Annual Crops Contribute More Predators than Perennial Habitats during an Aphid Outbreak
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection
2.2. Insect Sampling
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iuliano, B.; Gratton, C. Temporal resource (dis)continuity for conservation biological control: From field to landscape scales. Front. Sust. Food Syst. 2020, 4. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.G. Relationships of Natural Enemies and Non-Prey Foods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; Volume 7. [Google Scholar]
- Schellhorn, N.A.; Gagic, V.; Bommarco, R. Time will tell: Resource continuity bolsters ecosystem services. Trends. Ecol. Evol. 2015, 30, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Pons, X.; Núñez, E.; Lumbierres, B.; Albajes, R. Epigeal aphidophagous predators and the role of alfalfa as a reservoir of aphid predators for arable crops. Eur. J. Entomol. 2005, 102, 519–525. [Google Scholar] [CrossRef]
- Heimoana, V.; Pilkington, L.J.; Raman, A.; Mitchell, A.; Nicol, H.I.; Johnson, A.C.; Gurr, G.M. Integrating spatially explicit molecular and ecological methods to explore the significance of non-crop vegetation to predators of brassica pests. Agric. Ecosyst. Environ. 2017, 239, 12–19. [Google Scholar] [CrossRef]
- Villegas, C.M.; Verdugo, J.A.; Grez, A.A.; Tapia, J.; Lavandero, B. Movement between crops and weeds: Temporal refuges for aphidophagous insects in Central Chile. Ciencia Investig. Agr. 2013, 40, 317–326. [Google Scholar] [CrossRef]
- Rand, T.A.; Tylianakis, J.M.; Tscharntke, T. Spillover edge effects: The dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol. Lett. 2006, 9, 603–614. [Google Scholar] [CrossRef]
- Blitzer, E.J.; Dormann, C.F.; Holzschuh, A.; Klein, A.-M.; Rand, T.A.; Tscharntke, T. Spillover of functionally important organisms between managed and natural habitats. Agric. Ecosyst. Environ. 2012, 146, 34–43. [Google Scholar] [CrossRef]
- Frost, C.M.; Didham, R.K.; Rand, T.A.; Peralta, G.; Tylianakis, J.M. Community-level net spillover of natural enemies from managed to natural forest. Ecology 2015, 96, 193–202. [Google Scholar] [CrossRef]
- Gonzalez, E.; Salvo, A.; Defago, M.T.; Valladares, G. A moveable feast: Insects moving at the forest-crop interface are affected by crop phenology and the amount of forest in the landscape. PLoS ONE 2016, 11, e0158836. [Google Scholar] [CrossRef]
- Tscharntke, T.; Rand, T.A.; Bianchi, F. The landscape context of trophic interactions: Insect spillover across the crop-noncrop interface. Ann. Zool. Fenn. 2005, 42, 421–432. [Google Scholar]
- Macfadyen, S.; Hopkinson, J.; Parry, H.; Neave, M.; Bianchi, F.; Zalucki, M.; Schellhorn, N. Early-season movement dynamics of phytophagous pest and natural enemies across a native vegetation-crop ecotone. Agric. Ecosyst. Environ. 2015, 200, 110–118. [Google Scholar] [CrossRef]
- Macfadyen, S.; Muller, W. Edges in agricultural landscapes: Species interactions and movement of natural enemies. PLoS ONE 2013, 8, e59659. [Google Scholar] [CrossRef] [PubMed]
