Effects of Temperature and Nutrition during the Larval Period on Life History Traits in an Invasive Malaria Vector Anopheles stephensi
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Malaria Parasite
2.2. Effects of Larval Rearing Conditions on Larval Survival and Development and Female Wing Size
2.3. Effects of Larval Rearing Temperature on Egg Production and Egg Size
2.4. Effects of Larval Developmental Conditions on Plasmodium berghei Infection and Adult Survival
2.5. Statistics
3. Results
3.1. Effects of Larval Rearing Conditions on Larval Mortality and Period and Female Wing Size
3.2. Effects of Larval Rearing Temperature on Egg Production and Egg Size
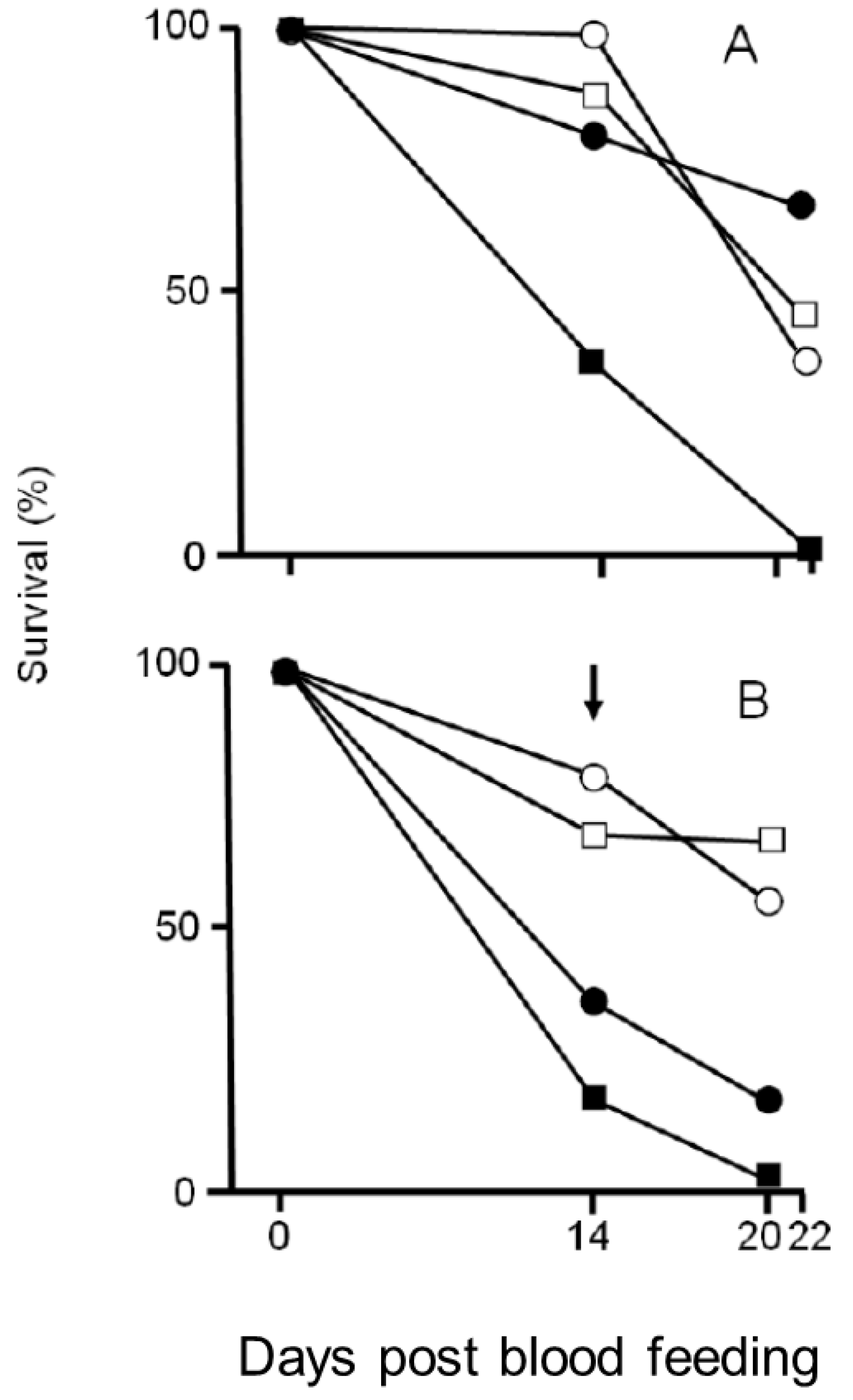
3.3. Effects of Larval Rearing Conditions on Plasmodium berghei Infection and Adult Survival
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cator, L.J.; Thomas, S.; Paaijmans, K.P.; Ravishankaran, S.; Justin, J.A.; Mathai, M.T.; Read, A.F.; Thomas, M.B.; Eapen, A. Characterizing microclimate in urban malaria transmission settings: A case study from Chennai, India. Malar. J. 2013, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Herrel, N.; Amerasinghe, F.P.; Ensink, J.; Mukhtar, M.; Van Der Hoek, W.; Konradsen, F. Breeding of Anopheles mosquitoes in irrigated areas of South Punjab, Pakistan. Med. Vet. Entomol. 2001, 15, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Herrel, N.; Amerasinghe, F.P.; Ensink, J.; Mukhtar, M.; Van Der Hoek, W.; Konradsen, F. Adult anopheline ecology and malaria transmission in irrigated areas of South Punjab, Pakistan. Med. Vet. Entomol. 2004, 18, 141–152. [Google Scholar] [CrossRef]
- Nagpal, B.N.; Srivastava, A.; Dash, A.P. Resting behaviour of Anopheles stephensi type form to assess its amenability to control malaria through indoor residual spray. J. Vector Borne Dis. 2012, 49, 175–180. [Google Scholar] [PubMed]
- Rastogi, M.; Pal, N.L.; Sen, A.B. Effect of variation in temperature on development of Plasmodium berghei (NK 65 strain) in Anopheles stephensi. Folia Parasitol. 1987, 34, 289–297. [Google Scholar]
- Santos-Vega, M.; Bouma, M.J.; Kohli, V.; Pascual, M. Population Density, Climate Variables and Poverty Synergistically Structure Spatial Risk in Urban Malaria in India. PLoS Negl. Trop. Dis. 2016, 10, e0005155. [Google Scholar] [CrossRef]
- Thomas, S.; Ravishankaran, S.; Justin, J.A.; Asokan, A.; Mathai, M.T.; Valecha, N.; Thomas, M.B.; Eapen, A. Overhead tank is the potential breeding habitat of Anopheles stephensi in an urban transmission setting of Chennai, India. Malar. J. 2016, 15, 274. [Google Scholar] [CrossRef]
- Thomas, S.; Ravishankaran, S.; Justin, N.A.; Asokan, A.; Kalsingh, T.; Mathai, M.T.; Valecha, N.; Montgomery, J.; Thomas, M.B.; Eapen, A. Microclimate variables of the ambient environment deliver the actual estimates of the extrinsic incubation period of Plasmodium vivax and Plasmodium falciparum: A study from a malaria-endemic urban setting, Chennai in India. Malar. J. 2018, 17, 201. [Google Scholar] [CrossRef]
