Expression of Molecular Markers of Resilience against Varroa destructor and Bee Viruses in Ethiopian Honey Bees (Apis mellifera simensis) Focussing on Olfactory Sensing and the RNA Interference Machinery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
2.1. Study Area and Honey Bee Samples
2.2. Homogenization and RNA Extraction
2.3. cDNA Synthesis
2.4. Viral Load Quantification
2.5. Gene Expression Analysis
2.6. Scanning Electron Microscopy
2.7. Statistical Analysis
3. Results
3.1. Gene Expression and DWV Loads in the Antennae
3.2. RNAi Activation and DWV Loads in Whole Bees
3.3. External Morphology of the Antennae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Currie, R.W.; Pernal, S.F.; Guzman-Novoa, E. Honey bee colony losses in Canada. J. Apic. Res. 2010, 49, 104–106. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Hayes, J.; Underwood, R.M.; Pettis, J.S. A survey of honey bee colony losses in the United States, fall 2008 to spring 2009. J. Apic. Res. 2010, 49, 7–14. [Google Scholar] [CrossRef]
- Brodschneider, R.; Gray, A.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charriere, J.D.; Chlebo, R.; Coffey, M.F.; Dahle, B.; de Graaf, D.C.; et al. Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J. Apic. Res. 2018, 57, 452–457. [Google Scholar] [CrossRef]
- Gray, A.; Brodschneider, R.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrire, J.D.; Chlebo, R.; Coffey, M.F.; Cornelissen, B.; da Costa, C.A.; et al. Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J. Apic. Res. 2019, 58, 479–485. [Google Scholar] [CrossRef]
- Vanengelsdorp, D.; Caron, D.; Hayes, J.; Underwood, R.; Henson, M.; Rennich, K.; Spleen, A.; Andree, M.; Snyder, R.; Lee, K.; et al. A national survey of managed honey bee 2010-11 winter colony losses in the USA: Results from the bee informed partnership. J. Apic. Res. 2012, 51, 115–124. [Google Scholar] [CrossRef]
- Kajobe, R.; Marris, G.; Budge, G.; Laurenson, L.; Cordoni, G.; Jones, B.; Wilkins, S.; Cuthbertson, A.G.S.; Brown, M.A. First molecular detection of a viral pathogen in Ugandan honey bees. J. Invertebr. Pathol. 2010, 104, 153–156. [Google Scholar] [CrossRef]
- Amakpe, F.; De Smet, L.; Brunain, M.; Ravoet, J.; Jacobs, F.J.; Reybroeck, W.; Sinsin, B.; de Graaf, D.C. Discovery of Lake Sinai virus and an unusual strain of acute bee paralysis virus in West African apiaries. Apidologie 2016, 47, 35–47. [Google Scholar] [CrossRef]
- Muli, E.; Patch, H.; Frazier, M.; Frazier, J.; Torto, B.; Baumgarten, T.; Kilonzo, J.; Kimani, J.N.; Mumoki, F.; Masiga, D.; et al. Evaluation of the distribution and impacts of parasites, pathogens, and pesticides on honey bee (Apis mellifera) populations in East Africa. PLoS ONE 2014, 9, e94459. [Google Scholar] [CrossRef]
