Breeding Polyommatus icarus Serves as a Large-Scale and Environmentally Friendly Source of Precisely Tuned Photonic Nanoarchitectures
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Butterfly
2.2. Laboratory Population
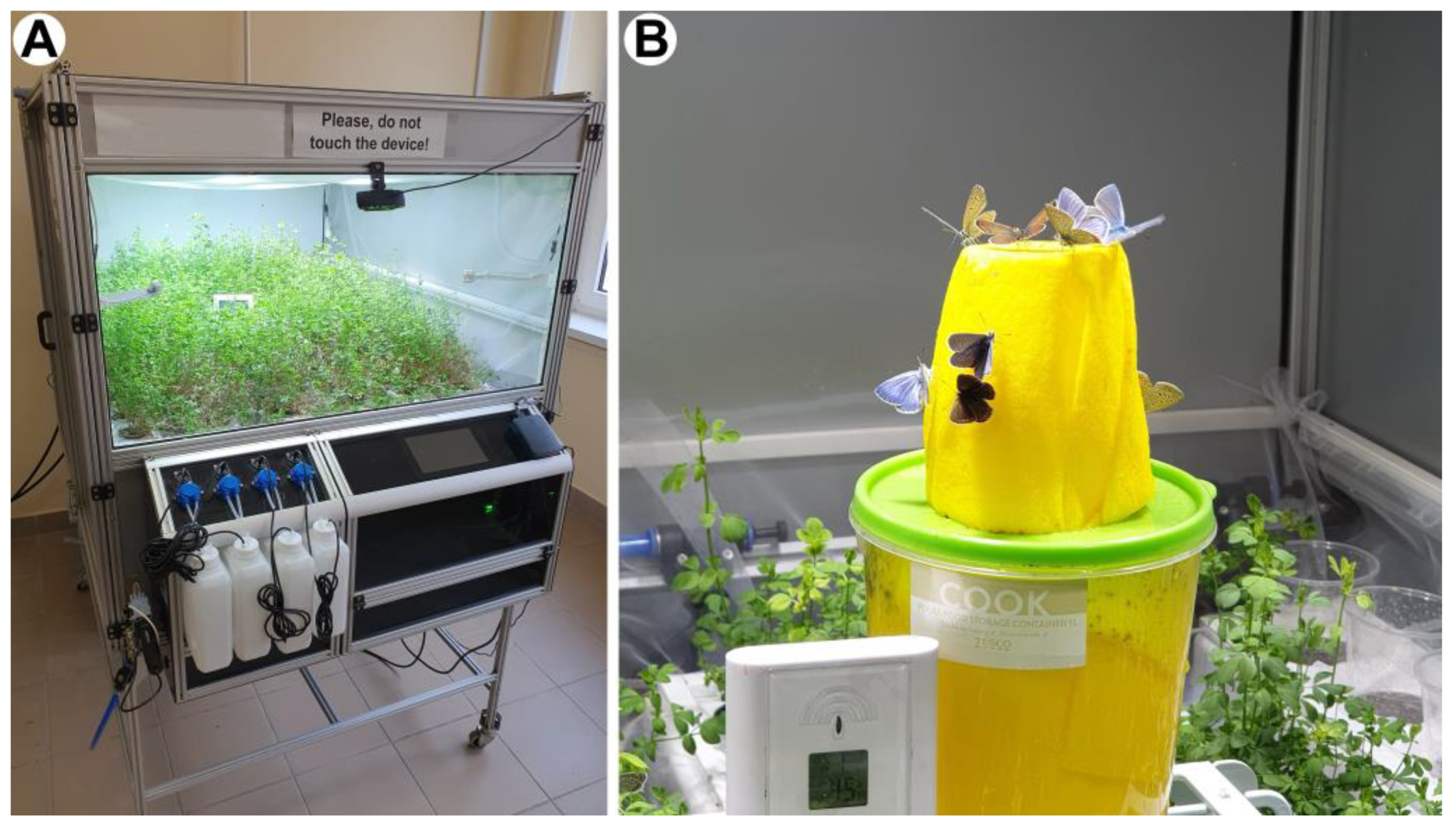
2.3. The Insectarium
2.4. Reflectance Spectrophotometry
2.5. Population Genetics
3. Results and Discussion
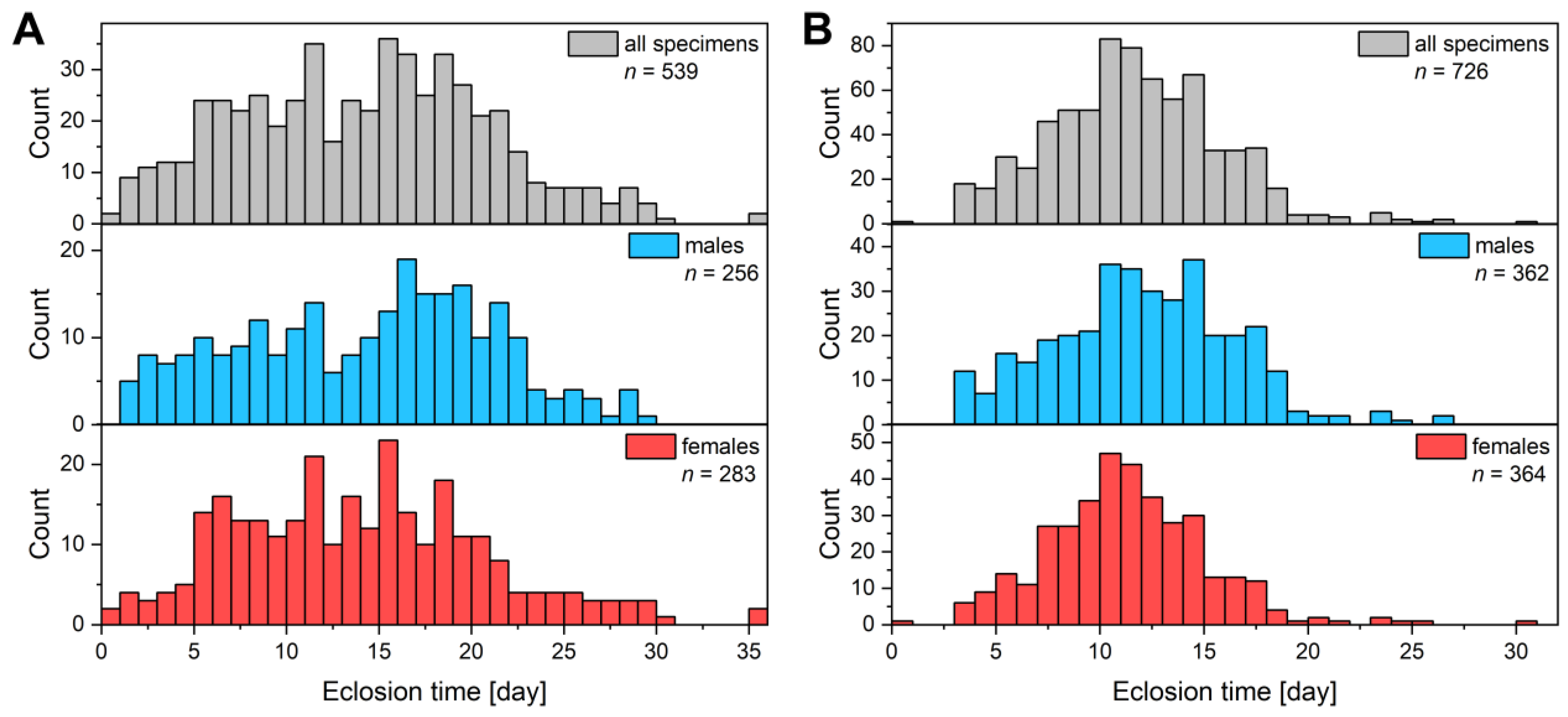
3.1. Breeding
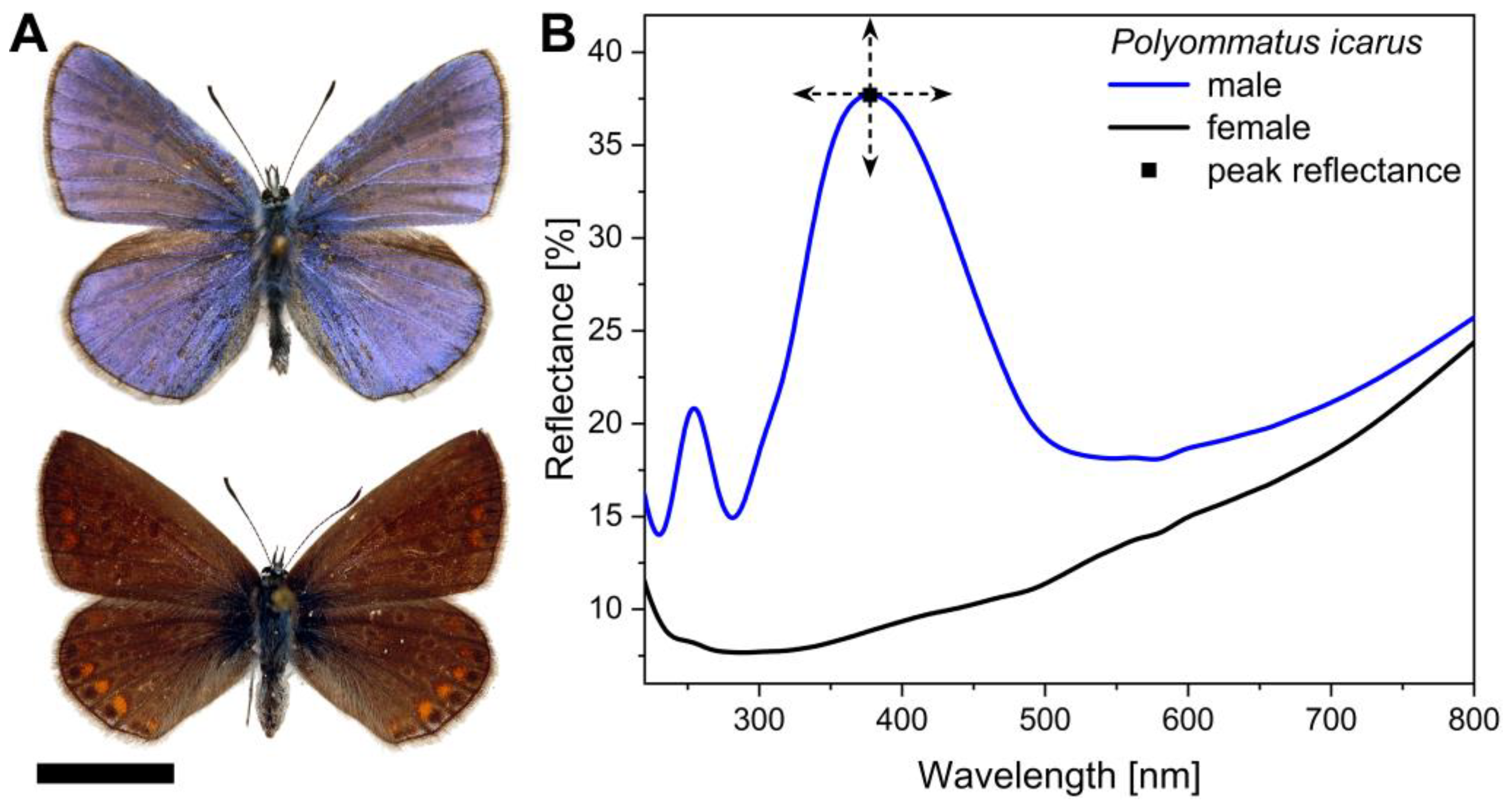
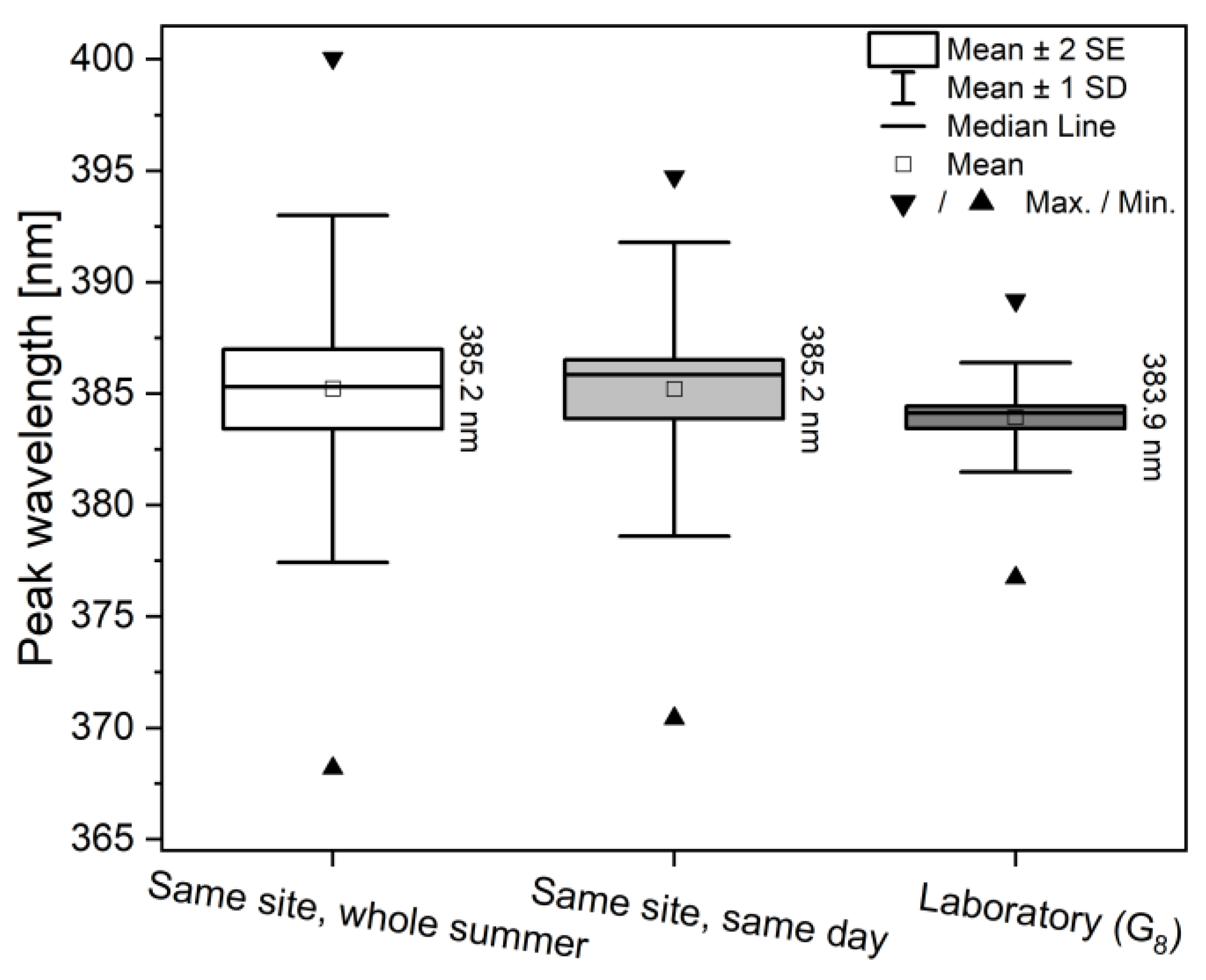
3.2. Spectral Properties
3.3. Population Genetics
3.4. Biotemplating for Industrial Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basalla, G. The Evolution of Technology; Cambridge University Press: Cambridge, UK, 1988; p. 248. [Google Scholar]
- Crane, E. The World History of Beekeeping and Honey Hunting, 1st ed.; Taylor & Francis: Abingdon-on-Thames, UK, 1999; Part xxii; p. 681. [Google Scholar]
- Klein, K. (Ed.) The Unbroken Thread: Conserving the Textile Traditions of Oaxaca; The Getty Conservation Institute: Los Angeles, CA, USA, 1997; p. 162. [Google Scholar]
- Badola, K.; Peigler, S.R. Eri Silk Cocoon to Cloth; Bisher Singh & Mehadra Pal Singh Publication: Dehra Dun, India, 2013; p. 110. [Google Scholar]
- Czaplicki, Z.; Gliścińska, E.; Machnowski, W. Natural Silk—An Unusual Fibre: Origin, Processing and World Production. Fibres Text. East. Eur. 2021, 29, 22–28. [Google Scholar] [CrossRef]