- Mojahedi, A.R.; Safari, R.; Yarian, M.; Pakari, A.; Raeisi, A.; Edalat, H.; Beniardelan, M.; Poudat, A.; Zaim, M.; Basseri, H.R. Biting and resting behaviour of malaria vectors in Bandar-Abbas County, Islamic Republic of Iran. East. Mediterr. Health J. 2020, 26, 1218–1226. [Google Scholar] [CrossRef]
- Pakdad, K.; Hanafi-Bojd, A.A.; Vatandoost, H.; Sedaghat, M.M.; Raeisi, A.; Moghaddam, A.S.; Foroushani, A.R. Predicting the potential distribution of main malaria vectors Anopheles stephensi, An. culicifacies s.l. and An. fluviatilis s.l. in Iran based on maximum entropy model. Acta Trop. 2017, 169, 93–99. [Google Scholar]
- Salahi-Moghaddam, A.; Khoshdel, A.; Dalaei, H.; Pakdad, K.; Nutifafa, G.G.; Sedaghat, M.M. Spatial changes in the distribution of malaria vectors during the past 5 decades in Iran. Acta Trop. 2017, 166, 45–53. [Google Scholar] [CrossRef]
- Faulde, M.K.; Rueda, L.M.; Khaireh, B.A. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop. 2014, 139, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.E.; Pironon, S.; Massey, N.C.; Longbottom, J.; Hemingway, J.; Moyes, C.L.; Willis, K.J. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. USA 2020, 117, 24900–24908. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.; Hamlet, A.; Sherrard-Smith, E.; Winskill, P.; Cuomo-Dannenburg, G.; Walker, P.G.; Sinka, M.; Pironon, S.; Kumar, A.; Ghani, A.; et al. Seasonal dynamics of Anopheles stephensi and its implications for mosquito detection and emergent malaria control in the Horn of Africa. Proc. Natl. Acad. Sci. USA 2023, 120, e2216142120. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Initiative to Stop the Spread of Anopheles Stephensi in Africa; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Fazeli-Dinan, M.; Azarnoosh, M.; Özgökçe, M.S.; Chi, H.; Hosseini-Vasoukolaei, N.; Haghi, F.M.; Zazouli, M.A.; Nikookar, S.H.; Dehbandi, R.; Enayati, A.; et al. Global water quality changes posing threat of increasing infectious diseases, a case study on malaria vector Anopheles stephensi coping with the water pollutants using age-stage, two-sex life table method. Malar. J. 2022, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Lippi, C.A.; Villena, O.C.; Singh, A.; Murdock, C.C.; Johnson, L.R. Mapping current and future thermal limits to suitability for malaria transmission by the invasive mosquito Anopheles stephensi. Malar. J. 2023, 22, 104. [Google Scholar] [CrossRef]
- Garrett-Jones, C. Prognosis for Interruption of Malaria Transmission Through Assessment of the Mosquito’s Vectorial Capacity. Nature 1964, 204, 1173–1175. [Google Scholar] [CrossRef]
- Molineaux, L.; Shidrawi, G.R.; Clarke, J.L.; Boulzaguet, J.R.; Ashkar, T.S. Assessment of insecticidal impact on the malaria mosquito’s vectorial capacity, from data on the man-biting rate and age-composition. Bull. World Health Organ. 1979, 57, 265–274. [Google Scholar]