- Mumoki, F.N.; Fombong, A.; Muli, E.; Muigai, W.T.; Masiga, D. An inventory of documented diseases of African honeybees. Afr. Entomol. 2014, 22, 473–487. [Google Scholar] [CrossRef]
- Ongus, J.R.; Fombong, A.T.; Irungu, J.; Masiga, D.; Raina, S. Prevalence of common honey bee pathogens at selected apiaries in Kenya, 2013/2014. Int. J. Trop. Insect Sci. 2018, 38, 58–70. [Google Scholar] [CrossRef]
- Strauss, U.; Dietemann, V.; Human, H.; Crewe, R.M.; Pirk, C.W.W. Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite Varroa destructor. Parasitology 2016, 143, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Begna, D.; Gela, A.; Negera, T.; Bezabeh, A. Identifying the species, effects and seasonal dynamics of honeybee varroa Page mites: A newly emerging parasite to Ethiopian honeybee. Int. J. Sci. Res. Environ. Sci. Toxicol. 2016, 2, 4. [Google Scholar]
- Gebremedhn, H.; Amssalu, B.; De Smet, L.; de Graaf, D.C. Factors restraining the population growth of Varroa destructor in Ethiopian honey bees (Apis mellifera simensis). PLoS ONE 2019, 14, e0223236. [Google Scholar] [CrossRef] [PubMed]
- Nganso, B.T.; Fombong, A.T.; Yusuf, A.A.; Pirk, C.W.W.; Stuhl, C.; Torto, B. Hygienic and grooming behaviors in African and European honeybees—New damage categories in Varroa destructor. PLoS ONE 2017, 12, e0179329. [Google Scholar] [CrossRef]
- Chemurot, M.; Akol, A.M.; Masembe, C.; de Smet, L.; Descamps, T.; de Graaf, D.C. Factors influencing the prevalence and infestation levels of Varroa destructor in honeybee colonies in two highland agro-ecological zones of Uganda. Exp. Appl. Acarol. 2016, 68, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Pirk, C.W.W.; Strauss, U.; Yusuf, A.A.; Demares, F.; Human, H. Honeybee health in Africa—A review. Apidologie 2016, 47, 276–300. [Google Scholar] [CrossRef]
- Ruttner, F.; Marx, H.; Marx, G. Observation about a Possible Adaptation of Varroa jacobsoni to Apis mellifera L in Uruguay. Apidologie 1984, 15, 43–62. [Google Scholar] [CrossRef]
- Peng, Y.S.; Fang, Y.Z.; Xu, S.Y.; Ge, L.S. The resistance mechanism of the Asian honey bee, Apis cerana Fabr, to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Invertebr. Pathol. 1987, 49, 54–60. [Google Scholar] [CrossRef]
- Boecking, O.; Spivak, M. Behavioral defenses of honey bees against I. Apidologie 1999, 30, 141–158. [Google Scholar] [CrossRef]
- Boecking, O.; Drescher, W. Response of Apis mellifera L. colonies infested with Varroa jacobsoni Oud. Apidologie 1991, 22, 237–241. [Google Scholar] [CrossRef]
