Conferring High IAA Productivity on Low-IAA-Producing Organisms with PonAAS2, an Aromatic Aldehyde Synthase of a Galling Sawfly, and Identification of Its Inhibitor
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Construction of a Plasmid Vector for the Expression of PonAAS2::GFP in C. elegans
2.3. Expression of PonAAS2::GFP in C. elegans
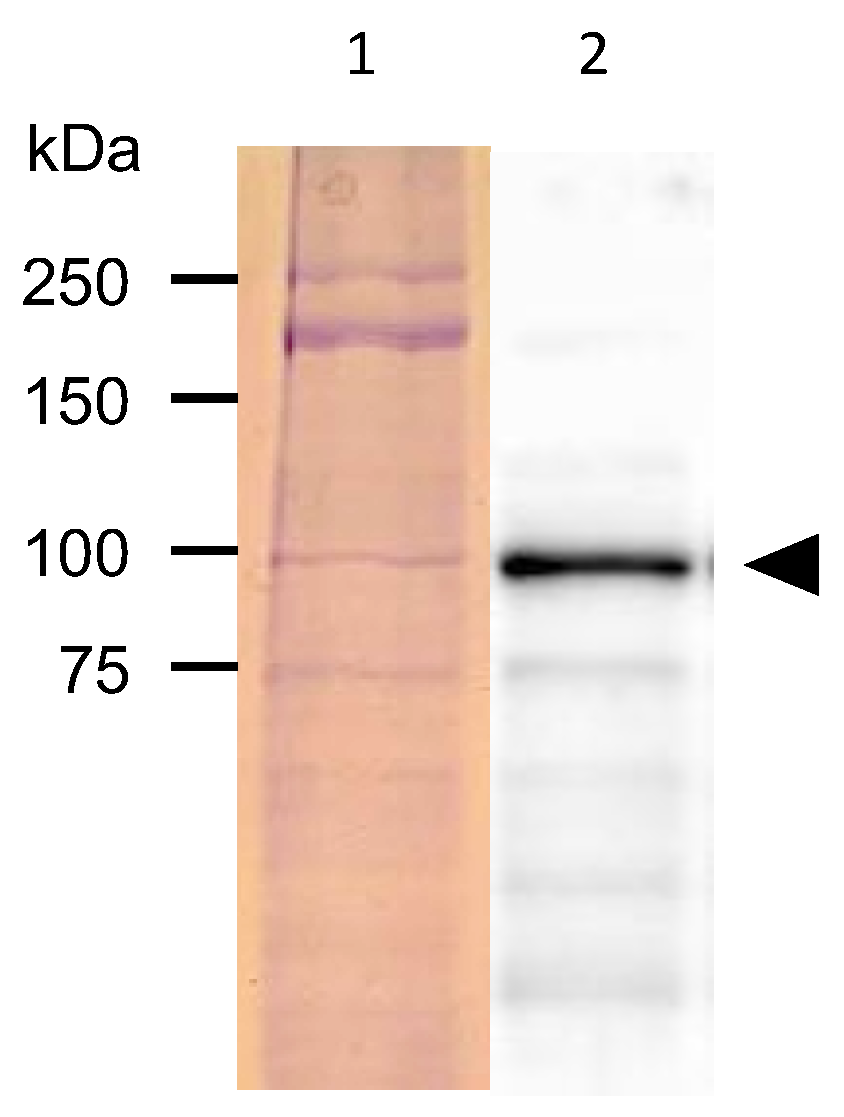
2.4. Protein Analysis of C. elegans Expressing PonAAS2::GFP
2.5. Large-Scale Analysis of Endogenous IAA and Conversion Activity in Wild-Type C. elegans
2.6. Analysis of Endogenous IAA and Conversion Activity in C. elegans Expressing PonAAS2::GFP
2.7. Screening a Chemical Library for Inhibitors of PonAAS2
3. Results
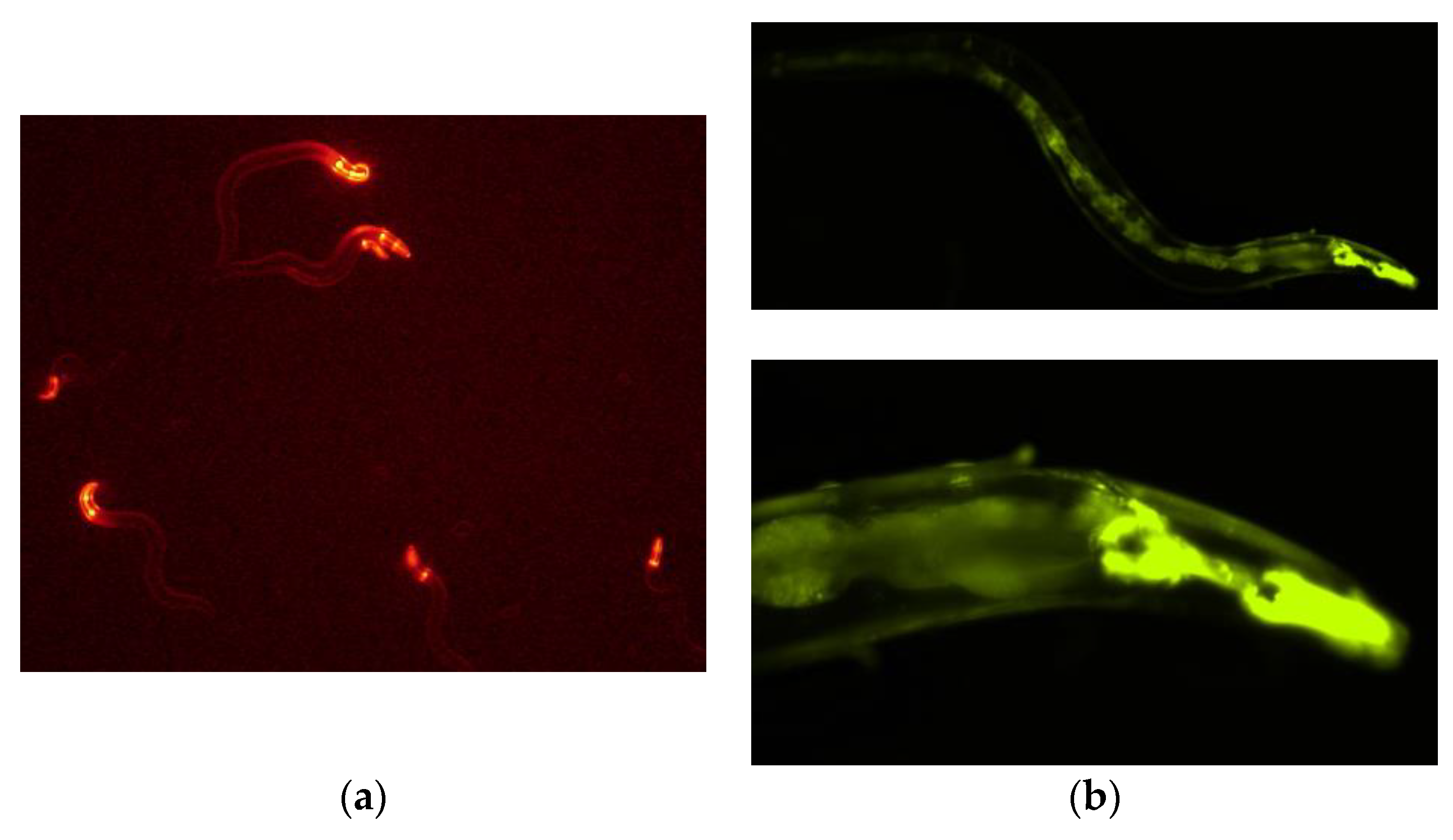
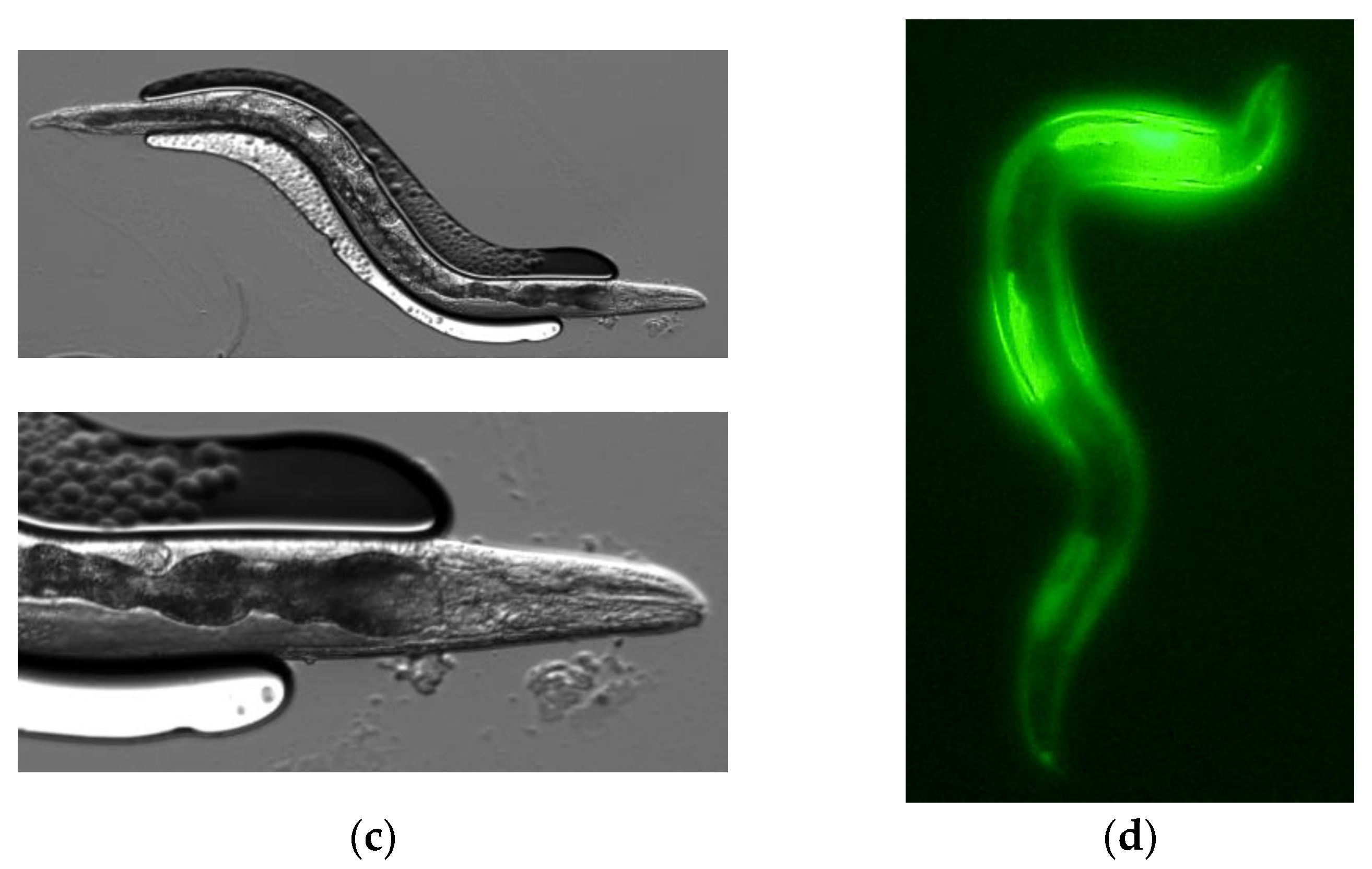
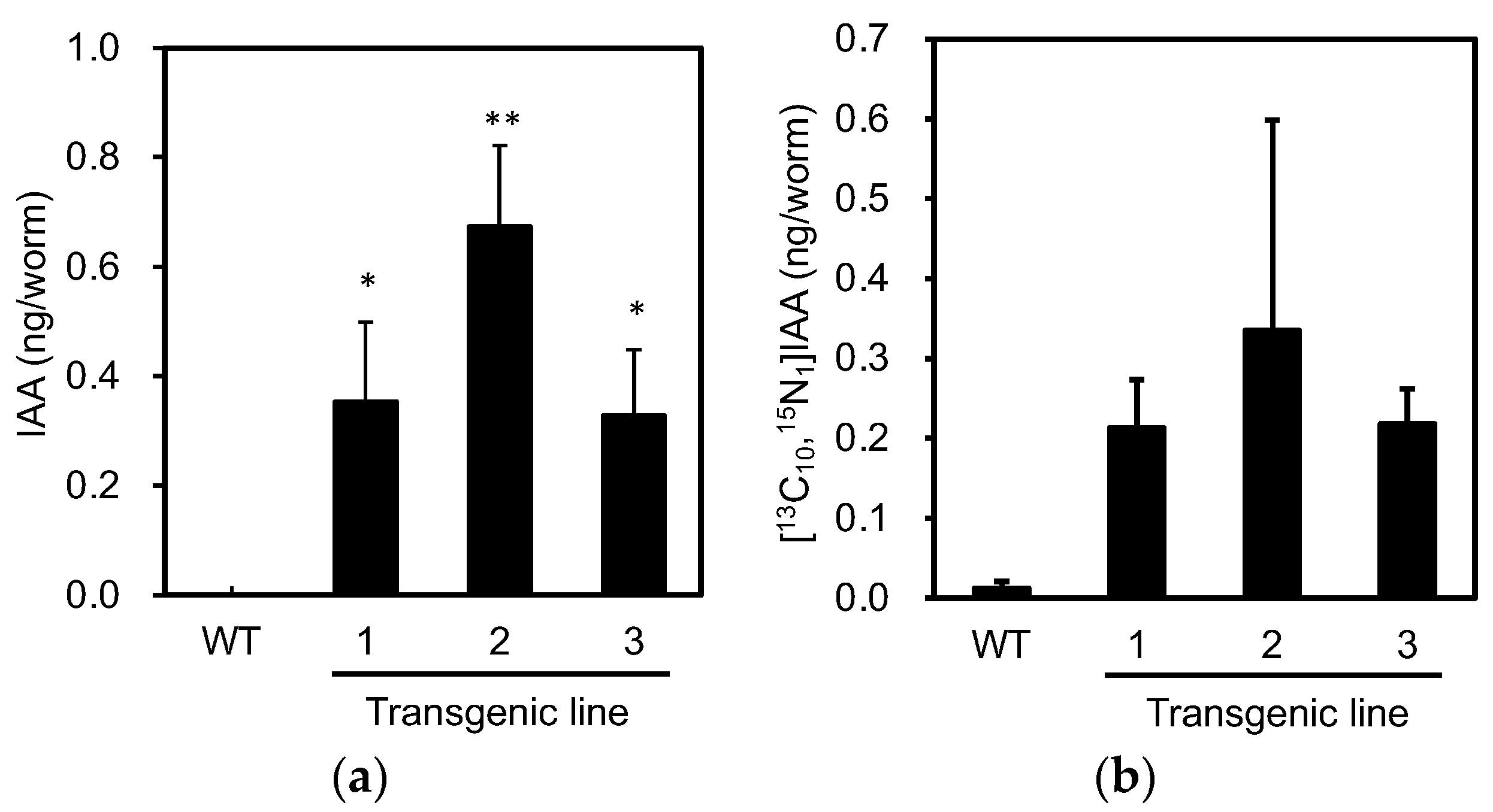
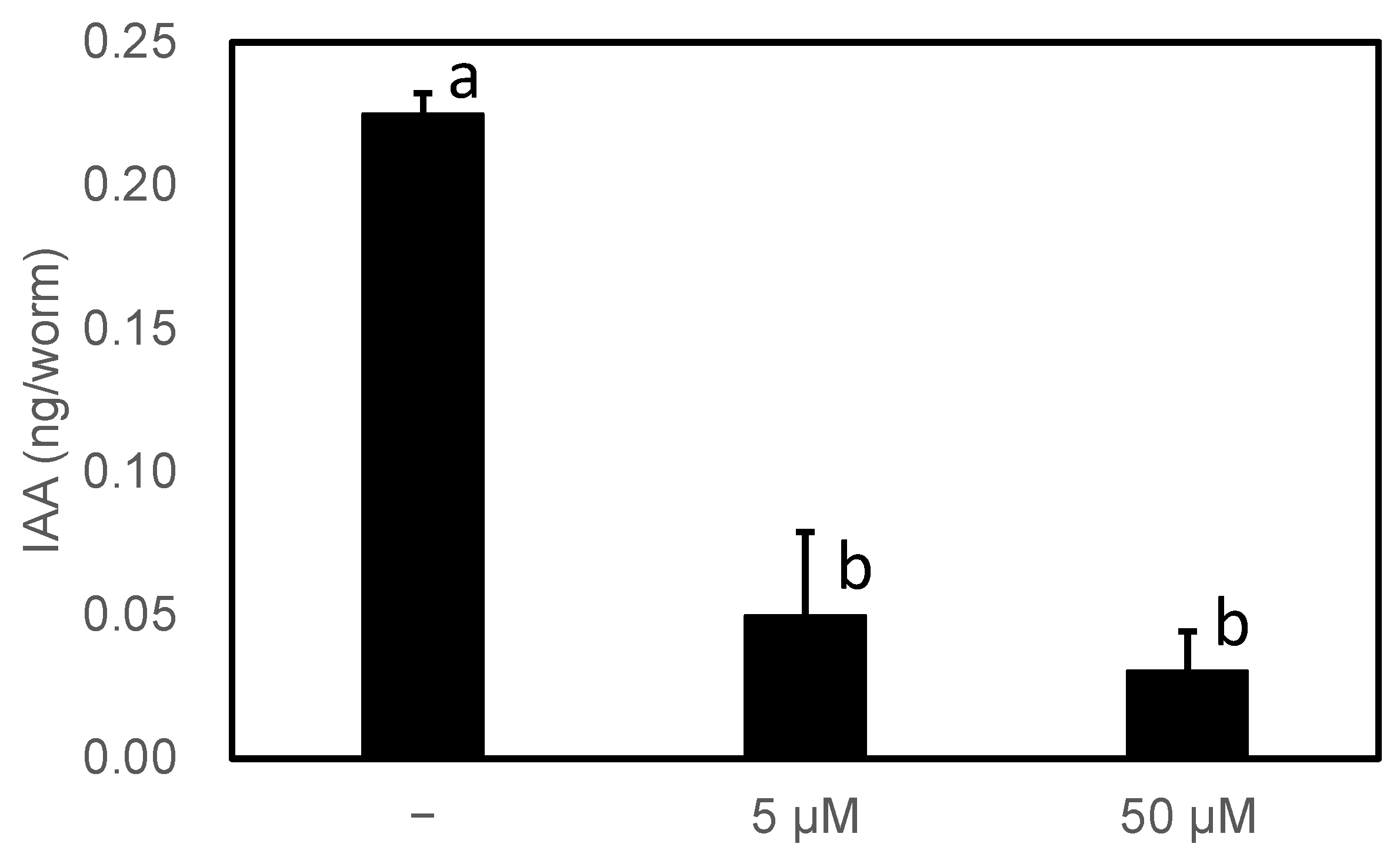
3.1. PonAAS2 Confers High IAA Productivity on C. elegans
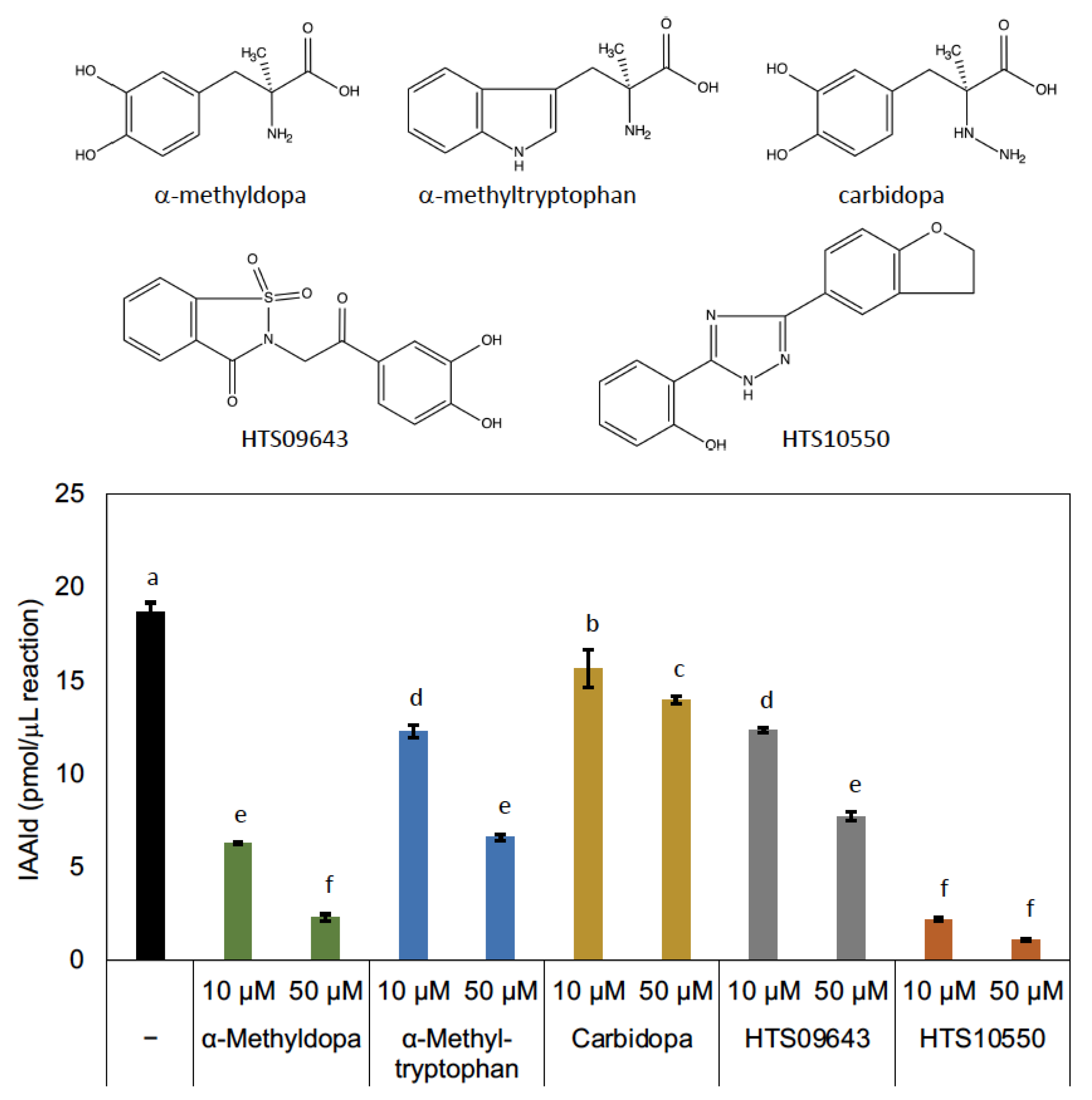
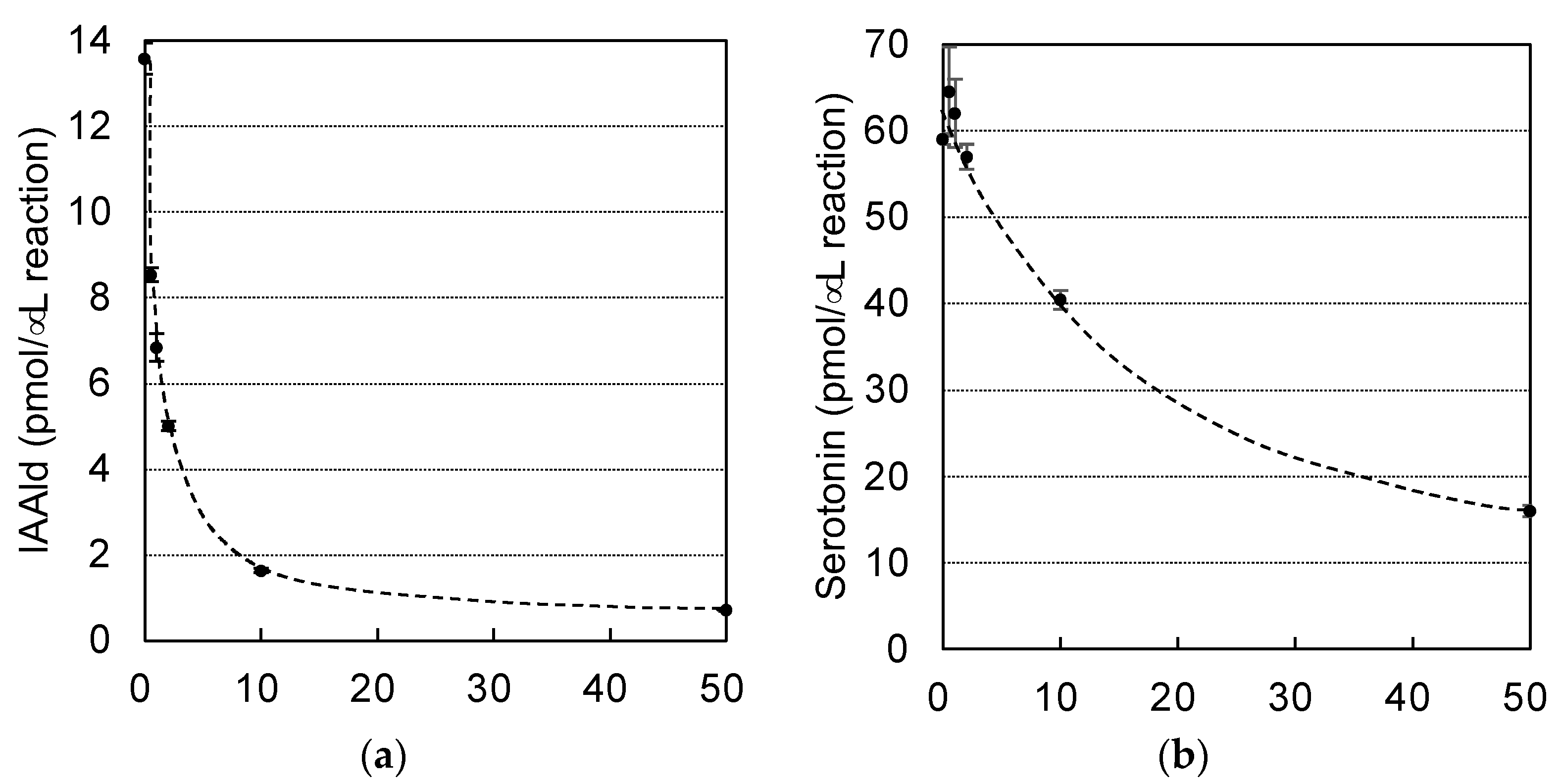
3.2. Characterization of PonAAS2 Inhibitor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tooker, J.F.; Helms, A.M. Phytohormone dynamics associated with gall insects, and their