- Molineaux, L.; Gramiccia, G.; World Health Organization. The Garki Project: Research on the Epidemiology and Control of Malaria in the Sudan Savanna of West Africa/by L. Molineaux and G. Gramiccia; World Health Organization: Geneva, Switzerland, 1980. [Google Scholar]
- Agyekum, T.P.; Arko-Mensah, J.; Botwe, P.K.; Hogarh, J.N.; Issah, I.; Dwomoh, D.; Billah, M.K.; Dadzie, S.K.; Robins, T.G.; Fobil, J.N. Effects of Elevated Temperatures on the Growth and Development of Adult Anopheles gambiae (s.l.) (Diptera: Culicidae) Mosquitoes. J. Med. Entomol. 2022, 59, 1413–1420. [Google Scholar] [CrossRef]
- Agyekum, T.P.; Botwe, P.K.; Arko-Mensah, J.; Issah, I.; Acquah, A.A.; Hogarh, J.N.; Dwomoh, D.; Robins, T.G.; Fobil, J.N. A Systematic Review of the Effects of Temperature on Anopheles Mosquito Development and Survival: Implications for Malaria Control in a Future Warmer Climate. Int. J. Environ. Res. Public Health 2021, 18, 7255. [Google Scholar] [CrossRef]
- Murdock, C.C.; Sternberg, E.D.; Thomas, M.B. Malaria transmission potential could be reduced with current and future climate change. Sci. Rep. 2016, 6, 27771. [Google Scholar] [CrossRef]
- Shapiro, L.L.; Whitehead, S.A.; Thomas, M.B. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017, 15, e2003489. [Google Scholar] [CrossRef]
- Takken, W.; Smallegange, R.C.; Vigneau, A.J.; Johnston, V.; Brown, M.; Mordue-Luntz, A.J.; Billingsley, P.F. Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasites Vectors 2013, 6, 345. [Google Scholar] [CrossRef]
- Villena, O.C.; Ryan, S.J.; Murdock, C.C.; Johnson, L.R. Temperature impacts the environmental suitability for malaria transmission by Anopheles gambiae and Anopheles stephensi. Ecology 2022, 103, e3685. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.L.; Suh, E.; Lynch, P.A.; Thomas, M.B. Exploring the lower thermal limits for development of the human malaria parasite, Plasmodium falciparum. Biol. Lett. 2019, 15, 20190275. [Google Scholar] [CrossRef] [PubMed]
- Ameneshewa, B.; Service, M.W. The relationship between female body size and survival rate of the malaria vector Anopheles arabiensis in Ethiopia. Med. Vet. Entomol. 1996, 10, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Mwangangi, J.M.; Mbogo, C.M.; Nzovu, J.G.; Kabiru, E.W.; Mwambi, H.; Githure, J.I.; Beier, J.C. Relationships between body size of anopheles mosquitoes and Plasmodium falciparum sporozoite rates along the Kenya coast. J. Am. Mosq. Control Assoc. 2004, 20, 390–394. [Google Scholar]
- Lyimo, E.O.; Koella, J.C. Relationship between body size of adult Anopheles gambiae s.l. and infection with the malaria parasite Plasmodium falciparum. Parasitology 1992, 104 Pt 2, 233–237. [Google Scholar]
- Fernandez-Salas, I.; Rodriguez, M.H.; Roberts, D.R. Gonotrophic cycle and survivorship of Anopheles pseudopunctipennis (Diptera: Culicidae) in the Tapachula foothills of southern Mexico. J. Med. Entomol. 1994, 31, 340–347. [Google Scholar] [CrossRef]