- Boecking, O.; Drescher, W. The removal response of Apis mellifera L. colonies to brood in wax and plastic cells after artificial and natural infestation with Varroa jacobsoni Oud. and to freeze-killed brood. Exp. Appl. Acarol. 1992, 16, 321–329. [Google Scholar] [CrossRef]
- Ritter, W.; Dejong, D. Reproduction of Varroa jacobsoni O. in Europe, the Middle-East and Tropical South-America. Z. Angew. Entomol. J. Appl. Entomol. 1984, 98, 55–57. [Google Scholar] [CrossRef]
- Martin, S.; Holland, K.; Murray, M. Non-reproduction in the honeybee mite Varroa jacobsoni. Exp. Appl. Acarol. 1997, 21, 539–549. [Google Scholar] [CrossRef]
- Pritchard, D.J. Grooming by honey bees as a component of varroa resistant behavior. J. Apic. Res. 2016, 55, 38–48. [Google Scholar] [CrossRef]
- Kurze, C.; Routtu, J.; Moritz, R.F.A. Parasite resistance and tolerance in honeybees at the individual and social level. Zoology 2016, 119, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Harbo, J.R.; Harris, J.W. Heritability in honey bees (Hymenoptera: Apidae) of characteristics associated with resistance to Varroa jacobsoni (Mesostigmata: Varroidae). J. Econ. Entomol. 1999, 92, 261–265. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Suppressed mite reproduction explained by the behaviour of adult bees. J. Apic. Res. 2005, 44, 21–23. [Google Scholar] [CrossRef]
- Broeckx, B.J.G.; De Smet, L.; Blacquiere, T.; Maebe, K.; Khalenkow, M.; Van Poucke, M.; Dahle, B.; Neumann, P.; Nguyen, K.B.; Smagghe, G.; et al. Honey bee predisposition of resistance to ubiquitous mite infestations. Sci. Rep. 2019, 9, 7794. [Google Scholar] [CrossRef]
- Mondet, F.; Parejo, M.; Meixner, M.D.; Costa, C.; Kryger, P.; Andonov, S.; Servin, B.; Basso, B.; Bienkowska, M.; Bigio, G.; et al. Evaluation of suppressed mite reproduction (SMR) reveals potential for varroa resistance in European honey bees (Apis mellifera L.). Insects 2020, 11, 595. [Google Scholar] [CrossRef]
- Kefuss, J. Breeding Bees Tolerant to Varroa Mites. Am. Bee J. 1993, 133, 53–54. [Google Scholar]
- Harbo, J.R.; Hoopingarner, R.A. Honey bees (Hymenoptera:Apidae) in the United States that express resistance to Varroa jacobsoni (Mesostigmata:Varroidae). J. Econ. Entomol. 1997, 90, 893–898. [Google Scholar] [CrossRef]
- Buchler, R.; Berg, S.; Le Conte, Y. Breeding for resistance to Varroa destructor in Europe. Apidologie 2010, 41, 393–408. [Google Scholar] [CrossRef]
- DeJong, D.; Soares, A.E.E. An isolated population of Italian bees that has survived Varroa jacobsoni infestation without treatment for over 12 years. Am. Bee J. 1997, 137, 742–745. [Google Scholar]
- Fries, I.; Bommarco, R. Possible host-parasite adaptations in honey bees infested by Varroa destructor mites. Apidologie 2007, 38, 525–533. [Google Scholar] [CrossRef]