potential role in the evolution of the gall-inducing habit. J. Chem. Ecol. 2014, 40, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Tanaka, H.; Hasegawa, M.; Tokuda, M.; Asami, T.; Suzuki, Y. Phytohormones and willow gall induction by a gall-inducing sawfly. New Phytol. 2012, 196, 586–595. [Google Scholar] [CrossRef]
- Tanaka, Y.; Okada, K.; Asami, T.; Suzuki, Y. Phytohormones in Japanese mugwort gall induction by a gall-inducing gall midge. Biosci. Biotechnol. Biochem. 2013, 77, 1942–1948. [Google Scholar] [CrossRef]
- Takei, M.; Yoshida, S.; Kawai, T.; Hasegawa, M.; Suzuki, Y. Adaptive significance of gall formation for a gall-inducing aphids on Japanese elm trees. J. Insect Physiol. 2015, 72, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yokokura, J.; Ito, T.; Arai, R.; Yokoyama, C.; Toshima, H.; Nagata, S.; Asami, T.; Suzuki, Y. Biosynthetic pathway of the phytohormone auxin in insects and screening of its inhibitors. Insect Biochem. Mol. Biol. 2014, 53, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, C.; Takei, M.; Kouzuma, Y.; Nagata, S.; Suzuki, Y. Novel tryptophan metabolic pathways in auxin biosynthesis in silkworm. J. Insect Physiol. 2017, 101, 91–96. [Google Scholar] [CrossRef]