- Harberd, R. A Manual of Tropical Butterfly Farming; The Darwin Initiative: London, UK, 2005. [Google Scholar]
- Høegh-Guldberg, O. North European Groups of Aricia allous G.-Hb: Their Variability and Relationship to A. agestis Schiff; Naturhistorisk Museum: Aarhus, Denmark, 1966; p. 184. [Google Scholar]
- Mattoni, R.; Longcore, T.; Krenova, Z.; Lipman, A. Mass rearing of the endangered Palos Verdes blue butterfly (Glaucophyte lygdamus palosverdesensis: Lycaenidae). J. Res. Lepid. 2003, 37, 55–67. [Google Scholar] [CrossRef]
- Fiedler, K. Effects of larval diet on myrmecophilous qualities of Polyommatus icarus caterpillars (Lepidoptera: Lycaenidae). Oecologia 1990, 83, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Physiologically induced color-pattern changes in butterfly wings: Mechanistic and evolutionary implications. J. Insect Physiol. 2008, 54, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Kertész, K.; Piszter, G.; Horváth, Z.E.; Bálint, Z.; Biró, L.P. Changes in structural and pigmentary colours in response to cold stress in Polyommatus icarus butterflies. Sci. Rep. 2017, 7, 1118. [Google Scholar] [CrossRef] [PubMed]
- Piszter, G.; Kertész, K.; Horváth, Z.E.; Bálint, Z.; Biró, L.P. Reproducible phenotype alteration due to prolonged cooling of the pupae of Polyommatus icarus butterflies. PLoS ONE 2019, 14, e0225388. [Google Scholar] [CrossRef]
