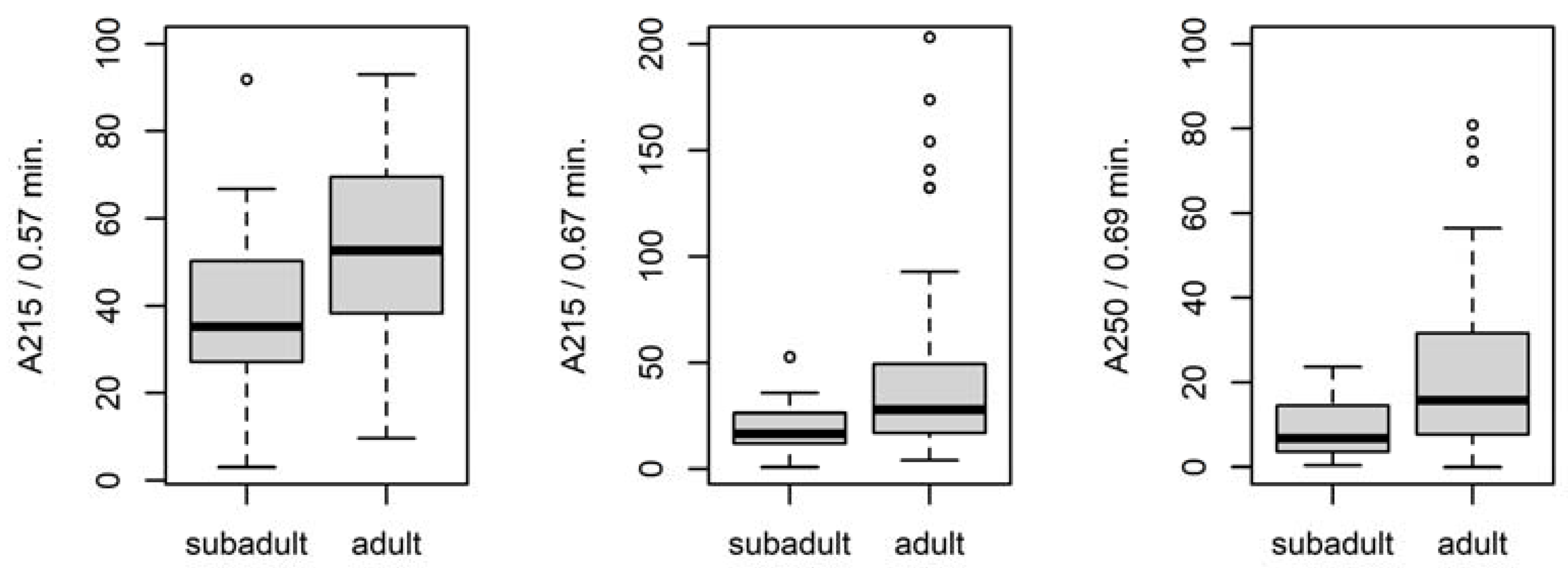
3.4.1. Differences between Adult and Subadult Individuals
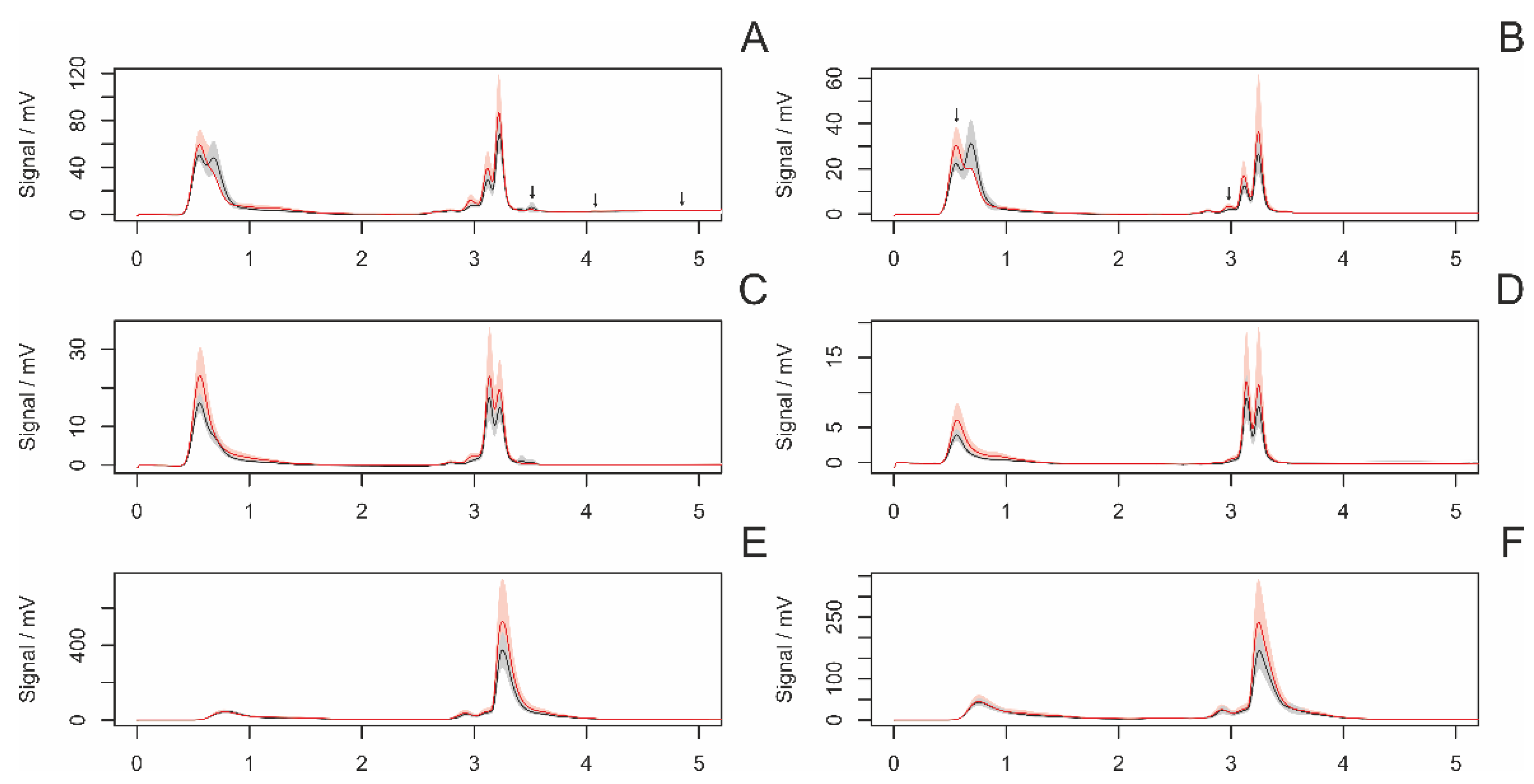
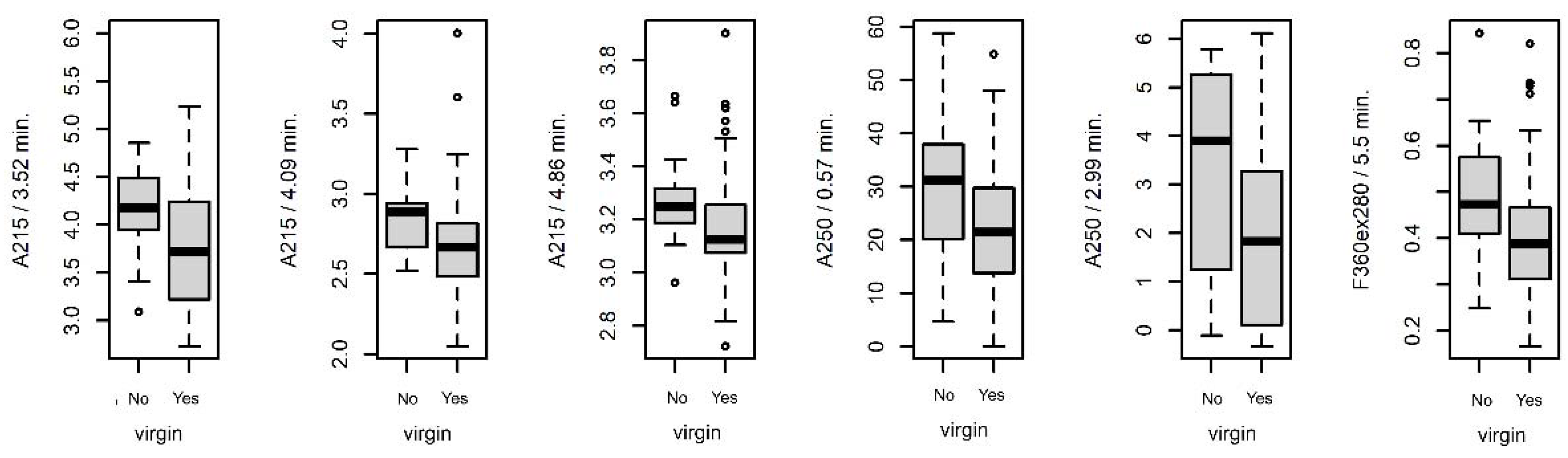
3.4.2. Differences between Virgin and Nonvirgin Females
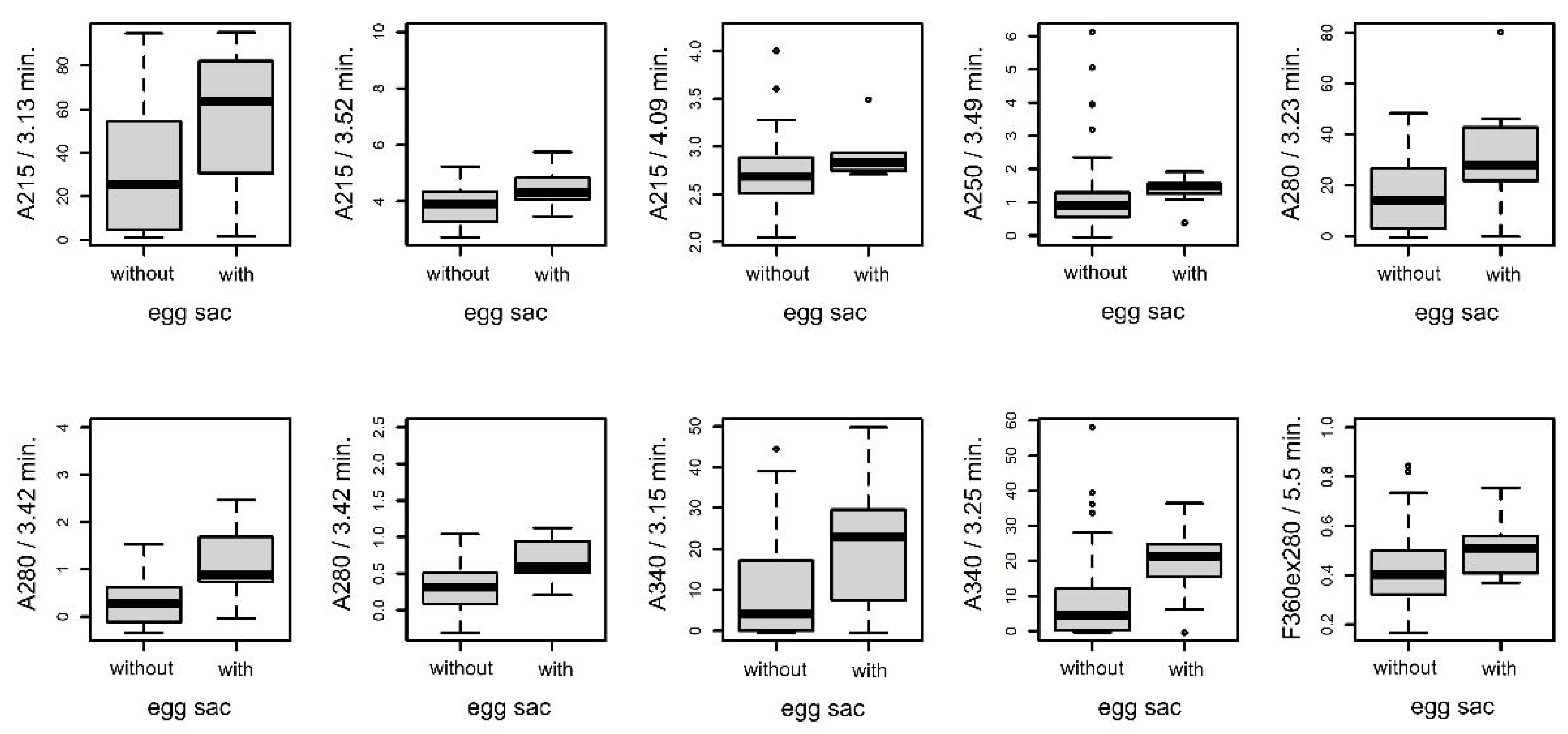
3.4.3. Females with and without Eggsacs
3.4.4. Hungry and Fed Females
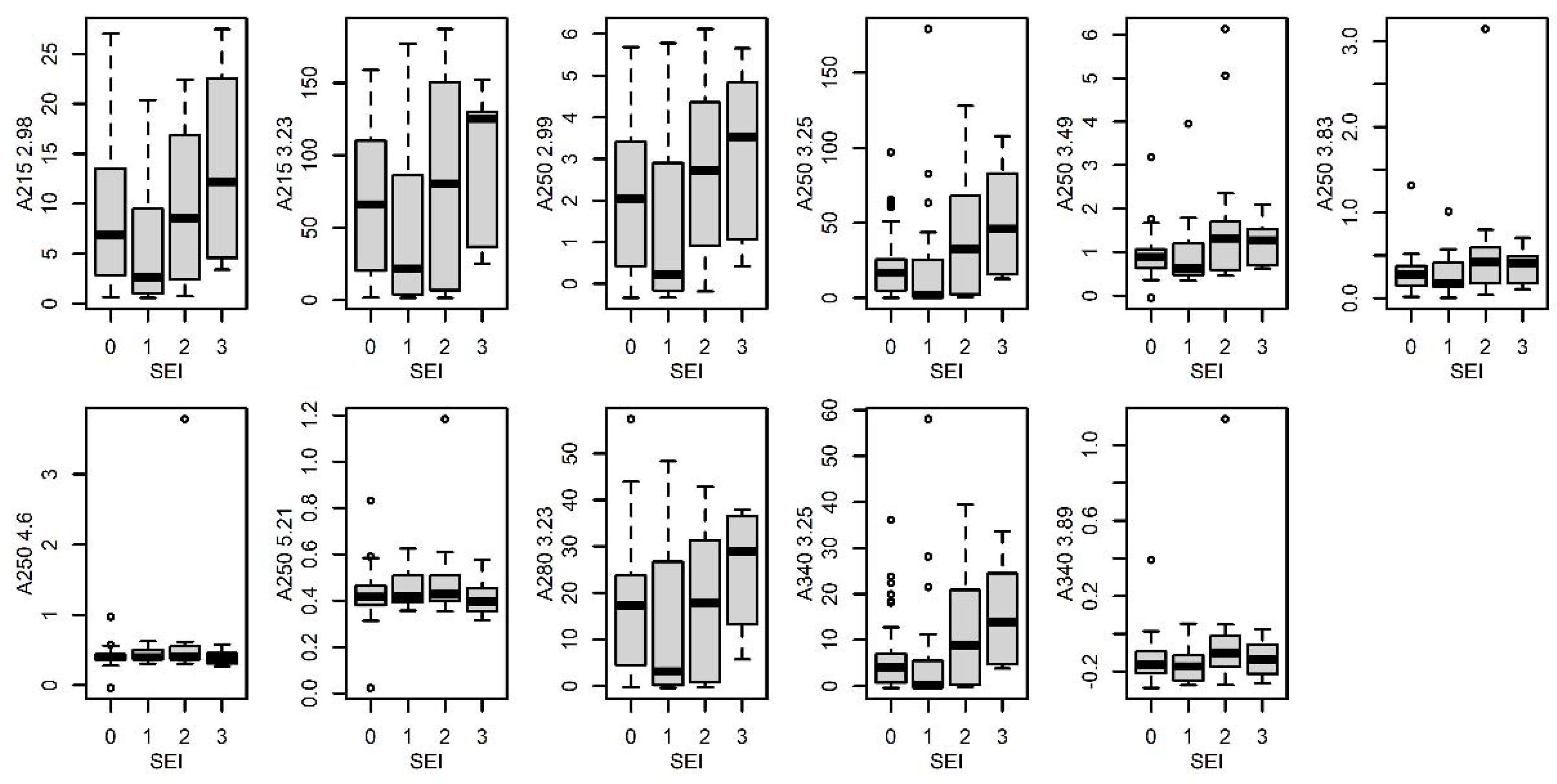
3.4.5. Sexual Excitement Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, G.; Russell, I.J. Flying in tune: Sexual recognition in mosquitoes. Curr. Biol. 2006, 16, R131–R134. [Google Scholar] [CrossRef] [PubMed]
- Zuk, M.; Kolluru, G.R. Exploitation of sexual signals by predators and parasitoids. Quart. Rev. Biol. 1998, 73, 415–438. [Google Scholar] [CrossRef]
- Jiggins, C.D. The Ecology and Evolution of Heliconius Butterflies; Oxford University Press: Oxford, UK, 2017; p. 288. [Google Scholar]
- Tigreros, N. Linking nutrition and sexual selection across life stages in a model butterfly system. Funct. Ecol. 2013, 27, 145–154. [Google Scholar] [CrossRef]
- Obara, Y.; Ozawa, G.; Fukano, Y.; Watanabe, K.; Satoh, T. Mate preference in males of the cabbage butterfly, Pieris rapae crucivora, changes seasonally with the change in female UV color. Zool. Sci. 2008, 25, 1–5. [Google Scholar] [CrossRef]
- Andersson, M.B. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Cardé, R.T.; Willis, M.A. Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 2008, 34, 854–866. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and Animal Behavior: Chemical Signals and Signatures, 2nd ed.; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Reinhold, K. Variation in acoustic signalling traits exhibits footprints of sexual selection. Evolution 2011, 65, 738–745. [Google Scholar] [CrossRef]
- Kelley, L.A.; Endler, J.A. Illusions promote mating success in great bowerbirds. Science 2012, 335, 335–338. [Google Scholar] [CrossRef]