- Takei, M.; Kogure, S.; Yokoyama, C.; Kouzuma, Y.; Suzuki, Y. Identification of an aldehyde oxidase involved in indole-3-acetic acid synthesis in Bombyx mori silk gland. Biosci. Biotechnol. Biochem. 2019, 83, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Koshiba, T.; Yamauchi, K.; Matsuyama, H.; Miyakado, M.; Sori, I.; Sato, M. Flavin-photosensitized production of indole-3-acetaldehyde from tryptophan. Tetrahedron Lett. 1993, 34, 7603–7604. [Google Scholar] [CrossRef]
- Miyata, U.; Arakawa, K.; Takei, M.; Asami, T.; Asanbou, K.; Toshima, H.; Suzuki, Y. Identification of an aromatic aldehyde synthase involved in indole-3-acetic acid biosynthesis in the galling sawfly (Pontania sp.) and screening of an inhibitor. Insect Biochem. Mol. Biol. 2021, 137, 103639. [Google Scholar] [CrossRef]
- Vavricka, C.; Han, Q.; Huang, Y.; Erickson, S.M.; Harich, K.; Christensen, B.M.; Li, J. From L-dopa to dihydroxyphenylacetaldehyde: A toxic biochemical pathway plays a vital physiological function in insects. PLoS ONE 2011, 6, e16124. [Google Scholar] [CrossRef]