- Grech, K.; Maung, L.A.; Read, A.F. The effect of parental rearing conditions on offspring life history in Anopheles stephensi. Malar. J. 2007, 6, 130. [Google Scholar] [CrossRef]
- Kittayapong, P.; Edman, J.D.; Harrison, B.A.; Delorme, D.R. Female body size, parity, and malaria infection of Anopheles maculatus (Diptera: Culicidae) in peninsular Malaysia. J. Med. Entomol. 1992, 29, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Barreaux, A.M.; Stone, C.M.; Barreaux, P.; Koella, J.C. The relationship between size and longevity of the malaria vector Anopheles gambiae (s.s.) depends on the larval environment. Parasites Vectors 2018, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Miazgowicz, K.L.; Shocket, M.S.; Ryan, S.J.; Villena, O.C.; Hall, R.J.; Owen, J.; Adanlawo, T.; Balaji, K.; Johnson, L.R.; Mordecai, E.A.; et al. Age influences the thermal suitability of Plasmodium falciparum transmission in the Asian malaria vector Anopheles stephensi. Proc. R. Soc. B 2020, 287, 20201093. [Google Scholar] [CrossRef]
- Moller-Jacobs, L.L.; Murdock, C.C.; Thomas, M.B. Capacity of mosquitoes to transmit malaria depends on larval environment. Parasites Vectors 2014, 7, 593. [Google Scholar] [CrossRef]
- Alam, M.S.; Tuno, N. Reduction of Reproductive Capacity in Aedes albopictus (Diptera: Culicidae) in Hot, Dry Summer. J. Med. Entomol. 2019, 56, 1729–1733. [Google Scholar] [CrossRef] [PubMed]
- de Zulueta, J. Changes in the geographical distribution of malaria throughout history. Parassitologia 1987, 29, 193–205. [Google Scholar]
- de Zulueta, J. Malaria and ecosystems: From prehistory to posteradication. Parassitologia 1994, 36, 7–15. [Google Scholar]
- Tuno, N.; Kjaerandsen, J.; Badu, K.; Kruppa, T. Blood-feeding behavior of Anopheles gambiae and Anopheles melas in Ghana, western Africa. J. Med. Entomol. 2010, 47, 28–31. [Google Scholar] [CrossRef]
- Tuno, N.; Githeko, A.K.; Nakayama, T.; Minakawa, N.; Takagi, M.; Yan, G. The Association between the Phytoplankton, Rhopalosolen Species (Chlorophyta; Chlorophyceae), and Anopheles gambiae Sensu Lato (Diptera: Culicidae) Larval Abundance in Western Kenya. Ecol. Res. 2006, 21, 476–482. [Google Scholar] [CrossRef]
- Tuno, N.; Kohzu, A.; Tayasu, I.; Nakayama, T.; Githeko, A.; Yan, G. An Algal Diet Accelerates Larval Growth of Anopheles gambiae (Diptera: Culicidae) and Anopheles arabiensis (Diptera: Culicidae). J. Med. Entomol. 2018, 55, 600–608. [Google Scholar] [CrossRef]
- Tuno, N.; Okeka, W.; Minakawa, N.; Takagi, M.; Yan, G. Survivorship of Anopheles gambiae sensu stricto (Diptera: Culicidae) larvae in western Kenya highland forest. J. Med. Entomol. 2005, 42, 270–277. [Google Scholar] [CrossRef] [PubMed]
| Food Supply | Temp. (°C) | Larval Mortality | Larval Period (Days) | Wing Length (mm) | |||
|---|---|---|---|---|---|---|---|
| N | % | N | Mean ± SD | N | Mean ± SD | ||