- Dettner, K.; Liepert, C. Chemical mimicry and camouflage. Annu. Rev. Entomol. 1994, 39, 129–154. [Google Scholar] [CrossRef]
- Henneken, J.; Jones, T.M. Pheromones-based sexual selection in a rapidly changing world. Curr. Ins. Sci. 2017, 24, 84–88. [Google Scholar] [CrossRef]
- Steiger, S.; Stökl, J. The Role of Sexual Selection in the Evolution of Chemical Signals in Insects. Insects 2014, 5, 423–438. [Google Scholar] [CrossRef]
- Gaskett, A.C. Spider sex pheromones: Emission, reception, structures, and functions. Biol. Rev. 2007, 82, 26–48. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Toft, S. Identification of a sex pheromone from a spider. Science 1993, 260, 1635–1637. [Google Scholar] [CrossRef] [PubMed]
- Kasumovic, M.M.; Andrade, M.C.B. Discrimination of airborne pheromones by mate-searching male western black widow spiders (Latrodectus hesperus): Species- and population-specific responses. Can. J. Zool. 2004, 82, 1027–1034. [Google Scholar] [CrossRef]
- Ross, K.; Smith, R.L. Aspects of the courtship behavior of the black widow spider, Latrodectus hesperus (Araneae: Theridiidae), with evidence for the existence of a contact sex pheromone. J. Arachnol. 1979, 7, 69–77. [Google Scholar]
- Aisenberg, A.; Costa, F.G. Reproductive isolation and sex-role reversal in two sympatric sand-dwelling wolf spiders of the genus Allocosa. Can. J. Zool. 2008, 86, 648. [Google Scholar] [CrossRef]
- Pruitt, J.N.; Riechert, S.E. Male mating preference is associated with risk of pre-copulatory cannibalism in a socially polymorphic spider. Behav. Ecol. Sociobiol. 2009, 63, 1573–1580. [Google Scholar] [CrossRef]
- Pollard, S.D.; Macnab, A.M.; Jackson, R.R. Communication with chemicals: Pheromones and spiders. In Ecophysiology of Spiders; Nentwig, W., Ed.; Springer Verlag: Berlin/Heidelberg, Germany, 1987; pp. 133–141. [Google Scholar]
- Schulz, S. Spider pheromones a structural perspective. J. Chem. Ecol. 2013, 39, 1–14. [Google Scholar] [CrossRef]