- Vavricka, C.J.; Yoshida, T.; Kuriya, Y.; Takahashi, S.; Ogawa, T.; Ono, F.; Agari, K.; Kiyota, H.; Li, J.; Ishii, J.; et al. Mechanism-based tuning of insect 3,4-dihydroxyphenylacetaldehyde synthase for synthetic bioproduction of benzylisoquinoline alkaloids. Nat. Commun. 2019, 10, 2015. [Google Scholar] [CrossRef]
- Tokuda, M.; Suzuki, Y.; Fujita, S.; Matsuda, H.; Adachi-Fukunaga, S.; Elsayed, A.K. Terrestrial arthropods broadly possess endogenous phytohormones auxin and cytokinins. Sci. Rep. 2022, 12, 4750. [Google Scholar] [CrossRef]
- Liang, J.; Han, Q.; Ding, H.; Li, J. Biochemical identification of residues that discriminate between 3,4-dihydroxyphenylalanine decarboxylase and 3,4-dihydroxyphenylacetaldehyde synthase-mediated reactions. Insect Biochem. Mol. Biol. 2017, 91, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.H. Insect neurotransmission: Neurotransmitters and their receptors. Pharmacol. Ther. 1996, 69, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Ferdig, M.T.; Li, J.; Severson, D.W.; Christensen, B.M. Mosquito dopa decarboxylase cDNA characterization and blood-meal-induced ovarian expression. Insect Mol. Biol. 1996, 5, 119–126. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chou, S.Y.; Bartholomay, L.C.; Christensen, B.M.; Chen, C.C. The use of gene silencing to study the role of dopa decarboxylase in mosquito melanization reactions. Insect Mol. Biol. 2005, 14, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Paskewitz, S.M.; Andreev, O. Silencing the genes for dopa decarboxylase or dopachrome conversion enzyme reduces melanization of foreign targets in Anopheles gambiae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.M.; Primrose, D.A.; Hodgetts, R.B. A member of the p38 mitogen-activated protein kinase family is responsible for transcriptional induction of Dopa decarboxylase in the epidermis of Drosophila melanogaster during the innate immune response. Mol. Cell Biol. 2008, 28, 4883–4895. [Google Scholar] [CrossRef] [PubMed]
- Sideri, M.; Tsakas, S.; Markoutsa, E.; Lampropoulou, M.; Marmaras, V.J. Innate immunity in insects: Surface-associated dopa decarboxylase-dependent pathways regulate phagocytosis, nodulation and melanization in medfly haemocytes. Immunology 2008, 123, 528–537. [Google Scholar] [CrossRef]
- Nappi, A.J.; Carton, Y.; Vass, E. Reduced cellular immune competence of a temperature-sensitive dopa decarboxylase mutant strain of Drosophila melanogaster against the parasite Leptopilina boulardi. Comp. Biochem. Physiol. B 1992, 101, 453–460. [Google Scholar] [CrossRef]