- Otaki, J.M.; Hiyama, A.; Iwata, M.; Kudo, T. Phenotypic plasticity in the range-margin population of the lycaenid butterfly Zizeeria maha. BMC Evol. Biol. 2010, 10, 252. [Google Scholar] [CrossRef]
- Hiyama, A.; Nohara, C.; Kinjo, S.; Hiyama, A.; Nohara, C.; Kinjo, S.; Taira, W.; Gima, S.; Tanahara, A.; Otaki, J.M. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012, 2, 570. [Google Scholar] [CrossRef]
- Deuerling, S.; Kugler, S.; Klotz, M.; Zollfrank, C.; Van Opdenbosch, D. A Perspective on Bio-Mediated Material Structuring. Adv. Mater. 2018, 30, 1703656. [Google Scholar] [CrossRef]
- Bálint, Z.; Wojtusiak, J.; Kertész, K.; Piszter, G.; Biró, L.P. Spectroboard: An instrument for measuring spectral characteristics of butterfly wings—A new tool for taxonomists. Genus 2010, 21, 163–168. [Google Scholar]
- Bálint, Z.; Kertész, K.; Piszter, G.; Vértesy, Z.; Biro, L.P. The well-tuned blues: The role of structural colours as optical signals in the species recognition of a local butterfly fauna (Lepidoptera: Lycaenidae: Polyommatinae). J. R. Soc. Interface 2012, 9, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Piszter, G.; Kertész, K.; Bálint, Z.; Biró, L.P. Variability of the structural coloration in two butterfly species with different prezygotic mating strategies. PLoS ONE 2016, 11, e0165857. [Google Scholar] [CrossRef] [PubMed]
- Piszter, G.; Kertész, K.; Bálint, Z.; Biró, L.P. Wide-gamut structural colours on oakblue butterflies by naturally tuned photonic nanoarchitectures. R. Soc. Open Sci. 2023, 10, 221487. [Google Scholar] [CrossRef]
