Aedes aegypti, Ae. albopictus and Culex quinquefasciatus Adults Found Coexisting in Urban and Semiurban Dwellings of Southern Chiapas, Mexico
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Mosquito Collection
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, E.W.J.; Wang, X.; Lin, L.; Xuan, Z.; Wu, D.; Lin, H.; Shen, P. Infectious diseases prevention and control using an integrated health big data system in China. BMC Infect. Dis. 2022, 22, 344. [Google Scholar] [CrossRef]
- Thongsripong, P.; Chandler, J.A.; Green, A.B.; Kittayapong, P.; Wilcox, B.A.; Kapan, D.D.; Bennett, S.N. Mosquito vector-associated microbiota: Metabarcoding bacteria and eukaryotic symbionts across habitat types in Thailand endemic for dengue and other arthropod-borne diseases. Ecol. Evol. 2017, 8, 1352–1368. [Google Scholar] [CrossRef]
- Torres Muñoz, A. La fiebre amarilla en México. Erradicación del Aedes aegypti [Yellow fever in Mexico. Eradication of Aedes aegypti]. Salud Publica Mex. 1966, 8, 561–570. (In Spanish) [Google Scholar]
- Narro-Robles, J.; Gómez-Dantés, H. El dengue en México: Un problema prioritario de salud pública [Dengue in Mexico: A priority problem of public health]. Salud Publica Mex. 1995, 37, S12–S20. (In Spanish) [Google Scholar]
- Díaz-González, E.E.; Kautz, T.F.; Dorantes-Delgado, A.; Malo-García, I.R.; Laguna-Aguilar, M.; Langsjoen, R.M.; Chen, R.; Auguste, D.I.; Sánchez Casas, R.M.; Danis-Lozano, R.; et al. First Report of Aedes aegypti Transmission of Chikungunya Virus in the Americas. Am. J. Trop. Med. Hyg. 2015, 93, 1325–1329. [Google Scholar] [CrossRef]
- Guerbois, M.; Fernandez-Salas, I.; Azar, S.R.; Danis-Lozano, R.; Alpuche-Aranda, C.M.; Leal, G.; Garcia-Malo, I.R.; Diaz-Gonzalez, E.E.; Casas-Martinez, M.; Rossi, S.L.; et al. Outbreak of Zika Virus Infection, Chiapas State, Mexico, 2015, and First Confirmed Transmission by Aedes aegypti Mosquitoes in the Americas. J. Infect. Dis. 2016, 214, 1349–1356. [Google Scholar] [CrossRef]
- Ortega-Morales, A.; Moreno Garcia, M.; Gonzalez-Acosta, C.; Correa-Morales, F. Mosquito suerveillance in Mexico: The use of ovitraps for Aedes aegypti, Ae. albopictus, and non-target species. Fla. Entomol. 2018, 101, 623–626. [Google Scholar] [CrossRef]
- Salomón-Grajales, J.; Lugo-Moguel, G.V.; Tinal-Gordillo, V.R.; de La Cruz-Velázquez, J.; Beaty, B.J.; Eisen, L.; Lozano-Fuentes, S.; Moore, C.G.; García-Rejón, J.E. Aedes albopictus mosquitoes, Yucatán Peninsula, Mexico. Emerg. Infect. Dis. 2012, 18, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Casas-Martínez, M.; Torres-Estrada, J.L. First evidence of Aedes albopictus (Skuse) in southern Chiapas, Mexico. Emerg. Infect. Dis. 2003, 9, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Boletín Epidemiológico; Secretaria de Salud. Panorama Epidemiológico de Dengue. Semana epidemiológica 52 de 2022; Dirección General de Epidemiología: Mexico City, Mexico, 2022.