- Fischer, A. Chemical communication in spiders a methodological review. J. Arachnol. 2019, 47, 1–27. [Google Scholar] [CrossRef]
- Schneider, J.; Andrade, M. Mating behaviour and sexual selection. In Spider Behaviour Flexibility, and Versatility; Herberstein, M.E., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 215–274. [Google Scholar]
- Foelix, R.F. Biology of Spiders, 2nd ed.; Oxford University Press: Oxford, UK; Georg Thieme Verlag: Berlin, Germany, 1996; p. 336. [Google Scholar]
- Avile’s, L. Causes and consequences of cooperation and permanent-sociality in spiders. In Evolution of Social Behaviour in Insects and Arachnids; Choe, J., Crespi, B., Eds.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Anderson, J.T.; Morse, D.H. Pick-up lines: Cues used by male crab spiders to find reproductive females. Behav. Ecol. 2001, 12, 360–366. [Google Scholar] [CrossRef][Green Version]
- Schulz, S. Semiochemistry of spiders. In Advances in Chemical Ecology; Carde, R., Millar, J., Eds.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Clark, R.J.; Jackson, R.R. Araneophagic jumping spiders discriminate between the draglines of familiar and unfamiliar conspecifics. Ethol. Ecol. Evol. 1995, 7, 185–190. [Google Scholar] [CrossRef]
- Clark, R.J.; Jackson, R.R.; Waas, J.R. Draglines and assessment of fighting ability in cannibalistic jumping spiders. J. Ins. Behav. 1999, 12, 753–766. [Google Scholar] [CrossRef]
- Eberhard, M.J.B.; Machnis, A.; Uhl, G. Condition dependent differences in male vibratory precopulatory and copulatory courtship in a nuptial gift-giving spider. Behav. Ecol. Sociobiol. 2020, 74, 138. [Google Scholar] [CrossRef]
- Lang, A. Silk investment in gifts by males of the nuptial feeding spider Pisaura mirabilis (Araneae: Pisauridae). Behaviour 1996, 133, 697–716. [Google Scholar] [CrossRef]
- Nitzsche, R.O.M. Courtship, mating and agonistic behaviour in Pisaura mirabilis (Clerck, 1757). Arachnology 2011, 15, 93–120. [Google Scholar] [CrossRef]