- Bálint, Z.; Katona, G.; Sáfián, S.; Collins, S.; Piszter, G.; Kertész, K.; Biró, L.P. Measuring and Modelling Structural Colours of Euphaedra neophron (Lepidoptera: Nymphalidae) Finely Tuned by Wing Scale Lower Lamina in Various Subspecies. Insects 2023, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Bálint, Z.; Moser, A.; Kertész, K.; Biró, L.P.; Parker, A.R. A supposition: Structural colours resulting from both natural and sexual selection on an individual wing in the butterfly genus Cyanophrys (Lepidoptera: Lycaenidae). Ann. Hist. Musei Natl. Hungarici 2009, 101, 63–79. [Google Scholar]
- Kertész, K.; Bálint, Z.; Vértesy, Z.; Márk, G.I.; Lousse, V.; Vigneron, J.P.; Rassart, M.; Biró, L.P. Gleaming and dull surface textures from photonic-crystal-type nanostructures in the butterfly Cyanophrys remus. Phys. Rev. E 2006, 74, 021922. [Google Scholar] [CrossRef]
- Piszter, G.; Kertész, K.; Sramkó, G.; Krízsik, V.; Bálint, Z.; Biró, L.P. Concordance of the spectral properties of dorsal wing scales with the phylogeographic structure of European male Polyommatus icarus butterflies. Sci. Rep. 2021, 11, 16498. [Google Scholar] [CrossRef]
- Kertész, K.; Piszter, G.; Bálint, Z.; Biró, L.P. Biogeographical patterns in the structural blue of male Polyommatus icarus butterflies. Sci. Rep. 2019, 9, 2338. [Google Scholar] [CrossRef]