- Ibáñez-Bernal, S.; Briseño, B.; Mutebi, J.P.; Argot, E.; Rodríguez, G.; Martínez-Campos, C.; Paz, R.; de la Fuente-San Román, P.; TapiaConyer, R.; Flisser, A. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med. Vet. Entomol. 1997, 11, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodríguez, O.S.; Sanchez-Casas, R.M.; Laguna-Aguilar, M.; Alvarado-Moreno, M.S.; Zarate-Nahon, E.A.; Ramirez-Jimenez, R.; de la Garza, C.E.M.; Torres-Zapata, R.; Dominguez-Galera, M.; Mis-Avila, P. Natural transmission of dengue virus by Aedes albopictus at Monterrey, Northeastern Mexico. Southwest. Entomol. 2014, 39, 459–468. [Google Scholar] [CrossRef][Green Version]
- Angelini, P.; Macini, P.; Finarelli, A.C.; Pol, C.; Venturelli, C.; Bellini, R.; Dottori, M. Chikungunya epidemic outbreak in Emilia-Romagna (Italy) during summer 2007. Parassitologia 2008, 50, 97–98. [Google Scholar] [PubMed]
- Smartt, C.T.; Stenn, T.M.S.; Chen, T.Y.; Teixeira, M.G.; Queiroz, E.P.; Souza Dos Santos, L.; Queiroz, G.A.N.; Ribeiro Souza, K.; Kalabric Silva, L.; Shin, D.; et al. Evidence of Zika Virus RNA Fragments in Aedes albopictus (Diptera: Culicidae) Field-Collected Eggs From Camaçari, Bahia, Brazil. J. Med. Entomol. 2017, 54, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, C.; Toma, L.; Remoli, M.E.; Amendola, A.; Severini, F.; Boccolini, D.; Romi, R.; Venturi, G.; Rezza, G.; Di Luca, M. Vector competence of Aedes albopictus for the Indian Ocean lineage (IOL) chikungunya viruses of the 2007 and 2017 outbreaks in Italy: A comparison between strains with and without the E1:A226V mutation. Euro Surveill. 2018, 23, 1800246. [Google Scholar] [CrossRef]
- Vega-Rúa, A.; Zouache, K.; Girod, R.; Failloux, A.B.; Lourenço-de-Oliveira, R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J. Virol. 2014, 88, 6294–6306. [Google Scholar] [CrossRef]
- Lambrechts, L.; Scott, T.W.; Gubler, D.J. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 2010, 4, e646. [Google Scholar] [CrossRef]
- Huerta, H.; González-Roldán, J.F.; Sánchez-Tejeda, G.; Correa-Morales, F.; Romero-Contreras, F.E.; Cárdenas-Flores, R.; Rangel-Martínez, M.L.; Mata-Rivera, J.M.; Siller-Martínez, J.J.; Vazquez-Prokopec, G.M.; et al. Detection of Zika virus in Aedes mosquitoes from Mexico. Trans. R Soc. Trop. Med. Hyg. 2017, 111, 328–331. [Google Scholar] [CrossRef]
- Braks, M.A.; Honório, N.A.; Lourençqo-De-Oliveira, R.; Juliano, S.A.; Lounibos, L.P. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J. Med. Entomol. 2003, 40, 785–794. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Schaffner, F.; Versteirt, V.; Hendrickx, G.; Zeller, H.; Van Bortel, W. A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef]
- Bond, J.G.; Moo-Llanes, D.A.; Ortega-Morales, A.I.; Marina, C.F.; CasasMartínez, M.; Danis-Lozano, R. Diversity and potential distribution of culicids of medical importance of the Yucatan Peninsula, Mexico. Salud Publica Mex. 2020, 62, 379–387. [Google Scholar] [CrossRef]
- WHO. Lymphatic Filariasis. Global Program to Eliminate Limphatic Filariasis: A Handbook of Practical Entomology for National Lymphatic Filariasis Elimination Programmes; WHO: Geneva, Switzerland, 2013.