- Prokop, P. Male preferences for nuptial gifts and gift weight loss amongst the nursery web spider, Pisaura mirabilis. J. Ethol. 2019, 37, 363–370. [Google Scholar] [CrossRef]
- Beyer, M.; Czaczkes, T.J.; Tuni, C. Does silk mediate chemical communication between the sexes in a nuptial feeding spider? Behav. Ecol. Sociobiol. 2018, 72, 49. [Google Scholar] [CrossRef]
- Bristowe, W.S.; Lockett, G.H. The courtship of British lycosid spiders, and its probable significance. Proc. Zool. Soc. Lond. 1926, 22, 317–347. [Google Scholar] [CrossRef]
- Stålhandske, P. Nuptial gift in the spider Pisaura mirabilis maintained by sexual selection. Behav. Ecol. 2001, 12, 691–697. [Google Scholar] [CrossRef]
- Albo, M.J.; Toft, S.; Bilde, T. Condition dependence of male nuptial gift construction in the spider Pisaura mirabilis (Pisauridae). J. Ethol. 2011, 29, 473–479. [Google Scholar] [CrossRef]
- Tuni, C.; Albo, M.J.; Bilde, T. Polyandrous females acquire indirect benefits in a nuptial feeding species. J. Evol. Biol. 2013, 26, 1307–1316. [Google Scholar] [CrossRef]
- Scott, C.E.; Anderson, A.G.; Andrade, M.C.B. A review of the mechanisms and functional roles of male silk use in spider courtship and mating. J. Arachnol. 2018, 46, 173–206. [Google Scholar] [CrossRef]
- Stålhandske, S. Nuptial gifts of male spiders function as sensory traps. Proc. Roy. Soc. Lond. B 2002, 269, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Trillo, M.C.; Melo-Gonzalez, V.; Albo, M.J. Silk wrapping of nuptial gifts as visual signal for female attraction in a crepuscular spider. Naturwissenschaften 2014, 101, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.L.; Trillo, M.C.; Costa, F.G.; Albo, M.J. Nuptial gift size, mating duration and remating success in a Neotropical spider. Ethol. Ecol. Evol. 2014, 26, 29–39. [Google Scholar] [CrossRef]