- Abramova, V.; Sinitskii, A. Large-scale ZnO inverse opal films fabricated by a sol–gel technique. Superlattices Microstruct. 2009, 45, 624–629. [Google Scholar] [CrossRef]
- Thomas, J.; Lewington, J. The Butterflies of Britain & Ireland; Bloomsbury Publishing: London, UK, 2010; p. 288. [Google Scholar]
- Rivest, S.A.; Kharouba, H.M. Anthropogenic disturbance promotes the abundance of a newly introduced butterfly, the European common blue (Polyommatus icarus; Lepidoptera: Lycaenidae), in Canada. Can. J. Zool. 2021, 99, 642–652. [Google Scholar] [CrossRef]
- Settele, J.; Shreeve, T.; Konvicka, M.; van Dyck, H. (Eds.) Ecology of Butterflies in Europe; Cambridge University Press: Cambridge, UK, 2009; p. 513. [Google Scholar]
- Dincă, V.; Dapporto, L.; Vila, R. A combined genetic-morphometric analysis unravels the complex biogeographical history of Polyommatus icarus and Polyommatus celina Common Blue butterflies. Mol. Ecol. 2011, 20, 3921–3935. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.; Gerth, M.; Hone-Millard, W.G.; Nunes, M.D.S.; Dapporto, L.; Shreeve, T.G. Evidence for multiple colonisations and Wolbachia infections shaping the genetic structure of the widespread butterfly Polyommatus icarus in the British Isles. Mol. Ecol. 2021, 30, 5196–5213. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.O.D.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 12 July 2023).