- Blitvich, B.J.; Fernandez-Salas, I.; Contreras-Cordero, J.F.; Marlenee, N.L.; Gonzalez-Rojas, J.I.; Komar, N.; Gubler, D.J.; Calisher, C.H.; Beaty, B.J. Serologic evidence of West Nile virus infection in horses, Coahuila State, Mexico. Emerg. Infect. Dis. 2003, 9, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Loroño-Pino, M.A.; Blitvich, B.J.; Farfán-Ale, J.A.; Puerto, F.I.; Blanco, J.M.; Marlenee, N.L.; Rosado-Paredes, E.P.; García-Rejón, J.E.; Gubler, D.J.; Calisher, C.H.; et al. Serologic evidence of West Nile virus infection in horses, Yucatan State, Mexico. Emerg. Infect. Dis. 2003, 9, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.A.; Granados, O.A. Distribución geoespacial del mosquito Culex quinquefasciatus (diptera:culicidae) principal vector del Virus del oeste del Nilo, en la zona urbana de ciudad Juárez, Chihuahua, México. Rev. Salud Publica Nutr. 2007, 8, 1–13. [Google Scholar]
- Elizondo-Quiroga, D.; Ramírez-Medina, M.; Gutiérrez-Ortega, A.; Elizondo-Quiroga, A.; Muñoz-Medina, J.E.; Sánchez-Tejeda, G.; González-Acosta, C.; Correa-Morales, F. Vector competence of Aedes aegypti and Culex quinquefasciatus from the metropolitan area of Guadalajara, Jalisco, Mexico for Zika virus. Sci. Rep. 2019, 9, 16955. [Google Scholar] [CrossRef]
- Janaki, M.D.S.; Aryaprema, V.S.; Fernando, N.; Handunnetti, S.M.; Weerasena, O.V.D.S.J.; Pathirana, P.P.S.L.; Tissera, H.A. Prevalence and resting behaviour of dengue vectors, Aedes aegypti and Aedes albopictus in dengue high risk urban settings in Colombo, Sri Lanka. J. Asia-Pac. Entomol. 2022, 25, 101961. [Google Scholar] [CrossRef]
- Dzul-Manzanilla, F.; Ibarra-López, J.; Bibiano Marín, W.; Martini-Jaimes, A.; Leyva, J.T.; Correa-Morales, F.; Huerta, H.; Manrique-Saide, P.; Vazquez-Prokopec, G.M. Indoor Resting Behavior of Aedes aegypti (Diptera: Culicidae) in Acapulco, Mexico. J. Med. Entomol. 2017, 54, 501–504. [Google Scholar] [CrossRef]
- Rueda, L.M. Zootaxa 589: Pictorial Keys for the Identification of Mosquitoes (Diptera: Culicinidae) Associated with Dengue Virus Transmission; Magnolia Press: Auckland, New Zealand, 2004; p. 60. [Google Scholar]
- Darsie, R.F., Jr.; Ward, R.A. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. Mosq. Syst. 1981, 1, 1–313. [Google Scholar] [CrossRef]