- Henneken, J.; Goodger, J.Q.D.; Jones, T.M.; Elgar, M.A. Diet-mediated pheromones and signature mixtures can enforce signal reliability. Front. Ecol. Evol. 2017, 4, 145. [Google Scholar] [CrossRef]
- Fisher, H.S.; Rosenthal, G.G. Female swordtail fish use chemical cues to select well-fed mates. Anim. Behav. 2006, 72, 721–725. [Google Scholar] [CrossRef]
- Giaquinto, P.C. Female pintado catfish choose well-fed males. Behaviour 2010, 147, 319–332. [Google Scholar] [CrossRef]
- Baruffaldi, L.; Andrade, M.C.B. Contact pheromones mediate male preference in black widow spiders: Avoidance of hungry sexual cannibals? Anim. Behav. 2015, 102, 25–32. [Google Scholar] [CrossRef]
- Arnqvist, G.; Nilsson, T. The evolution of polyandry: Multiple mating and female fitness in insects. Anim. Behav. 2000, 60, 145–164. [Google Scholar] [CrossRef]
- Bilde, T.; Tuni, C.; Elsayed, R.; Pekar, S.; Toft, S. Nuptial gifts of male spiders: Sensory exploitation of the female’s maternal care instinct or foraging motivation? Anim. Behav. 2007, 73, 267–273. [Google Scholar] [CrossRef]
- Gwynne, D.T. Testing parental investment and the control of sexual selection in katydids: The operational sex ratio. Am. Natur. 1990, 136, 474–484. [Google Scholar] [CrossRef]
- Lewis, S.; South, A. The evolution of animal nuptial gifts. Advan. Stud. Behav. 2012, 44, 53–97. [Google Scholar] [CrossRef]
- Boggs, C.L. A general model of the role of male-donated nutrients in female insects’ reproduction. Am. Natur. 1990, 136, 598–617. [Google Scholar] [CrossRef]
- Simmons, L.W.; Gwynne, D.T. The refractory period of female katydids (Orthoptera: Tettigoniidae): Sexual conflict over the remating interval? Behav. Ecol. 1991, 2, 276–282. [Google Scholar] [CrossRef][Green Version]
- Edvardsson, M. Female Callosobruchus maculates mate when they are thirsty: Resource-rich ejaculates as mating effort in a beetle. Anim. Behav. 2007, 74, 183–188. [Google Scholar] [CrossRef]