- Le Conte, Y.; De Vaublanc, G.; Crauser, D.; Jeanne, F.; Rousselle, J.C.; Becard, J.M. Honey bee colonies that have survived Varroa destructor. Apidologie 2007, 38, 566–572. [Google Scholar] [CrossRef]
- Seeley, T.D. Honey bees of the Arnot Forest: A population of feral colonies persisting with Varroa destructor in the northeastern United States. Apidologie 2007, 38, 19–29. [Google Scholar] [CrossRef]
- Kruitwagen, A.; van Langevelde, F.; van Dooremalen, C.; Blacquiere, T. Naturally selected honey bee (Apis mellifera) colonies resistant to Varroa destructor do not groom more intensively. J. Apic. Res. 2017, 56, 354–365. [Google Scholar] [CrossRef]
- Neumann, P.; Blacquiere, T. The Darwin cure for apiculture? Natural selection and managed honeybee health. Evol. Appl. 2017, 10, 226–230. [Google Scholar] [CrossRef]
- Oddie, M.A.Y.; Dahle, B.; Neumann, P. Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ 2017, 5, e3956. [Google Scholar] [CrossRef]
- Mondet, F.; Alaux, C.; Severac, D.; Rohmer, M.; Mercer, A.R.; Le Conte, Y. Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci. Rep. 2015, 5, 10454. [Google Scholar] [CrossRef]
- Martin, C.; Provost, E.; Bagneres, A.G.; Roux, M.; Clement, J.L.; Le Conte, Y. Potential-mechanism for detection by Apis mellifera of the parasitic mite Varroa destructor inside sealed brood cells. Physiol. Entomol. 2002, 27, 175–188. [Google Scholar] [CrossRef]
- Gramacho, K.P.; Spivak, M. Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav. Ecol. Sociobiol. 2003, 54, 472–479. [Google Scholar] [CrossRef]
- Navajas, M.; Migeon, A.; Alaux, C.; Martin-Magniette, M.L.; Robinson, G.E.; Evans, J.D.; Cros-Arteil, S.; Crauser, D.; Le Conte, Y. Differential gene expression of the honey bee Apis mellifera associated with Varroa destructor infection. BMC Genom. 2008, 9, 301. [Google Scholar] [CrossRef]
- Parker, R.; Guarna, M.M.; Melathopoulos, A.P.; Moon, K.M.; White, R.; Huxter, E.; Pernal, S.F.; Foster, L.J. Correlation of proteome-wide changes with social immunity behaviors provides insight into resistance to the parasitic mite, Varroa destructor, in the honey bee (Apis mellifera). Genome Biol. 2012, 13, R81. [Google Scholar] [CrossRef] [PubMed]
- Guarna, M.M.; Melathopoulos, A.P.; Huxter, E.; Iovinella, I.; Parker, R.; Stoynov, N.; Tam, A.; Moon, K.M.; Chan, Q.W.T.; Pelosi, P.; et al. A search for protein biomarkers links olfactory signal transduction to social immunity. BMC Genom. 2015, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, J.M.; Harris, J.W.; Bourgeois, L.; Danka, R.G.; Hunt, G.J. High-resolution linkage analyses to identify genes that influence varroa sensitive hygiene behavior in honey bees. PLoS ONE 2012, 7, e48276. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.L.; Rinderer, T.E.; de Guzman, L.I.; Holloway, B. Molecular genetic analysis of Varroa destructor mites in brood, fallen injured mites, and worker bee longevity in honey bees. J. Apic. Res. 2016, 54, 328–334. [Google Scholar] [CrossRef]