- Câmara, D.C.P.; Codeço, C.T.; Ayllón, T.; Nobre, A.A.; Azevedo, R.C.; Ferreira, D.F.; da Silva Pinel, C.; Rocha, G.P.; Honório, N.A. Entomological Surveillance of Aedes Mosquitoes: Comparison of Different Collection Methods in an Endemic Area in RIO de Janeiro, Brazil. Trop. Med. Infect. Dis. 2022, 7, 114. [Google Scholar] [CrossRef]
- Pereira-Dos-Santos, T.; Roiz, D.; Lourenço-de-Oliveira, R.; Paupy, C. A Systematic Review: Is Aedes albopictus an Efficient Bridge Vector for Zoonotic Arboviruses? Pathogens 2020, 9, 266. [Google Scholar] [CrossRef]
- Chadee, D.D. Resting behaviour of Aedes aegypti in Trinidad: With evidence for the re-introduction of indoor residual spraying (IRS) for dengue control. Parasit. Vectors 2013, 6, 255. [Google Scholar] [CrossRef]
- Marina, C.F.; Bond, J.G.; Hernández-Arriaga, K.; Valle, J.; Ulloa, A.; Fernández-Salas, I.; Carvalho, D.O.; Bourtzis, K.; Dor, A.; Williams, T.; et al. Population Dynamics of Aedes aegypti and Aedes albopictus in Two Rural Villages in Southern Mexico: Baseline Data for an Evaluation of the Sterile Insect Technique. Insects 2021, 12, 58. [Google Scholar] [CrossRef]
- Forattini, O.P. Mosquitos culicidae como vectores emergentes de infectiones [Culicidae mosquitoes as emerging vectors of diseases]. Rev. Saude Publica 1998, 32, 497–502. (In Portuguese) [Google Scholar] [CrossRef]
- Contreras-Perera, Y.J.; Briceño-Mendez, M.; Flores-Suárez, A.E.; Manrique-Saide, P.; Palacio-Vargas, J.A.; Huerta-Jimenez, H.; MartinPark, A. New Record of Aedes albopictus In A Suburban Area Of Merida, Yucatan, Mexico. J. Am. Mosq. Control Assoc. 2019, 35, 210–213. [Google Scholar] [CrossRef]
- Castro, M.G.; Nogueira, R.M.; Schatzmayr, H.G.; Miagostovich, M.P.; Lourenço-de-Oliveira, R. Dengue virus detection by using reverse transcription-polymerase chain reaction in saliva and progeny of experimentally infected Aedes albopictus from Brazil. Mem. Inst. Oswaldo Cruz 2004, 99, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Arguedas, O.; Troyo, A.; Moreira-Soto, R.D.; Marín, R.; Taylor, L. Dengue viruses in Aedes albopictus Skuse from a pineapple plantation in Costa Rica. J. Vector Ecol. 2015, 40, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Delatte, H.; Toty, C.; Boyer, S.; Bouetard, A.; Bastien, F.; Fontenille, D. Evidence of habitat structuring Aedes albopictus populations in Réunion Island. PLoS Negl. Trop. Dis. 2013, 7, e2111. [Google Scholar] [CrossRef]