- Gwynne, D.T. Sexual conflict over nuptial gifts in insects. Ann. Rev. Entomol. 2008, 53, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Prokop, P.; Maxwell, M.R. Female feeding regime and polyandry in the nuptially feeding nursery-web spider, Pisaura mirabilis. Naturwissenschaften 2009, 96, 259–265. [Google Scholar] [CrossRef]
- Albo, M.J.; Franco-Trecu, V.; Wojciechowski, F.J.; Toft, S.; Bilde, T. Maintenance of deceptive gifts in a natural spider population: Ecological and demographic factors. Behav. Ecol. 2019, 30, 993–1000. [Google Scholar] [CrossRef]
- Buchar, J.; Babrakzai, H.; Hodek, I. Life-cycle and phenology of the spider Pisaura mirabilis (Araneae) in central Europe. Acta Entomol. Bohemos. 1989, 86, 414–418. [Google Scholar]
- Austad, S.N.; Thornhill, R. Female reproductive variation in a nuptial feeding spider, Pisaura mirabilis. Bull. Brit. Arachnol. Soc. 1986, 7, 48–52. [Google Scholar]
- Andersen, T.; Bollerup, K.; Toft, S.; Bilde, T. Why do males of the spider Pisaura mirabilis wrap their nuptial gifts in silk: Female preference or male control? Ethology 2008, 114, 775–781. [Google Scholar] [CrossRef]
- Bristowe, W.S. The World of Spiders; Collins: London, UK, 1958; p. 304. [Google Scholar]
- Kotiaho, J.S.; Alatalo, R.V.; Mappes, J.; Parri, S. Sexual signalling and viability in a wolf spider (Hygrolycosa rubrofasciata): Measurements under laboratory and field conditions. Behav. Ecol. Sociobiol. 1999, 46, 123–128. [Google Scholar] [CrossRef]
- Prokop, P.; Semelbauer, M. Biometrical and behavioural associations with offering nuptial gifts by males in the spider Pisaura mirabilis. Anim. Behav. 2017, 129, 189–196. [Google Scholar] [CrossRef]
- Prokop, P.; Maxwell, M.R. Gift -carrying in the spider Pisaura mirabilis: Nuptial gift contents in nature and effects on male running speed and fighting success. Anim. Behav. 2012, 83, 1395–1399. [Google Scholar] [CrossRef]