- Spotter, A.; Gupta, P.; Mayer, M.; Reinsch, N.; Bienefeld, K. Genome-wide association study of a varroa-specific defense behavior in honeybees (Apis mellifera). J. Hered. 2016, 107, 220–227. [Google Scholar] [CrossRef]
- Conlon, B.H.; Frey, E.; Rosenkranz, P.; Locke, B.; Moritz, R.F.A.; Routtu, J. The role of epistatic interactions underpinning resistance to parasitic Varroa mites in haploid honey bee (Apis mellifera) drones. J. Evol. Biol. 2018, 31, 801–809. [Google Scholar] [CrossRef]
- Foret, S.; Maleszka, R. Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res. 2006, 16, 1404–1413. [Google Scholar] [CrossRef]
- Oxley, P.R.; Spivak, M.; Oldroyd, B.P. Six quantitative trait loci influence task thresholds for hygienic behaviour in honeybees (Apis mellifera). Mol. Ecol. 2010, 19, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Robertson, T.; Mostajeran, M.; Robertson, A.J.; Qiu, X. Differential gene expression of two extreme honey bee (Apis mellifera) colonies showing varroa tolerance and susceptibility. Insect Mol. Biol. 2016, 25, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Guarna, M.M.; Hoover, S.E.; Huxter, E.; Higo, H.; Moon, K.M.; Domanski, D.; Bixby, M.E.F.; Melathopoulos, A.P.; Ibrahim, A.; Peirson, M.; et al. Peptide biomarkers used for the selective breeding of a complex polygenic trait in honey bees. Sci. Rep. 2017, 7, 8381. [Google Scholar] [CrossRef]
- Silva, D.; Ceballos, R.; Arismendi, N.; Dalmon, A.; Vargas, M. Variant A of the deformed wings virus alters the olfactory sensitivity and the expression of odorant binding proteins on antennas of Apis mellifera. Insects 2021, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, D.C.; Laget, D.; De Smet, L.; Bouuaert, D.C.; Brunain, M.; Veerkamp, R.F.; Brascamp, E.W. Heritability estimates of the novel trait ‘suppressed in ovo virus infection’ in honey bees (Apis mellifera). Sci. Rep. 2020, 10, 14310. [Google Scholar] [CrossRef]
- Raberg, L.; Graham, A.L.; Read, A.F. Decomposing health: Tolerance and resistance to parasites in animals. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 37–49. [Google Scholar] [CrossRef]
- Thaduri, S.; Stephan, J.G.; de Miranda, J.R.; Locke, B. Disentangling host-parasite-pathogen interactions in a varroa-resistant honeybee population reveals virus tolerance as an independent, naturally adapted survival mechanism. Sci. Rep. 2019, 9, 6221. [Google Scholar] [CrossRef] [PubMed]