- Dalpadado, R.; Amarasinghe, D.; Gunathilaka, N.; Ariyarathna, N. Bionomic aspects of dengue vectors Aedes aegypti and Aedes albopictus at domestic settings in urban, suburban and rural areas in Gampaha District, Western Province of Sri Lanka. Parasit. Vectors 2022, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Gratz, N.G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004, 18, 215–227. [Google Scholar] [CrossRef]
- Marra, P.P.; Griffing, S.; Caffrey, C.; Kilpatrick, A.; Mclean, R.; Brand, C.; Saito, E.; Dupuis, A.P.; Kramer, L.; Novak, R. West Nile virus and wildlife. BioScience 2004, 54, 393–402. [Google Scholar]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- López-Solís, A.D.; Castillo-Vera, A.; Cisneros, J.; Solis-Santoyo, F.; Penilla-Navarro, R.P.; Black, I.V.W.C.; Torres-Estrada, J.L.; Rodríguez, A.D. Resistencia a Insecticidas en Aedes aegypti y Aedes albopictus (Diptera: Culicidae) de Tapachula, Chiapas, México. Salud Publica Mex. 2020, 62, 439–446. Available online: https://saludpublica.mx/in (accessed on 9 June 2023). [CrossRef]
- Janich, A.J.; Saavedra-Rodriguez, K.; Vera-Maloof, F.Z.; Kading, R.C.; Rodríguez, A.D.; Penilla-Navarro, P.; López-Solis, A.D.; Solis-Santoyo, F.; Perera, R.; Black, W.C. Permethrin Resistance Status and Associated Mechanisms in Aedes albopictus (Diptera: Culicidae) From Chiapas, Mexico. J. Med. Entomol. 2021, 58, 739–748. [Google Scholar] [CrossRef]
- Nazri, C.D.; Abu, H.A.; Rodziah, I. Habitat characterization of Aedes sp. breeding in urban hotspot area. Procedia Soc. Behav. Sci. 2013, 85, 100–109. [Google Scholar] [CrossRef]
- Madzlan, F.; Che Dom, N.; Chua, S.T.; Zakaria, N. Breeding characteristics of Aedes mosquitoes in dengue risk area. Procedia Soc. Behav. Sci. 2016, 234, 164–172. [Google Scholar] [CrossRef]