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Piszter, G.; Kertész, K.; Vértesy, Z.; Bálint, Z.; Biró, L.P. Color based discrimination of chitin–air nanocomposites in butterfly scales and their role in conspecific recognition. Anal. Methods 2011, 3, 78–83. [Google Scholar] [CrossRef]
- Lundgren, L. Role of Intra and Interspecific Male-Male Interactions in Polyommatus icarus Rott. and Some Other Species of Blues (Lycaenidae). J. Res. Lepid. 1977, 16, 249–264. [Google Scholar] [CrossRef]
- Newland, D.; Still, R.; Swash, A.; Tomlinson, D. Britain’s Butterflies: A Field Guide to the Butterflies of Britain and Ireland; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Hesselbarth, G.; van Oorschot, H.; Wagener, S. Die Tagfalter der Türkei unter Berücksichtigung der Angrenzenden Länder; ConchBook: Harxheim, Germany, 1995. [Google Scholar]
- Tennent, J. The Butterflies of Morocco, Algeria and Tunisia; Gem Publishing Co.: Oxfordshire, UK, 1996. [Google Scholar]
- Joannopoulos, J.D.; Meade, R.D.; Winn, J.N. Photonic Crystals: Molding the Flow of Light; Princeton University Press: Princeton, NJ, USA, 1995. [Google Scholar]
- Biró, L.P.; Vigneron, J.P. Photonic nanoarchitectures in butterflies and beetles: Valuable sources for bioinspiration. Laser Photon. Rev. 2011, 5, 27–51. [Google Scholar] [CrossRef]
- Shamim, G.; Ranjan, S.K.; Pandey, D.M.; Ramani, R. Biochemistry and biosynthesis of insect pigments. Eur. J. Entomol. 2014, 111, 149–164. [Google Scholar] [CrossRef]
- Burghardt, F.; Proksch, P.; Fiedler, K. Flavonoid sequestration by the common blue butterfly Polyommatus icarus: Quantitative intraspecific variation in relation to larval hostplant, sex and body size. Biochem. Syst. Ecol. 2001, 29, 875–889. [Google Scholar] [CrossRef]
- Potyrailo, R.; Ghiradella, H.; Vertiatchikh, A.; Dovidenko, K.; Cournoyer, J.R.; Olson, E. Morpho butterfly wing scales demonstrate highly selective vapour response. Nat. Photon. 2007, 1, 123–128. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable sensors for ubiquitous monitoring of gases in the era of internet of things and industrial internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Piszter, G.; Kertész, K.; Vértesy, Z.; Bálint, Z.; Biró, L.P. Substance specific chemical sensing with pristine and modified photonic nanoarchitectures occurring in blue butterfly wing scales. Opt. Express 2014, 22, 22649–22660. [Google Scholar] [CrossRef] [PubMed]