- Locke, B.; Forsgren, E.; de Miranda, J.R. Increased tolerance and resistance to virus infections: A possible factor in the survival of Varroa destructor resistant honey bees (Apis mellifera). PLoS ONE 2014, 9, e99998. [Google Scholar] [CrossRef]
- Gebremedhn, H.; Deboutte, W.; Schoonvaere, K.; Demaeght, P.; De Smet, L.; Amssalu, B.; Matthijnssens, J.; de Graaf, D.C. Metagenomic approach with the NetoVIR enrichment protocol reveals virus diversity within Ethiopian honey bees (Apis mellifera simensis). Viruses 2020, 12, 1218. [Google Scholar] [CrossRef]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global honey bee viral landscape altered by a parasitic mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Wilfert, L.; Martin, S.J.; Jones, I.M.; Schroeder, D.C. Diversity in a honey bee pathogen: First report of a third master variant of the Deformed Wing Virus quasispecies. Isme J. 2016, 10, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Kevill, J.L.; de Souza, F.S.; Sharples, C.; Oliver, R.; Schroeder, D.C.; Martin, S.J. DWV-A Lethal to honey bees (Apis mellifera): A colony level survey of DWV Variants (A, B, and C) in England, Wales, and 32 States across the US. Viruses 2019, 11, 426. [Google Scholar] [CrossRef]
- Beaurepaire, A.; Piot, N.; Doublet, V.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; Panziera, D.; et al. Diversity and global distribution of viruses of the Western honey bee, Apis mellifera. Insects 2020, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.P.; Natsopoulou, M.E.; Doublet, V.; Furst, M.; Weging, S.; Brown, M.J.F.; Gogol-Doring, A.; Paxton, R.J. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160811. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Childers, A.K.; Lopez, D.; Grubbs, K.; Posada-Florez, F.; Weaver, D.; Girten, W.; vanEngelsdorp, D.; Chen, Y.P.; Evans, J.D. Dynamic evolution in the key honey bee pathogen deformed wing virus: Novel insights into virulence and competition using reverse genetics. PLoS Biol. 2019, 17, e3000502. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.W. RNA-based antiviral immunity. Nat. Rev. Immunol. 2010, 10, 632–644. [Google Scholar] [CrossRef]
- Santos, D.; Wynant, N.; Van den Brande, S.; Verdonckt, T.W.; Mingels, L.; Peeters, P.; Kolliopoulou, A.; Swevers, L.; Broeck, J.V. Insights into RNAi-based antiviral immunity in Lepidoptera: Acute and persistent infections in Bombyx mori and Trichoplusia ni cell lines. Sci. Rep. 2018, 8, 2423. [Google Scholar] [CrossRef]
- De Smet, L.; Ravoet, J.; Wenseleers, T.; De Graaf, D.C. Expression of key components of the RNAi machinery are suppressed in Apis mellifera that suffer a high virus infection. Entomol. Sci. 2017, 20, 76–85. [Google Scholar] [CrossRef]