- Medeiros-Sousa, A.R.; Fernandes, A.; Ceretti-Junior, W.; Wilke, A.B.B.; Marrelli, M.T. Mosquitoes in urban green spaces: Using an island biogeographic approach to identify drivers of species richness and composition. Sci. Rep. 2017, 7, 17826. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.G.; Scavo, N.A.; Finney, M.; Fimbres-Macias, J.P.; Lively, M.T.; Strauss, B.H.; Hamer, G.L. Meta-Analysis of the Relative Abundance of Nuisance and Vector Mosquitoes in Urban and BlueGreen Spaces. Insects 2022, 13, 271. [Google Scholar] [CrossRef]

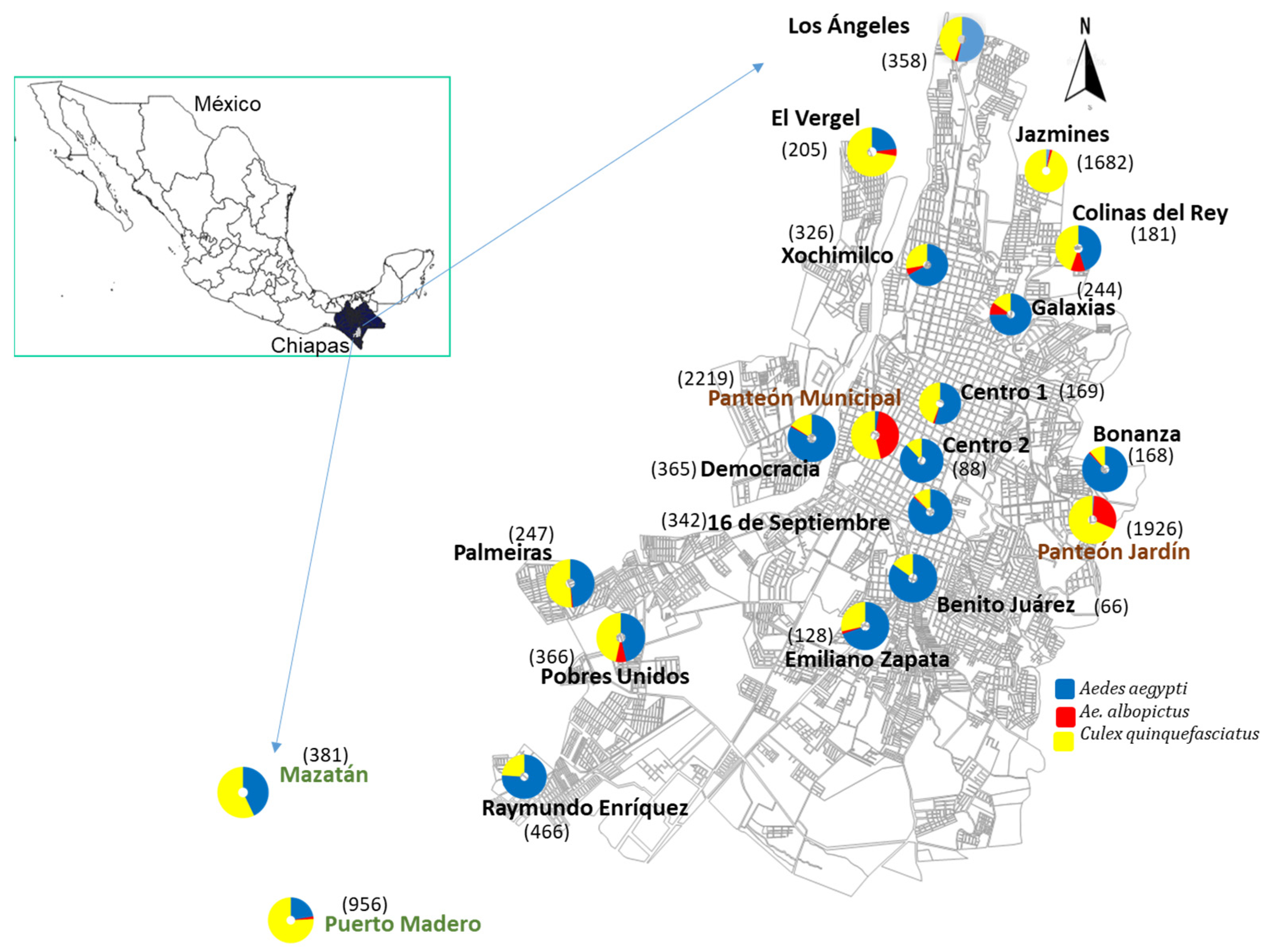
| No | Sites | Coordinates | |||
|---|---|---|---|---|---|
| Latitude | Longitude | Houses Collected | Months of Mosquito Collections | ||
| 1 | El Vergel | 14°56′21.2″ | 92°15′52.4″ | 18 | May, June, December |
| 2 | Los Ángeles | 14°56′42.3″ | 92°15′21.2″ | 24 | May, July, November |
| 3 | Jazmines | 14°53′32.2″ | 92°17′19.4″ | 22 | May, June, November |
| 4 | Xochimilco | 14°55′48.9″ | 92°15′37.8″ | 22 | May, June, November |
| 5 | Colinas del Rey | 14°55′50.9″ | 92°14′50.2″ | 17 | May, June, November |
| 6 | Galaxias | 14°55′11.2″ | 92°15″06,5″ | 13 | May, June, November |
| 7 | Centro 1 | 14°54′22.7″ | 92°15‘32.8″ | 15 | June, July, November |
| 8 | Democracia | 14°54′23.7″ | 92°16′33.5″ | 28 | June, July, November |
| 9 | Panteón Municipal | 14°54′15.3″ | 92°16′13.1″ | - | June, August, December |
| 10 | Centro 2 | 14°54′ 8.5″ | 92°15′47.3″ | 17 | June, July, November |
| 11 | Bonanza | 14°54′02.8″ | 92°14′31.7″ | 28 | May, July, November |
| 12 | Panteón Jardín | 14°53′41.7″ | 92°14′56.6″ | - | June, August, November |
| 13 | 16 de Septiembre | 14°53′44.0″ | 92°15′42.1″ | 16 | May, July |
| 14 | Benito Juárez | 14°53′21.8″ | 92°16′04.1″ | 7 | May, July |
| 15 | Emiliano Zapata | 14°53′02.1″ | 92°16′14.2″ | 16 | May, July |
| 16 | Raymundo Enríquez | 14°52′01.4″ | 92°18′48.8″ | 25 | May, June |
| 17 | Pobres Unidos | 4°53′14.0″ | 92°17′6.1″ | 25 | June, July |
| 18 | Palmeiras | 14°53′22.1″ | 92°18′06.4″ | 13 | June, July |
| 19 | Puerto Madero | 14°43′21.7″ | 92°25′38.7″ | 24 | June, August, November |
| 20 | Mazatán | 14°52′3.16″ | 92°26′59.88″ | 20 | Jule, August, November |
| House Area | Collection Time | Aedes aegypti (2807) | Ae. albopictus (195) | Culex quinquefasciatus (3736) | |||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | ||