- Schellhorn, N.A.; Bianchi, F.J.; Hsu, C.L. Movement of entomophagous arthropods in agricultural landscapes: Links to pest suppression. Annu. Rev. Entomol. 2014, 59, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, K.G.L.I.; Costamagna, A.C. Adjacent habitat type affects the movement of predators suppressing soybean aphids. PLoS ONE 2019, 14, e0218522. [Google Scholar] [CrossRef]
- Aguilera, G.; Roslin, T.; Miller, K.; Tamburini, G.; Birkhofer, K.; Caballero-Lopez, B.; Lindström, S.A.M.; Öckinger, E.; Rundlöf, M.; Rusch, A.; et al. Crop diversity benefits carabid and pollinator communities in landscapes with semi-natural habitats. J. Appl. Ecol. 2020, 57, 2170–2179. [Google Scholar] [CrossRef]
- Redlich, S.; Martin, E.A.; Steffan-Dewenter, I.; Willis, S. Landscape-level crop diversity benefits biological pest control. J. Appl. Ecol. 2018, 55, 2419–2428. [Google Scholar] [CrossRef]
- Sirami, C.; Gross, N.; Baillod, A.B.; Bertrand, C.; Carrie, R.; Hass, A.; Henckel, L.; Miguet, P.; Vuillot, C.; Alignier, A.; et al. Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc. Natl. Acad. Sci. USA 2019, 116, 16442–16447. [Google Scholar] [CrossRef]
- Bosem Baillod, A.; Tscharntke, T.; Clough, Y.; Batáry, P.; Marini, L. Landscape-scale interactions of spatial and temporal cropland heterogeneity drive biological control of cereal aphids. J. Appl. Ecol. 2017, 54, 1804–1813. [Google Scholar] [CrossRef]
- Kheirodin, A.; Cárcamo, H.A.; Costamagna, A.C. Contrasting effects of host crops and crop diversity on the abundance and parasitism of a specialist herbivore in agricultural landscapes. Land. Ecol. 2020, 35, 1073–1087. [Google Scholar] [CrossRef]
- Almdal, C.D.; Costamagna, A.C. Crop diversity and edge density benefit pest suppression through bottom-up and top-down processes, respectively. Agric. Ecosyst. Environ. 2023, 349, 108447. [Google Scholar] [CrossRef]
- Samaranayake, K.G.L.I.; Costamagna, A.C. Levels of predator movement between crop and neighboring habitats explain pest suppression in soybean across a gradient of agricultural landscape complexity. Agric. Ecosyst. Environ. 2018, 259, 135–146. [Google Scholar] [CrossRef]
- Carruthers, J.M.; Cook, S.M.; Wright, G.A.; Osborne, J.L.; Clark, S.J.; Swain, J.L.; Haughton, A.J. Oilseed rape (Brassica napus) as a resource for farmland insect pollinators: Quantifying floral traits in conventional varieties and breeding systems. Glob. Chang. Biol. Bioen. 2017, 9, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Elliott, N.C.; Kieckhefer, R.W.; Lee, J.H.; French, B.W. Influence of within-field and landscape factors on aphid predator populations in wheat. Land. Ecol. 1998, 14, 239–252. [Google Scholar] [CrossRef]
- Ximenez-Embun, M.G.; Zaviezo, T.; Grez, A. Seasonal, spatial and diel partitioning of Acyrthosiphon pisum (Hemiptera: Aphididae) predators and predation in alfalfa fields. Biol. Control 2014, 69, 1–7. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat Management to Suppress Pest Populations: Progress and Prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]