- Ghislandi, P.G.; Beyer, M.; Velado, P.; Tuni, C. Silk wrapping of nuptial gifts aids cheating behaviour in male spiders. Behav. Ecol. 2017, 28, 744–749. [Google Scholar] [CrossRef]
- Garcia-Berthou, E. On the misuse of residuals in ecology: Testing regression residuals vs. the analysis of covariance. J. Anim. Ecol. 2001, 70, 708–711. [Google Scholar] [CrossRef]
- Eberhard, M.J.B.; Möller, T.A.; Uhl, G. Dragline silk reveals female developmental stage and mediates male vibratory courtship in the nuptial gift-giving spider Pisaura mirabilis. Ethology 2021, 127, 267–277. [Google Scholar] [CrossRef]
- Prokop, P.; Okrouhlík, J. Metabolic cost of holding nuptial food gifts for male spiders. Ecol. Entomol. 2021, 46, 684–690. [Google Scholar] [CrossRef]
- Toft, S.; Albo, M.J. Optimal numbers of matings: The conditional balance between benefits and costs of mating for females of a nuptial gift-giving spider. J. Evol. Biol. 2015, 28, 457–467. [Google Scholar] [CrossRef]
- Prokop, P. Insemination does not affect female mate choice in a nuptial feeding spider. It. J. Zool 2006, 73, 197–201. [Google Scholar] [CrossRef]
- Tuni, C.; Bilde, T. No preference for novel mating partners in the polyandrous nuptial-feeding spider Pisaura mirabilis (Araneae: Pisauridae). Anim. Behav. 2010, 80, 435–442. [Google Scholar] [CrossRef]
- Matzke, M.; Toft, S.; Bechsgaard, J.; Pold Vilstrup, A.; Uhl, G.; Künzel, S.; Bilde, T. Sperm competition intensity affects sperm precedence patterns in a polyandrous gift-giving spider. Mol. Ecol. 2022, 31, 2435–2452. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.M.; Lubin, Y. Infanticidal male eresid spiders. Nature 1996, 381, 655–656. [Google Scholar] [CrossRef]
- Tuni, C.; Weber, S.; Bilde, T.; Uhl, G. Male spiders reduce pre-and postmating sexual investment in response to sperm competition risk. Behav. Ecol. 2017, 28, 1030–1036. [Google Scholar] [CrossRef]