| Indoors (3609) | 1 | 488 (1.19) | 407 (0.83) | 17 (1.30) | 13 (0.76) | 437 (1.12) | 389 (0.89) |
| 2 | 492 (1.17) | 417 (0.84) | 7 (1.00) | 7 (1.00) | 189 (1.35) | 139 (0.73) | |
| 3 | 164 (2.10) | 78 (0.47) | 5 (1.66) | 3 (0.60) | 230 (1.81) | 127 (0.55) | |
| Total | 1144 (1.26) | 902 (0.78) | 29 (1.26) | 23 (0.79) | 856 (1.30) | 655 (0.76) | |
| Outdoors (3129) | 1 | 202 (1.43) | 141 (0.69) | 51 (3.40) | 15 (0.29) | 418 (1.24) | 336 (0.80) |
| 2 | 225 (1.71) | 131 (0.58) | 29 (0.96) | 30 (1.03) | 274 (1.21) | 225 (0.82) | |
| 3 | 38 (1.58) | 24 (0.63) | 10 (1.25) | 8 (0.80) | 555 (1.33) | 417 (0.75) | |
| Total | 465 (1.57) | 296 (0.63) | 90 (1.69) | 53 (0.58) | 1247 (1.27) | 978 (0.78) | |
| Index Positive House | Mosquito Density/House | Bootstrap (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | Aedes aegypti | Ae. albopictus | Culex quinquefasciatus | Ae. aegypti | Ae. albopictus | Cx. quinquefasciatus | Ae. aegypti | Ae. albopictus | Cx. quinquefasciatus |
| Vergel | 78 | 22 | 94 | 5 | 2 | 8 | * | * | |
| Los Ángeles | 83 | 17 | 71 | 10 | 2 | 9 | * | ||
| Jazmines | 45 | 18 | 91 | 5 | 8 | 80 | |||
| Xochimilco | 95 | 36 | 45 | 11 | 3 | 8 | * | * | * |
| Colinas del Rey | 76 | 35 | 88 | 7 | 3 | 5 | * | ||
| Galaxias | 85 | 62 | 69 | 16 | 3 | 4 | * | * | |
| Centro 1 | 100 | 87 | 60 | 5 | 1 | 10 | * | ||
| Democracia | 100 | 14 | 46 | 11 | 1 | 4 | * | ||
| Centro 2 | 82 | 0 | 29 | 6 | 0 | 2 | |||
| Bonanza | 100 | 7 | 50 | 5 | 1 | 2 | * | ||
| 16 de Septiembre | 100 | 19 | 50 | 18 | 1 | 6 | |||
| Benito Juárez | 86 | 0 | 29 | 9 | 0 | 5 | |||
| Emiliano Zapata | 100 | 19 | 56 | 5 | 3 | 4 | * | ||
| Raymundo Enríquez | 92 | 0 | 60 | 15 | 0 | 8 | * | * | |
| Pobres Unidos | 88 | 24 | 56 | 7 | 7 | 12 | |||
| Palmeiras | 100 | 15 | 23 | 9 | 1 | 41 | |||
| Puerto Madero | 92 | 33 | 79 | 10 | 2 | 38 | |||
| Mazatán | 90 | 0 | 85 | 8 | 0 | 14 | |||
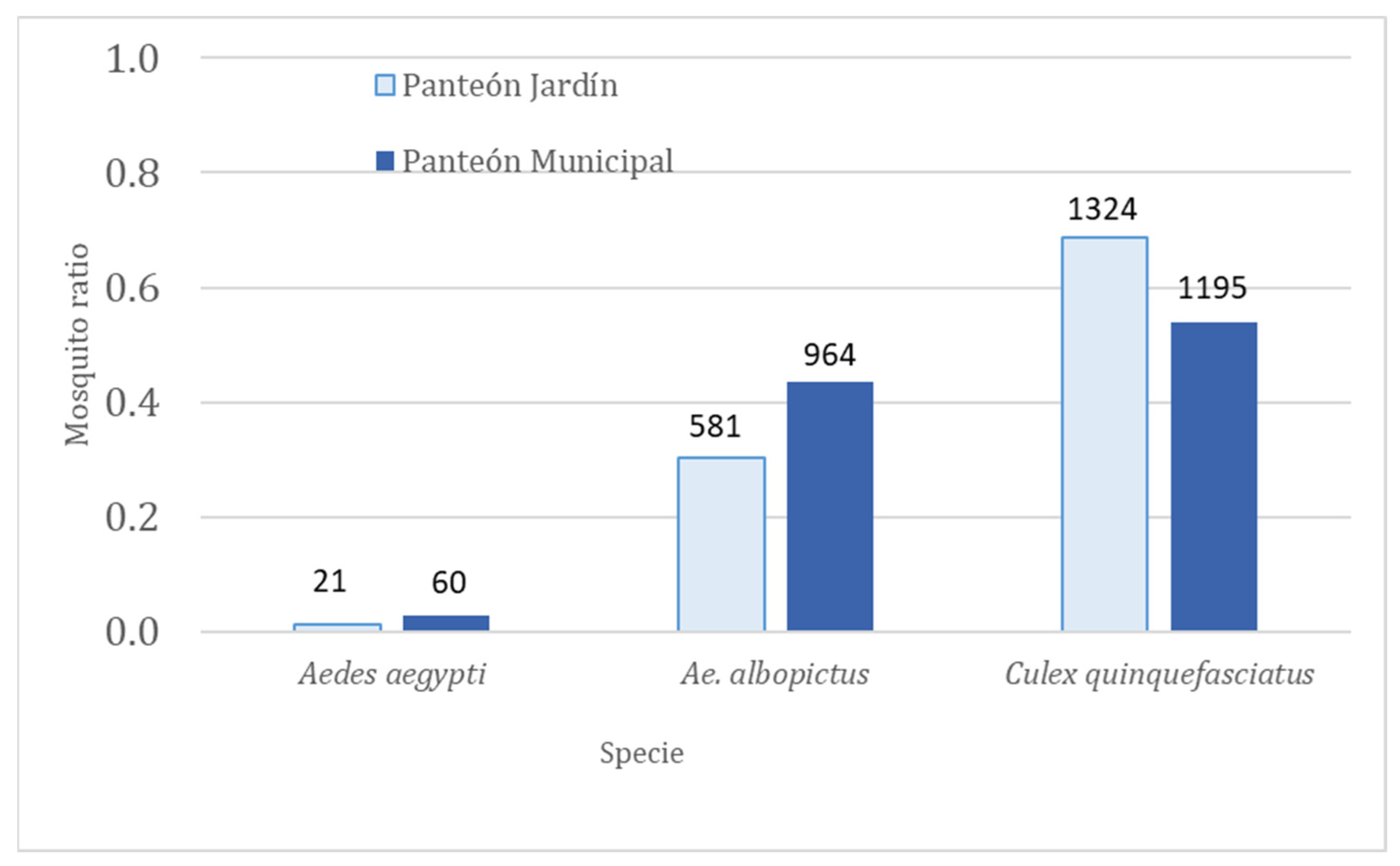
| Aedes aegypti (81) | Ae. albopictus (1545) | Culex quinquefasciatus (2519) | |||||
|---|---|---|---|---|---|---|---|
| Site | Collections Time | Male | Female | Male | Female | Male | Female |
| Panteón Jardín | 1 | 2 (0.66) | 3 (1.50) | 97 (0.98) | 98 (1.01) | 672 (1.23) | 543 (0.80) |