- Ragsdale, D.W.; McCornack, B.P.; Venette, R.C.; Potter, B.D.; MacRae, I.V.; Hodgson, E.W.; O’Neal, M.E.; Johnson, K.D.; O’Neil, R.J.; Difonzo, C.D.; et al. Economic threshold for soybean aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 1258–1267. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Schellhorn, N.A.; Marcora, A.; Batley, M. Movement and phenology of bees in a subtropical Australian agricultural landscape. Austral Ecol. 2013, 38, 456–464. [Google Scholar] [CrossRef]
- Hossain, Z.; Gurr, G.M.; Wratten, S.D.; Raman, A. Habitat manipulation in lucerne Medicago sativa: Arthropod population dynamics in harvested and ’refuge’ crop strips. J. Appl. Ecol. 2002, 39, 445–454. [Google Scholar] [CrossRef]
- Johnson, K.D.; O’Neal, M.E.; Ragsdale, D.W.; Difonzo, C.D.; Swinton, S.M.; Dixon, P.M.; Potter, B.D.; Hodgson, E.W.; Costamagna, A.C. Probability of cost-effective management of soybean aphid (Hemiptera: Aphididae) in North America. J. Econ. Entomol. 2009, 102, 2101–2108. [Google Scholar] [CrossRef]
- Costamagna, A.C.; Landis, D.A. Predators exert top-down control of soybean aphid across a gradient of agricultural management systems. Ecol. Appl. 2006, 16, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, A.C.; Landis, D.A. Quantifying predation on soybean aphid through direct field observations. Biol. Control 2007, 42, 16–24. [Google Scholar] [CrossRef]
- Costamagna, A.C.; Landis, D.A. Lack of strong refuges allows top-down control of soybean aphid by generalist natural enemies. Biol. Control 2011, 57, 184–192. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Landis, D.A.; Gratton, C.; DiFonzo, C.D.; O’Neal, M.; Chacon, J.M.; Wayo, M.T.; Schmidt, N.P.; Mueller, E.E.; Heimpel, G.E. Landscape diversity enhances biological control of an introduced crop pest in the north-central USA. Ecol. Appl. 2009, 19, 143–154. [Google Scholar] [CrossRef]
- Ragsdale, D.W.; Landis, D.A.; Brodeur, J.; Heimpel, G.E.; Desneux, N. Ecology and management of the soybean aphid in North America. Annu. Rev. Entomol. 2011, 56, 375–399. [Google Scholar] [CrossRef]
- Gordon, R.D. The Coccinellidae (Coleoptera) of America north of Mexico. J. N. Y. Entomol. Soc. 1985, 93. [Google Scholar]
- Gordon, R.D. The first North American records of Hippodamia variegata (Goeze)(Coleoptera: Coccinellidae). J. N. Y. Entomol. Soc. 1987, 95, 307–309. [Google Scholar]
- Garland, J.A.; Kevan, D.K.M. Chrysopidae of Canada and Alaska ( Insecta, Neuroptera): Revised checklist, new and noteworthy records, and geo-referenced localities. Zootaxa 2007, 1486, 1–84. [Google Scholar] [CrossRef]
- Penny, N.D.; Adams, P.A.; Stange, L.A. Species catalog of the Neuroptera, Megaloptera, and Raphidioptera of America north of Mexico. Proc. Calif. Acad. Sci. 1997, 50, 39–114. [Google Scholar]
- Klimaszewski, J.; McE. Kevan, D. Review of Canadian and Alaskan brown lacewing flies (Neuroptera: Hemerobiidae) with a key to the genera. Part IV: The genera Megalomus Rambur, Boriomyia Banks, Psectra Hagen and Sympherobius Banks. Ann. Transvaal Mus. 1992, 35, 435–457. [Google Scholar]
- Carpenter, F.M. A revision of the Nearctic Hemerobiidae, Berothidae, Sisyridae, Polystoechotidae and Dilaridae (Neuroptera). Proc. Am. Acad. Arts Sci. 1940, 74, 193–280. [Google Scholar] [CrossRef]