- Piszter, G.; Kertész, K.; Bálint, Z.; Biró, L.P. Optical detection of vapor mixtures using structurally colored butterfly and moth wings. Sensors 2019, 19, 3058. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.E.; Agarwal, S.P.; An, S.; Kazyak, E.; Das, D.; Shang, W.; Skye, R.; Deng, T.; Dasgupta, N.P. Biotemplated Morpho Butterfly Wings for Tunable Structurally Colored Photocatalysts. ACS Appl. Mater. Interfaces 2018, 10, 4614–4621. [Google Scholar] [CrossRef]
- Piszter, G.; Kertész, K.; Nagy, G.; Baji, Z.; Horváth, Z.E.; Bálint, Z.; Pap, J.S.; Biró, L.P. Spectral tuning of biotemplated ZnO photonic nanoarchitectures for photocatalytic applications. R. Soc. Open Sci. 2022, 9, 220090. [Google Scholar] [CrossRef] [PubMed]
- Piszter, G.; Nagy, G.; Kertész, K.; Baji, Z.; Kovács, K.; Bálint, Z.; Horváth, Z.E.; Pap, J.S.; Biró, L.P. Investigating the Effect of Reflectance Tuning on Photocatalytic Dye Degradation with Biotemplated ZnO Photonic Nanoarchitectures Based on Morpho Butterfly Wings. Materials 2023, 16, 3584. [Google Scholar] [CrossRef]
- Mu, Z.; Zhao, X.; Xie, Z.; Zhao, Y.; Zhong, Q.; Bo, L.; Gu, Z. In situ synthesis of gold nanoparticles (AuNPs) in butterfly wings for surface enhanced Raman spectroscopy (SERS). J. Mater. Chem. B 2013, 1, 1607–1613. [Google Scholar] [CrossRef]
- Takei, H.; Nagata, K.; Frese, N.; Gölzhäuser, A.; Okamoto, T. Surface-Enhanced Raman Spectroscopy for Molecule Characterization: HIM Investigation into Sources of SERS Activity of Silver-Coated Butterfly Scales. Nanomaterials 2021, 11, 1741. [Google Scholar] [CrossRef]
- Schlötterer, C. Evolutionary dynamics of microsatellite DNA. Chromosoma 2000, 109, 365–371. [Google Scholar] [CrossRef]
- Frankham, R. Conservation Genetics. Annu. Rev. Genet. 1995, 29, 305–327. [Google Scholar] [CrossRef] [PubMed]
- Kertész, K.; Bálint, Z.; Piszter, G.; Horváth, Z.E.; Biró, L.P. Multi-instrumental techniques for evaluating butterfly structural colors: A case study on Polyommatus bellargus (Rottemburg, 1775) (Lepidoptera: Lycaenidae: Polyommatinae). Arthropod Struct. Dev. 2021, 61, 101010. [Google Scholar] [CrossRef] [PubMed]