| High | 22 | 200 | 6.5 | 187 | 12.37 ± 1.20 | 30 | 3.44 ± 0.31 |
| 27 | 200 | 5.5 | 189 | 9.53 ± 1.18 | 28 | 3.54 ± 0.18 | |
| 30 | 200 | 9.5 | 182 | 8.80 ± 1.27 | 13 | 3.29 ± 0.12 | |
| 32 | 200 | 14.5 | 172 | 9.43 ± 1.26 | 11 | 3.19 ± 0.12 | |
| Low | 22 | 200 | 11.0 | 179 | 18.71 ± 1.83 | 21 | 3.35 ± 0.15 |
| 27 | 200 | 10.0 | 182 | 15.48 ± 2.87 | 24 | 3.11 ± 0.25 | |
| 30 | 200 | 12.5 | 175 | 14.38 ± 3.26 | 10 | 2.96 ± 0.12 | |
| 32 | 200 | 28.5 | 145 | 15.43 ± 2.08 | 3 | 2.74 ± 0.02 | |
| Traits | Model | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Larval mortality | 1136 | 1134 | 1140 | 1142 | 1147 |
| Larval development time | |||||
| 22–30 °C | 4281.8 | 4278.2 | 4370.3 | 4322.7 | 4380.3 |
| 22–32 °C | 5516.7 | 5515 | 5638.1 | 5559 | 5648.8 |
| 30–32 °C | 2722 | 2720.6 | 2784.6 | 2722.8 | 2783.5 |
| Female wing length | −13.8 | −7 | 32.4 | 20.6 | 50.5 |
| Egg number | - | - | 258.3 | - | 256.8 |
| Egg length | - | - | 2261.4 | - | 2263.4 |
| Frequency of females with oocysts | 20.9 | 22.5 | 20.8 | 20.7 | 18.9 |
| Temp. (°C) | No. of Eggs Oviposited | Egg Length (µm) | ||
|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | |
| 22 | 5 | 65.5 ± 5.2 | 47 | 559.7 ± 35.9 |
| 27 | 8 | 136.8 ± 64.2 | 80 | 558.6 ± 29.2 |
| 30 | 5 | 113.0 ± 17.0 | 49 | 525.9 ± 26.8 |
| 32 | 6 | 93.5 ± 27.7 | 60 | 528.1 ± 51.4 |
| One Blood Meal | Two Blood Meals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Food Supply | Temp. (°C) | Oocysts | N Oocyst | Sporozoits | Sporozoits | |||||||
| N | (%) | N Positive | Mean ± SE | N | (%) | N | (%) | |||||
| High | 27 | 12 | 25 | 3 | 13.8 ± 7.7 | 30 | 33 | 39 | 46 | |||
| 32 | 17 | 71 | 12 | 14.7 ± 9.5 | 28 | 39 | 17 | 29 | ||||
| Low | 27 | 16 | 50 | 8 | 2.8 ± 0.6 | 28 | 57 | 40 | 23 | |||
| 32 | 16 | 31 | 5 | 13.4 ± 3.4 | - | - | - | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuno, N.; Farjana, T.; Uchida, Y.; Iyori, M.; Yoshida, S. Effects of Temperature and Nutrition during the Larval Period on Life History Traits in an Invasive Malaria Vector Anopheles stephensi. Insects 2023, 14, 543. https://doi.org/10.3390/insects14060543
Tuno N, Farjana T, Uchida Y, Iyori M, Yoshida S. Effects of Temperature and Nutrition during the Larval Period on Life History Traits in an Invasive Malaria Vector Anopheles stephensi. Insects. 2023; 14(6):543. https://doi.org/10.3390/insects14060543
Chicago/Turabian StyleTuno, Nobuko, Thahsin Farjana, Yui Uchida, Mitsuhiro Iyori, and Shigeto Yoshida. 2023. "Effects of Temperature and Nutrition during the Larval Period on Life History Traits in an Invasive Malaria Vector Anopheles stephensi" Insects 14, no. 6: 543. https://doi.org/10.3390/insects14060543
APA StyleTuno, N., Farjana, T., Uchida, Y., Iyori, M., & Yoshida, S. (2023). Effects of Temperature and Nutrition during the Larval Period on Life History Traits in an Invasive Malaria Vector Anopheles stephensi. Insects, 14(6), 543. https://doi.org/10.3390/insects14060543