- Vockeroth, J.R. The Flower Flies of the Subfamily Syrphinae of Canada, Alaska, and Greenland: Diptera, Syrphidae; Agriculture Canada: Ottawa, ON, Canada, 1992; Volume 1867. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org (accessed on 1 July 2023).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Mazerolle, M.J.; Mazerolle, M.M.J. AICcmodavg, R Package Version 2.3-2. Available online: https://cran.pau.edu.tr/web/packages/AICcmodavg/AICcmodavg.pdf (accessed on 1 July 2023).
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; CRC press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models, R Package Version 3.1-162. Available online: https://CRAN.R-project.org/package=nlme (accessed on 1 July 2023).
- Lenth, R.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Estimated Marginal Means, aka Least-Squares Means, R Package Version 1.5.5-1. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 July 2023).
- Honěk, A. Population density of aphids at the time of settling and ovariole maturation in Coccinella septempunctata [Col.: Coccinellidae]. Entomophaga 1980, 25, 427–430. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Hesler, L.S.; Anderson, R.L. Preceding crop affects soybean aphid abundance and predator-prey dynamics in soybean. J. Appl. Entomol. 2017, 141, 669–676. [Google Scholar] [CrossRef]
- Maisonhaute, J.-É.; Labrie, G.; Lucas, E. Direct and indirect effects of the spatial context on the natural biocontrol of an invasive crop pest. Biol. Control 2017, 106, 64–76. [Google Scholar] [CrossRef]
- Woltz, J.M.; Landis, D.A. Coccinellid immigration to infested host patches influences suppression of Aphis glycines in soybean. Biol. Control 2013, 64, 330–337. [Google Scholar] [CrossRef]
- van der Werf, W.; Evans, E.W.; Powell, J. Measuring and modelling the dispersal of Coccinella septempunctata (Coleoptera: Coccinellidae) in alfalfa fields. Eur. J. Entomol. 2000, 97, 487–493. [Google Scholar] [CrossRef]
- Bahlai, C.A.; Colunga-Garcia, M.; Gage, S.H.; Landis, D.A. The role of exotic ladybeetles in the decline of native ladybeetle populations: Evidence from long-term monitoring. Biol. Invas. 2015, 17, 1005–1024. [Google Scholar] [CrossRef]
- Gardiner, M.M.; O’Neal, M.E.; Landis, D.A. Intraguild Predation and Native Lady Beetle Decline. PLoS ONE 2011, 6, e23576. [Google Scholar] [CrossRef]
- Lamb, R.J.; Bannerman, J.A.; Costamagna, A.C. Stability of native and exotic lady beetle populations in a diverse landscape. Ecosphere 2019, 10. [Google Scholar] [CrossRef]
- Eckberg, J.O.; Peterson, J.A.; Borsh, C.P.; Kaser, J.M.; Johnson, G.A.; Luhman, J.C.; Wyse, D.L.; Heimpel, G.E. Field abundance and performance of hoverflies (Diptera: Syrphidae) on soybean aphid. Ann. Entomol. Soc. Am. 2015, 108, 26–34. [Google Scholar] [CrossRef]
- Kaiser, M.E.; Noma, T.; Brewer, M.J.; Pike, K.S.; Vockeroth, J.R.; Gaimari, S.D. Hymenopteran parasitoids and dipteran predators found using soybean aphid after its midwestern United States invasion. Ann. Entomol. Soc. Am. 2007, 100, 196–205. [Google Scholar] [CrossRef]