- Hamiduzzaman, M.M.; Emsen, B.; Hunt, G.J.; Subramanyam, S.; Williams, C.E.; Tsuruda, J.M.; Guzman-Novoa, E. Differential gene expression associated with honey bee grooming behavior in response to varroa mites. Behav. Genet. 2017, 47, 335–344. [Google Scholar] [CrossRef]
- Scharlaken, B.; de Graaf, D.C.; Goossens, K.; Brunain, M.; Peelman, L.J.; Jacobs, F.J. Reference gene selection for insect expression studies using quantitative real-time PCR: The head of the honeybee, Apis mellifera, after a bacterial challenge. J. Insect Sci. 2008, 8, 33. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Zheng, H.Q.; Corona, M.; Pirk, C.; Meng, F.; Zheng, Y.F.; Hu, F.L. Comparative transcriptome analysis on the synthesis pathway of honey bee (Apis mellifera) mandibular gland secretions. Sci. Rep. 2017, 7, 4530. [Google Scholar] [CrossRef]
- Jeon, J.H.; Moon, K.; Kim, Y.; Kim, Y.H. Reference gene selection for qRT-PCR analysis of season- and tissue-specific gene expression profiles in the honey bee Apis mellifera. Sci. Rep. 2020, 10, 13935. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, E.; Anfora, G.; Trona, F.; Tessarolo, F.; Vallortigara, G. Morpho-functional asymmetry of the olfactory receptors of the honeybee (Apis mellifera). Behav. Brain Res. 2010, 209, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Fialho, M.D.Q.; Guss-Matiello, C.P.; Zanuncio, J.C.; Campos, L.A.O.; Serrao, J.E. A comparative study of the antennal sensilla in corbiculate bees. J. Apic. Res. 2014, 53, 392–403. [Google Scholar] [CrossRef]
- Jung, J.W.; Park, K.W.; Oh, H.W.; Kwon, H.W. Structural and functional differences in the antennal olfactory system of worker honey bees of Apis mellifera and Apis cerana. J. AsiaPac. Entomol. 2014, 17, 639–646. [Google Scholar] [CrossRef]
- Facchini, E.; Dell’Orco, F.; Morarino, M.; Rizzi, R. Expression monitoring of relevant sensitivity genes in honey bee antennae and their relationship with hygienic behavior. In Proceedings of the Veterinary and Animal Science Days, Milan, Italy, 6–8 June 2017. [Google Scholar]
- Hu, H.; Bienefeld, K.; Wegener, J.; Zautke, F.; Hao, Y.; Feng, M.; Han, B.; Fang, Y.; Wubie, A.J.; Li, J.K. Proteome analysis of the hemolymph, mushroom body, and antenna provides novel insight into honeybee resistance against varroa infestation. J. Proteome Res. 2016, 15, 2841–2854. [Google Scholar] [CrossRef]
- Xie, X.B.; Huang, Z.Y.; Zeng, Z.J. Why do Varroa mites prefer nurse bees? Sci. Rep. 2016, 6, 28228. [Google Scholar] [CrossRef]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef]