| (1926) | 2 | 7 (7.00) | 1(0.14) | 91 (1.04) | 87 (0.95) | 66 (2.53) | 26 (0.39) |
| 3 | 6 (3.00) | 2 (0.33) | 122 (1.41) | 86 (0.70) | 13 (3.25) | 4 (0.30) | |
| Total | 15 (2.50) | 6 (0.40) | 310 (1.14) | 271 (0.87) | 751 (1.31) | 573 (0.76) | |
| Panteón Municipal | 1 | 19 (3.16) | 6 (0.31) | 188 (0.89) | 209 ((1.11) | 459 (1.08) | 422 (0.91) |
| (2219) | 2 | 29 (7.25) | 4 (0.13) | 321 (1.58) | 203 (0.63) | 47 (0.90) | 52 (1.10) |
| 3 | 0 (0.00) | 2 (2.00) | 22 (1.00) | 21 (0.95) | 140 (1.86) | 75 (0.53) | |
| Total | 48 (4.00) | 12(0.25) | 531(1.22) | 433 (0.81) | 646 (1.17) | 549 (0.84) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Solis, A.D.; Solis-Santoyo, F.; Saavedra-Rodriguez, K.; Sanchez-Guillen, D.; Castillo-Vera, A.; Gonzalez-Gomez, R.; Rodriguez, A.D.; Penilla-Navarro, P. Aedes aegypti, Ae. albopictus and Culex quinquefasciatus Adults Found Coexisting in Urban and Semiurban Dwellings of Southern Chiapas, Mexico. Insects 2023, 14, 565. https://doi.org/10.3390/insects14060565
Lopez-Solis AD, Solis-Santoyo F, Saavedra-Rodriguez K, Sanchez-Guillen D, Castillo-Vera A, Gonzalez-Gomez R, Rodriguez AD, Penilla-Navarro P. Aedes aegypti, Ae. albopictus and Culex quinquefasciatus Adults Found Coexisting in Urban and Semiurban Dwellings of Southern Chiapas, Mexico. Insects. 2023; 14(6):565. https://doi.org/10.3390/insects14060565
Chicago/Turabian StyleLopez-Solis, Alma D., Francisco Solis-Santoyo, Karla Saavedra-Rodriguez, Daniel Sanchez-Guillen, Alfredo Castillo-Vera, Rebeca Gonzalez-Gomez, Americo D. Rodriguez, and Patricia Penilla-Navarro. 2023. "Aedes aegypti, Ae. albopictus and Culex quinquefasciatus Adults Found Coexisting in Urban and Semiurban Dwellings of Southern Chiapas, Mexico" Insects 14, no. 6: 565. https://doi.org/10.3390/insects14060565
APA StyleLopez-Solis, A. D., Solis-Santoyo, F., Saavedra-Rodriguez, K., Sanchez-Guillen, D., Castillo-Vera, A., Gonzalez-Gomez, R., Rodriguez, A. D., & Penilla-Navarro, P. (2023). Aedes aegypti, Ae. albopictus and Culex quinquefasciatus Adults Found Coexisting in Urban and Semiurban Dwellings of Southern Chiapas, Mexico. Insects, 14(6), 565. https://doi.org/10.3390/insects14060565