- Noma, T.; Brewer, M.J. Seasonal abundance of resident parasitoids and predatory flies and corresponding soybean aphid densities, with comments on classical biological control of soybean aphid in the Midwest. J. Econ. Entomol. 2008, 101, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Tooker, J.F.; Hauser, M.; Hanks, L.M. Floral host plants of Syrphidae and Tachinidae (Diptera) of central Illinois. Ann. Entomol. Soc. Am. 2006, 99, 96–112. [Google Scholar] [CrossRef]
- Gontijo, L.M.; Cockfield, S.D.; Beers, E.H. Natural enemies of woolly apple aphid (Hemiptera: Aphididae) in Washington State. Environ. Entomol. 2012, 41, 1364–1371. [Google Scholar] [CrossRef]
- Berthiaume, R.; Hébert, C.; Pelletier, G.; Cloutier, C. Seasonal natural history of aphidophagous Syrphidae (Diptera) attacking the balsam twig aphid in balsam fir (Pinaceae) Christmas tree plantations. Can. Entomol. 2016, 148, 466–475. [Google Scholar] [CrossRef]
- Rodríguez-Gasol, N.; Alins, G.; Veronesi, E.R.; Wratten, S. The ecology of predatory hoverflies as ecosystem-service providers in agricultural systems. Biol. Control 2020, 151, 104405. [Google Scholar] [CrossRef]
- van Rijn, P.C.; Kooijman, J.; Wackers, F. The impact of floral resources on syrphid performance and cabbage aphid biological control. IOBC WPRS Bull. 2006, 29, 149. [Google Scholar]
- Wäckers, F.L.; Van Rijn, P.C.; Heimpel, G.E. Honeydew as a food source for natural enemies: Making the best of a bad meal? Biol. Control 2008, 45, 176–184. [Google Scholar] [CrossRef]
- Haenke, S.; Kovacs-Hostyanszki, A.; Fruend, J.; Batary, P.; Jauker, B.; Tscharntke, T.; Holzschuh, A. Landscape configuration of crops and hedgerows drives local syrphid fly abundance. J. Appl. Ecol. 2014, 51, 505–513. [Google Scholar] [CrossRef]
- Haenke, S.; Scheid, B.; Schaefer, M.; Tscharntke, T.; Thies, C. Increasing syrphid fly diversity and density in sown flower strips within simple vs. complex landscapes. J. Appl. Ecol. 2009, 46, 1106–1114. [Google Scholar] [CrossRef]
- Meyer, B.; Jauker, F.; Steffan-Dewenter, I. Contrasting resource-dependent responses of hoverfly richness and density to landscape structure. Basic Appl. Ecol. 2009, 10, 178–186. [Google Scholar] [CrossRef]
- Almohamad, R.; Verheggen, F.; Haubruge, É. Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): A review. Biotech. Agron. Soc. Environ. 2009, 13, 467–481. [Google Scholar]
- Chambers, R.; Adams, T. Quantification of the impact of hoverflies (Diptera: Syrphidae) on cereal aphids in winter wheat: An analysis of field populations. J. Appl. Ecol. 1986, 23, 895–904. [Google Scholar] [CrossRef]
- Skevington, J.H.; Locke, M.M.; Young, A.D.; Moran, K.; Crins, W.J.; Marshall, S.A. Field Guide to the Flower Flies of Northeastern North America; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar]
- Bianchi, F.J.J.A.; Schellhorn, N.A.; Cunningham, S.A. Habitat functionality for the ecosystem service of pest control: Reproduction and feeding sites of pests and natural enemies. Agric. For. Entomol. 2013, 15, 12–23. [Google Scholar] [CrossRef]
- Duelli, P. Dispersal and oviposition strategies in Chrysoperla carnea. In Progress in world’s neuropterology: 1st International Symposium on Neuropterology (Insecta, Megaloptera, Raphidioptera, Planipennia) in Graz, Austria; Gepp, J., Aspock, H., Holzel, H., Eds.; Smithsonian Libraries: Washington, DC, USA, 1984; pp. 133–145. [Google Scholar]