- Armenti, S.T.; Lohmer, L.L.; Sherwood, D.R.; Nance, J. Repurposing an endogenous degradation system for rapid and targeted depletion of C. elegans proteins. Development 2014, 141, 4640–4647. [Google Scholar] [CrossRef] [PubMed]
- Frokjaer-Jensen, C.; Davis, M.W.; Hopkins, C.E.; Newman, B.J.; Thummel, J.M.; Olesen, S.P.; Grunnet, M.; Jorgensen, E.M. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 2008, 40, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.C.; Kramer, J.M.; Stinchcomb, D.; Ambros, V. Efficient gene transfer in C. elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991, 10, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Colaiacovo, M.P. CRISPR-Cas9-Guided Genome Engineering in C. elegans. Curr. Protoc. Mol. Biol. 2016, 115, 31–37. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Chiang, Y.C.; Smith, T.; Vicent, M.A.; Wang, Y.; Weng, J.K. Structural basis for divergent and convergent evolution of catalytic machineries in plant aromatic amino acid decarboxylase proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 10806–10817. [Google Scholar] [CrossRef]
- Han, Q.; Ding, H.; Robinson, H.; Christensen, B.M.; Li, J. Crystal structure and substrate specificity of Drosophila 3,4-dihydroxyphenylalanine decarboxylase. PLoS ONE 2010, 5, e8826. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Lazear, M.; von Guggenberg, R.; Ding, H.; Li, J. Investigation of a substrate-specifying residue within Papaver somniferum and Catharanthus roseus aromatic amino acid decarboxylases. Phytochemistry 2014, 106, 37–43. [Google Scholar] [CrossRef]
- Porter, C.C.; Watson, L.S.; Titus, D.C.; Totaro, J.A.; Byer, S.S. Inhibition of DOPA decarboxylase by the hydrazino analog of α-methyldopa. Biochem. Pharmacol. 1962, 11, 1067–1077. [Google Scholar] [CrossRef]
- Turnbull, I.F.; Howells, A.J. Larvicidal activity of inhibitors of DOPA decarboxylase on the Australian sheep blowfly, Lucilia cuprina. Aust. J. Biol. Sci. 1980, 33, 169–181. [Google Scholar] [CrossRef]
- Hirose, T.; Nakano, Y.; Nagamatsu, Y.; Misumi, T.; Ohta, H.; Ohshima, Y. Cyclic GMP-dependent protein kinase EGL-4 controls body size and lifespan in C elegans. Development 2003, 130, 1089–1099. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiura, T.; Yoshida, H.; Miyata, U.; Asami, T.; Suzuki, Y. Conferring High IAA Productivity on Low-IAA-Producing Organisms with PonAAS2, an Aromatic Aldehyde Synthase of a Galling Sawfly, and Identification of Its Inhibitor. Insects 2023, 14, 598. https://doi.org/10.3390/insects14070598
Hiura T, Yoshida H, Miyata U, Asami T, Suzuki Y. Conferring High IAA Productivity on Low-IAA-Producing Organisms with PonAAS2, an Aromatic Aldehyde Synthase of a Galling Sawfly, and Identification of Its Inhibitor. Insects. 2023; 14(7):598. https://doi.org/10.3390/insects14070598
Chicago/Turabian StyleHiura, Takeshi, Hibiki Yoshida, Umi Miyata, Tadao Asami, and Yoshihito Suzuki. 2023. "Conferring High IAA Productivity on Low-IAA-Producing Organisms with PonAAS2, an Aromatic Aldehyde Synthase of a Galling Sawfly, and Identification of Its Inhibitor" Insects 14, no. 7: 598. https://doi.org/10.3390/insects14070598
APA StyleHiura, T., Yoshida, H., Miyata, U., Asami, T., & Suzuki, Y. (2023). Conferring High IAA Productivity on Low-IAA-Producing Organisms with PonAAS2, an Aromatic Aldehyde Synthase of a Galling Sawfly, and Identification of Its Inhibitor. Insects, 14(7), 598. https://doi.org/10.3390/insects14070598