- Piszter, G.; Kertész, K.; Kovács, D.; Zámbó, D.; Baji, Z.; Illés, L.; Nagy, G.; Pap, J.S.; Bálint, Z.; Biró, L.P. Spectral Engineering of Hybrid Biotemplated Photonic/Photocatalytic Nanoarchitectures. Nanomaterials 2022, 12, 4490. [Google Scholar] [CrossRef] [PubMed]
- Kertész, K.; Piszter, G.; Horváth, Z.E.; Zámbó, D.; Deák, A.; Biró, L.P. Effect of Plasmonic Au and Ag/Au Nanoparticles and Sodium Citrate on the Optical Properties of Chitin-Based Photonic Nanoarchitectures in Butterfly Wing Scales. Photonics 2022, 9, 553. [Google Scholar] [CrossRef]

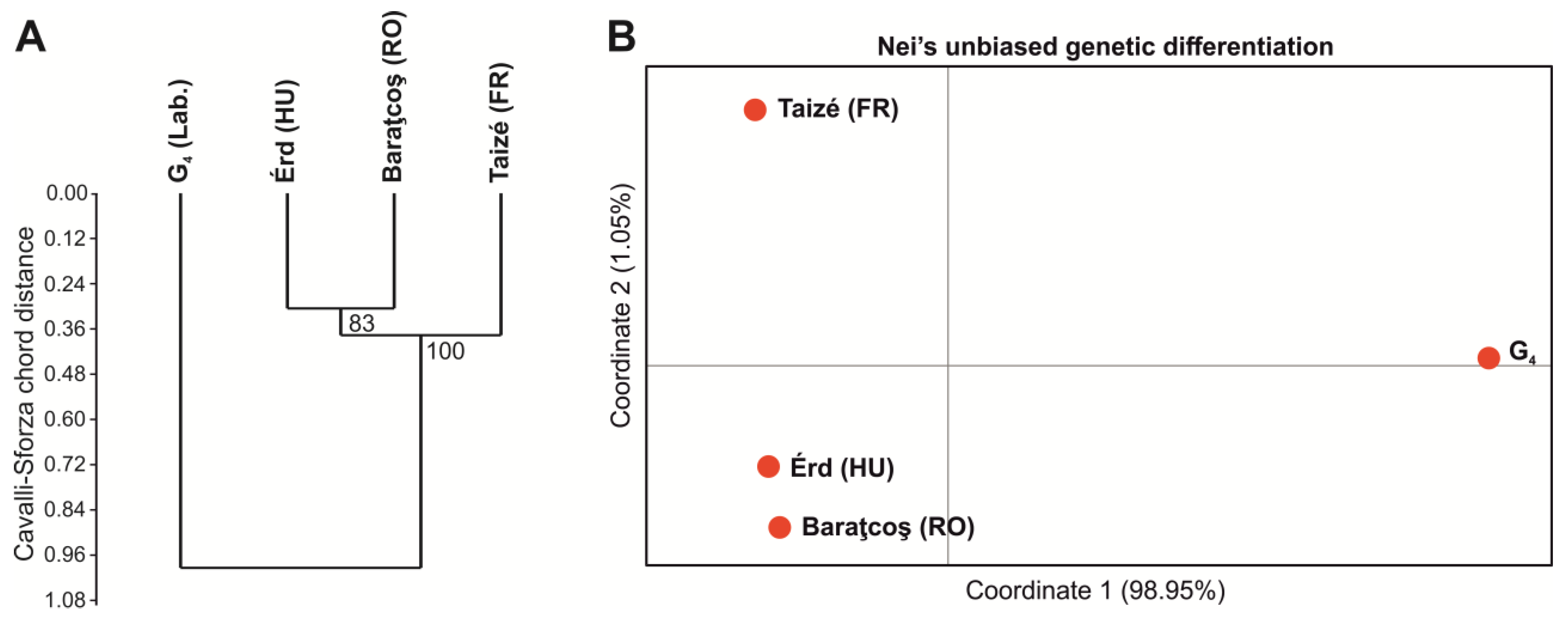
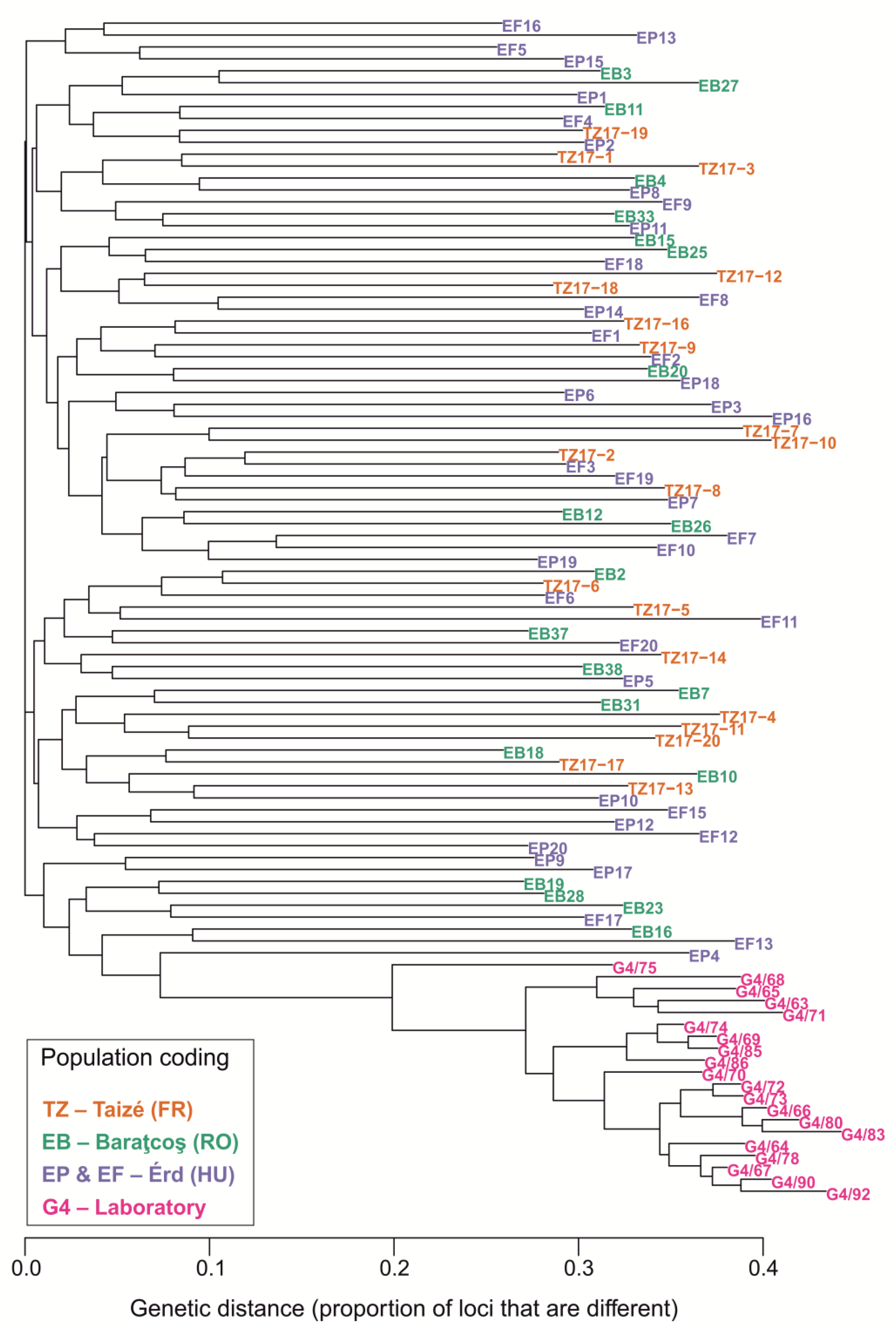
| Population | Na | Ho | He | uHe | Fis |
|---|---|---|---|---|---|
| Baraţcoş (RO) | 7.75 ± 0.864 | 0.617 ± 0.068 | 0.675 ± 0.064 | 0.692 ± 0.066 | 0.099 ± 0.039 |
| Taizé (FR) | 7.813 ± 0.918 | 0.557 ± 0.05 | 0.682 ± 0.054 | 0.701 ± 0.055 | 0.164 ± 0.046 |
| Érd (HU) | 9.688 ± 0.869 | 0.556 ± 0.05 | 0.682 ± 0.058 | 0.692 ± 0.059 | 0.179 ± 0.03 |
| Laboratory (G4) | 1.813 ± 0.164 | 0.361 ± 0.083 | 0.253 ± 0.052 | 0.26 ± 0.053 | −0.363 ± 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piszter, G.; Bálint, Z.; Kertész, K.; Szatmári, L.; Sramkó, G.; Biró, L.P. Breeding Polyommatus icarus Serves as a Large-Scale and Environmentally Friendly Source of Precisely Tuned Photonic Nanoarchitectures. Insects 2023, 14, 716. https://doi.org/10.3390/insects14080716
Piszter G, Bálint Z, Kertész K, Szatmári L, Sramkó G, Biró LP. Breeding Polyommatus icarus Serves as a Large-Scale and Environmentally Friendly Source of Precisely Tuned Photonic Nanoarchitectures. Insects. 2023; 14(8):716. https://doi.org/10.3390/insects14080716
Chicago/Turabian StylePiszter, Gábor, Zsolt Bálint, Krisztián Kertész, Lajos Szatmári, Gábor Sramkó, and László Péter Biró. 2023. "Breeding Polyommatus icarus Serves as a Large-Scale and Environmentally Friendly Source of Precisely Tuned Photonic Nanoarchitectures" Insects 14, no. 8: 716. https://doi.org/10.3390/insects14080716
APA StylePiszter, G., Bálint, Z., Kertész, K., Szatmári, L., Sramkó, G., & Biró, L. P. (2023). Breeding Polyommatus icarus Serves as a Large-Scale and Environmentally Friendly Source of Precisely Tuned Photonic Nanoarchitectures. Insects, 14(8), 716. https://doi.org/10.3390/insects14080716