- Janković, M.; Plećaš, M.; Sandić, D.; Popović, A.; Petrović, A.; Petrović-Obradović, O.; Tomanović, Ž.; Gagić, V. Functional role of different habitat types at local and landscape scales for aphids and their natural enemies. J. Pest Sci. 2016, 90, 261–273. [Google Scholar] [CrossRef]
- Almdal, C. Landscape Heterogeneity Impacts Aphid Suppression While Adjacent Habitats and Aphid Abundance Impact Predator Movement in Soybean; University of Manitoba: Winnipeg, MB, Canada, 2022. [Google Scholar]
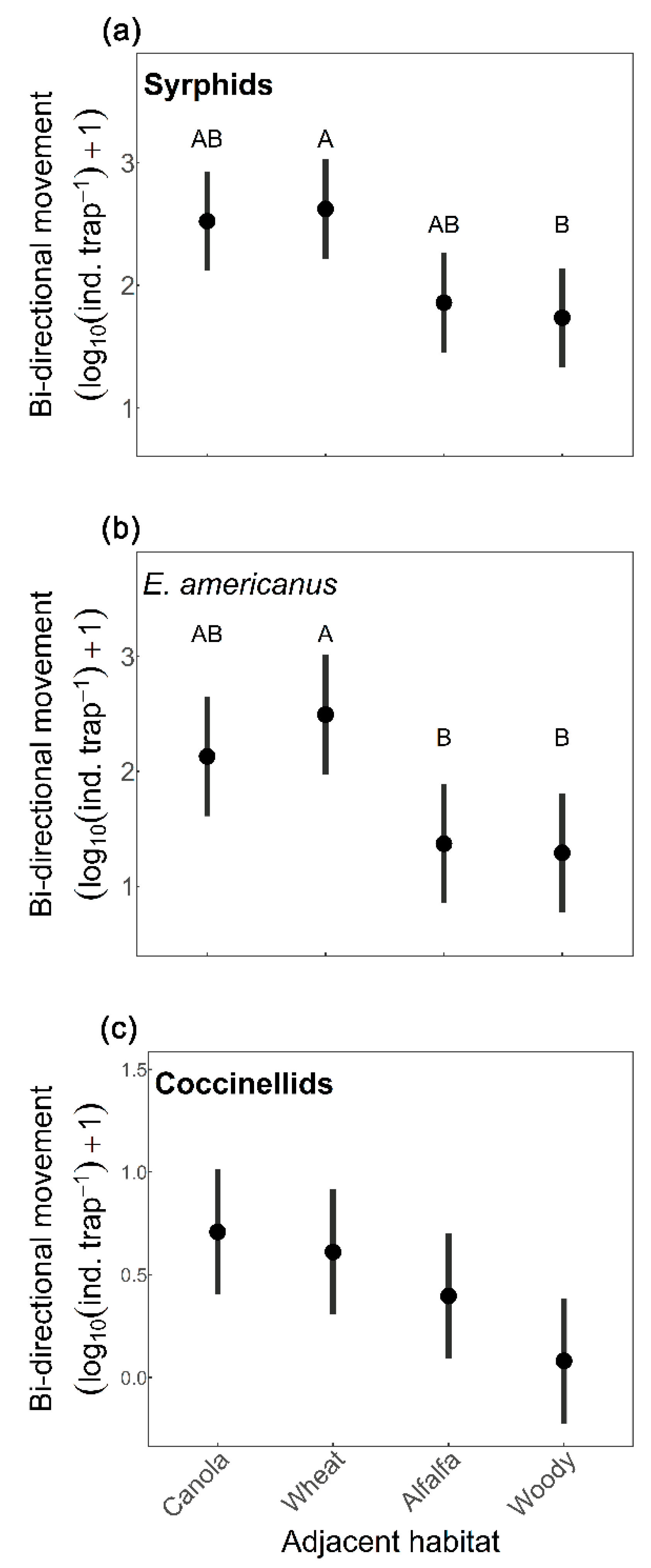

| Alfalfa | Canola | Wheat | Woody | |
|---|---|---|---|---|
| Soybean | ||||
| Syrphids | 529.7 ± 157.2 | 1809.0 ± 164.9 | 1085.7 ± 477.8 | 821.3 ± 575.8 |
| Toxomerus marginatus | 82.0 ± 40.3 | 287.3 ± 110.5 | 64.3 ± 22.4 | 201.3 ± 159.3 |
| Coccinellids | 36.0 ± 18.5 | 26.0 ± 5.2 | 50.7 ± 22.6 | 26.3 ± 20.9 |
| All other predators | 21.3 ± 8.7 | 30.7 ± 22.7 | 37.0 ± 18.7 | 21.7 ± 8.1 |
| Adjacent habitat | ||||
| Syrphids | 108.3 ± 6.2 a | 1210.0 ± 188.8 b | 525.7 ± 209.6 ab | 302.0 ± 272.2 a |
| Toxomerus marginatus | 18.3 ± 9.0 a | 149.3 ± 17.3 b | 43.0 ± 25.4 a | 3.7 ± 1.3 a |
| Coccinellids | 4.7 ± 2.4 ab | 2.3 ± 0.9 ab | 21.0 ± 4.4 b | 3.3 ± 2.9 a |
| All other predators a | 7.7 ± 3.8 a | 11.0 ± 4.4 a | 15.0 ± 9.5 a | 0.7 ± 0.7 a |
| Direction | Week | Adjacent Habitat | ||||||
|---|---|---|---|---|---|---|---|---|
| Est. | F | p | Est. | F | p | F | p | |
| Syrphids | 0.19 | 4.28 | 0.046 | 0.44 | 22.0 | 0.0001 | 5.7 | 0.022 |
| Eupeodes americanus | 0.32 | 9.6 | 0.0039 | 0.34 | 11.1 | 0.0021 | 5.7 | 0.022 |
| Toxomerus marginatus | 0.024 | 0.043 | 0.84 | 0.85 | 52.7 | 0.0001 | ||
| Coccinellids | 0.21 | 7.45 | 0.010 | 0.35 | 20.5 | 0.0001 | 3.9 | 0.055 |
| All other predators a | 0.060 | 0.45 | 0.51 | 0.14 | 2.4 | 0.13 | ||
| Aphid Abundance | Adjacent Habitat Abundance | Adjacent Habitat | ||||||
|---|---|---|---|---|---|---|---|---|
| Est. | F | p | Est. | F | p | F | p | |
| Syrphids | 0.66 | 3.8 | 0.079 | 0.57 | 7.8 | 0.019 | 4.3 | 0.044 |
| Eupeodes americanus | 1.16 | 10.2 | 0.0085 | - | - | - | 6.5 | 0.015 |
| Toxomerus marginatus | 0.83 | 0.62 | 0.45 | 1.0 | 10.2 | 0.010 | 0.68 | 0.59 |
| Coccinellids | 0.94 | 6.6 | 0.028 | 0.47 | 1.8 | 0.21 | 3.7 | 0.062 |
| All other predators a | 0.57 | 3.9 | 0.076 | 0.34 | 0.65 | 0.44 | 2.0 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almdal, C.D.; Costamagna, A.C. Annual Crops Contribute More Predators than Perennial Habitats during an Aphid Outbreak. Insects 2023, 14, 624. https://doi.org/10.3390/insects14070624
Almdal CD, Costamagna AC. Annual Crops Contribute More Predators than Perennial Habitats during an Aphid Outbreak. Insects. 2023; 14(7):624. https://doi.org/10.3390/insects14070624
Chicago/Turabian StyleAlmdal, Crystal D., and Alejandro C. Costamagna. 2023. "Annual Crops Contribute More Predators than Perennial Habitats during an Aphid Outbreak" Insects 14, no. 7: 624. https://doi.org/10.3390/insects14070624
APA StyleAlmdal, C. D., & Costamagna, A. C. (2023). Annual Crops Contribute More Predators than Perennial Habitats during an Aphid Outbreak. Insects, 14(7), 624. https://doi.org/10.3390/insects14070624