- Solano-Brenes, D.; Costa-Schmidt, L.E.; Albo, M.J.; Machado, G. Differential allocation in a gift-giving spider: Males adjust their reproductive investment in response to female condition. Ecol. Evol. 2021, 21, 140. [Google Scholar] [CrossRef]

| Estimate | Wald’s χ2 | −95% CI | +95% CI | p | |
|---|---|---|---|---|---|
| Intercept | 1.37 | 1.94 | |||
| Male mobility | 0.10 | 1.13 | 0.92 | 1.33 | 0.29 |
| Female mobility | 0.09 | 2.20 | 0.97 | 1.23 | 0.14 |
| Sexual excitement index | −1.00 | 6.93 | 0.17 | 0.77 | 0.008 |
| Hunger level (fed) | 0.87 | 3.93 | 1.02 | 32.37 | 0.047 |
| Mating status (virgin) | −0.003 | 0.00005 | 0.15 | 6.48 | 0.99 |
| Estimate | Wald’s χ2 | −95% CI | +95% CI | p | |
|---|---|---|---|---|---|
| Male mobility | −0.09 | 0.93 | −0.27 | 0.09 | 0.34 |
| Female mobility | −0.09 | 2.72 | −0.21 | 0.018 | 0.10 |
| Sexual excitement index | 0.86 | 5.83 | 0.16 | 1.56 | 0.02 |
| Hunger level (fed) | −0.55 | 1.64 | −1.40 | 0.29 | 0.12 |
| Mating status (virgin) | −0.15 | 0.09 | −1.14 | 0.84 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ježová, Z.; Prokop, P.; Zvaríková, M.; Zvarík, M. Unraveling the Significance of Draglines: Female Sexual Signalization in the Nursery-Web Spider, Pisaura mirabilis. Insects 2023, 14, 765. https://doi.org/10.3390/insects14090765
Ježová Z, Prokop P, Zvaríková M, Zvarík M. Unraveling the Significance of Draglines: Female Sexual Signalization in the Nursery-Web Spider, Pisaura mirabilis. Insects. 2023; 14(9):765. https://doi.org/10.3390/insects14090765
Chicago/Turabian StyleJežová, Zuzana, Pavol Prokop, Martina Zvaríková, and Milan Zvarík. 2023. "Unraveling the Significance of Draglines: Female Sexual Signalization in the Nursery-Web Spider, Pisaura mirabilis" Insects 14, no. 9: 765. https://doi.org/10.3390/insects14090765
APA StyleJežová, Z., Prokop, P., Zvaríková, M., & Zvarík, M. (2023). Unraveling the Significance of Draglines: Female Sexual Signalization in the Nursery-Web Spider, Pisaura mirabilis. Insects, 14(9), 765. https://doi.org/10.3390/insects14090765