- Amdam, G.V.; Fennern, F.; Havukainen, H. Vitellogenin in Honey Bee Behavior and Lifespan; Galizia, C.G., Eisenhardt, D., Giurfa, M., Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Hartfelder, K.; Engels, W. Social insect polymorphism: Hormonal regulation of plasticity in development and reproduction in the honeybee. Curr. Top. Dev. Biol. 1998, 40, 45–77. [Google Scholar] [CrossRef]
- Alaux, C.; Dantec, C.; Parrinello, H.; Le Conte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genom. 2011, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef]
- Smart, M.; Pettis, J.; Rice, N.; Browning, Z.; Spivak, M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS ONE 2016, 11, e0152685. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Schoning, C.; Gisder, S.; Geiselhardt, S.; Kretschmann, I.; Bienefeld, K.; Hilker, M.; Genersch, E. Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. J. Exp. Biol. 2012, 215, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Gramacho, K.P.; Goncalves, L.S. Comparative study of the hygienic behavior of Carniolan and Africanized honey bees directed towards grouped versus isolated dead brood cells. Genet. Mol. Res. 2009, 8, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, D.A.; Yang, X.Y.; Nino, E.L.; Yi, S.; Grozinger, C. Parallel epigenomic and transcriptomic responses to viral infection in honey bees (Apis mellifera). PLoS Pathog. 2015, 11, e1004713. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Jia, Q.D.; Li, F.; Han, Z.J. Identification of two piwi genes and their expression profile in honeybee, Apis mellifera. Arch. Insect. Biochem. 2010, 74, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Hill, K.; Hogendoorn, K.; Glatz, R.V.; Napier, K.R.; Bellgard, M.I.; Barrero, R.A. De novo assembly of honey bee RNA viral genomes by tapping into the innate insect antiviral response pathway. J. Invertebr. Pathol. 2018, 152, 38–47. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.D.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor mediated, or in vitro, transmission. PLoS Pathog. 2014, 10, e1004230. [Google Scholar] [CrossRef]
- Kim, S.H.; Mercer, A.; Mitchell, A.; de Miranda, J.R.; Ward, V.; Mondet, F.; Bostina, M. Viral infections alter antennal epithelium ultrastructure in honey bees. J. Invertebr. Pathol. 2019, 168, 107252. [Google Scholar] [CrossRef]
- Kim, S.H. Factors Influencing Varroa Sensitive Hygiene in European Honey Bees (Apis mellifera). Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2018. [Google Scholar]
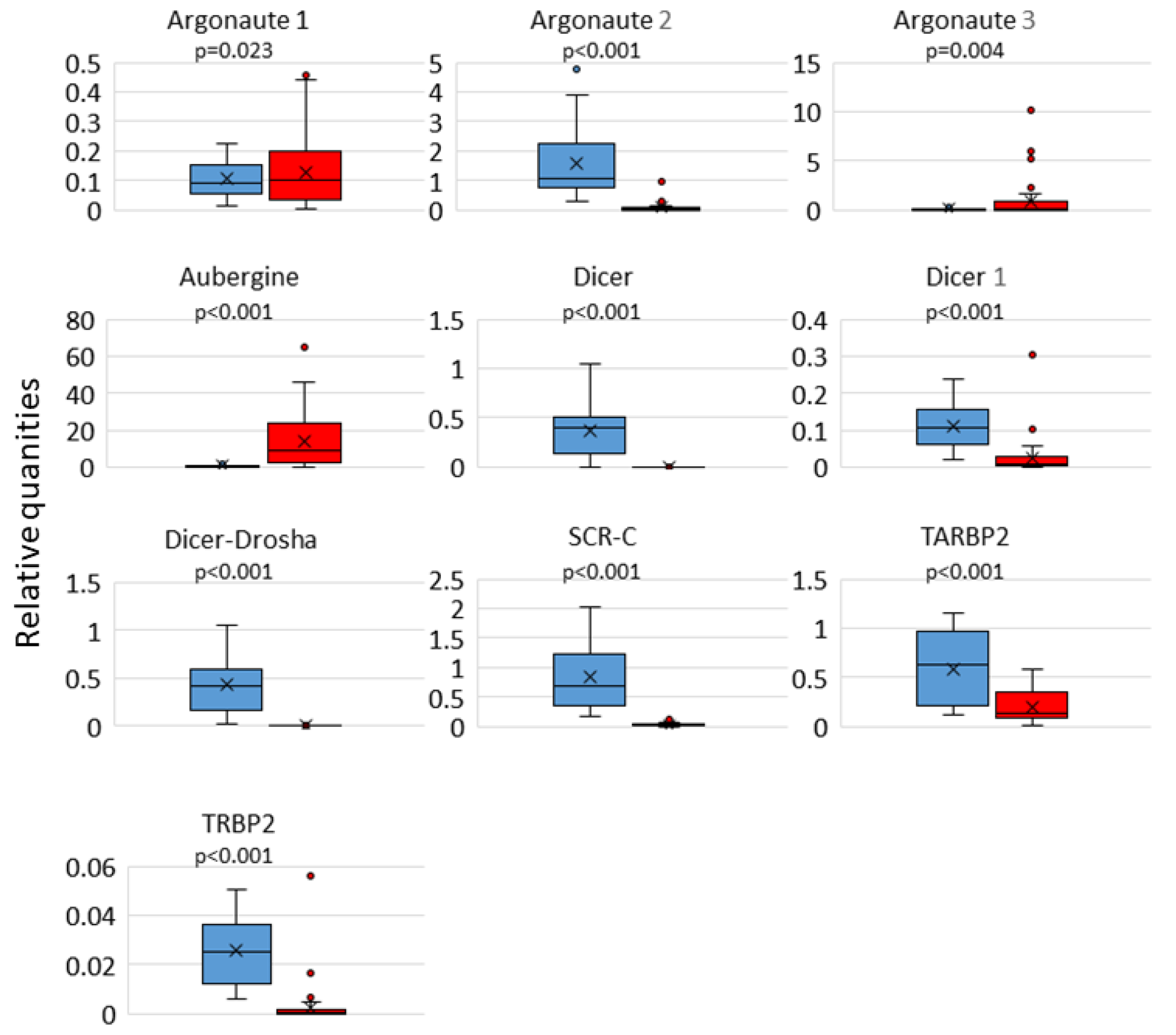

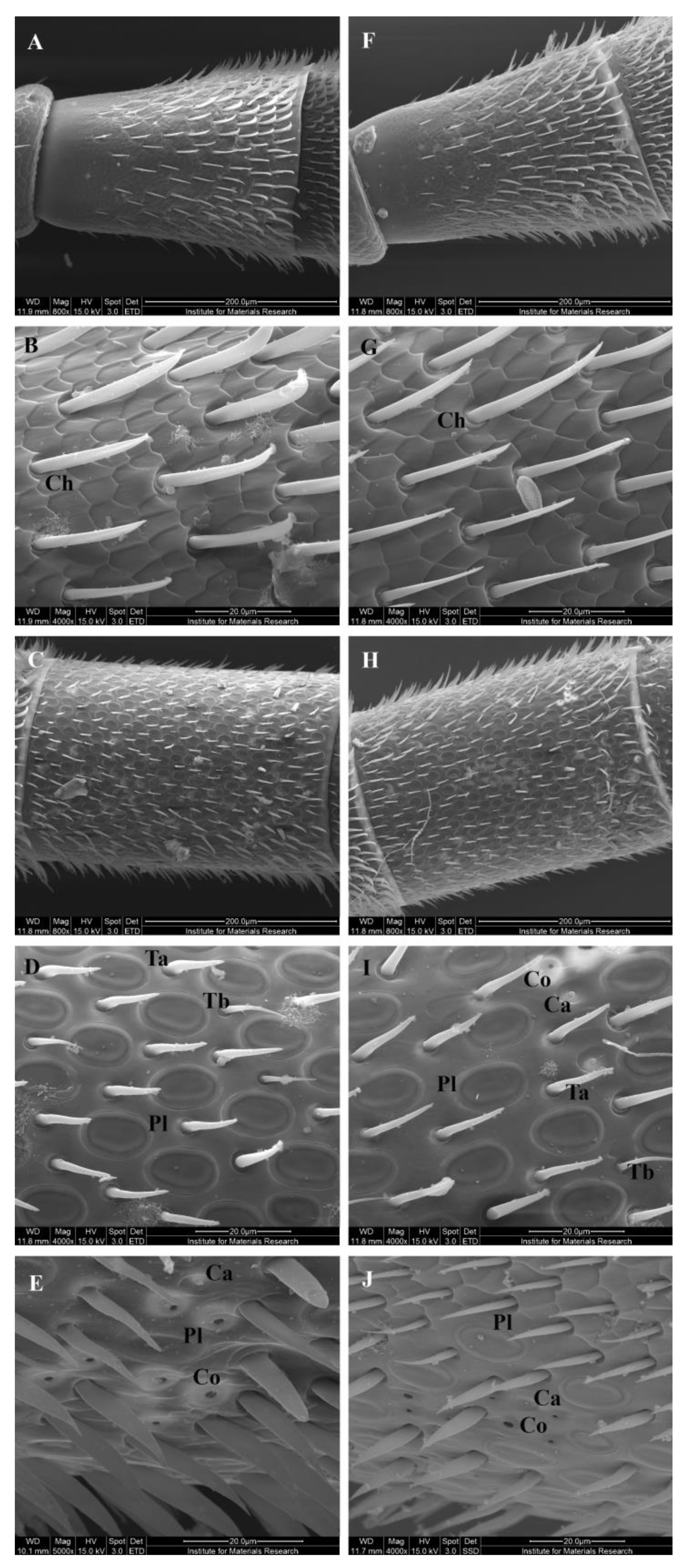
| Target | p-Value | f.c. |
|---|---|---|
| OBP14 | 0.0198 | 16.144 |
| Vg | 0.201 | 20.384 |
| GB43812 | 0.201 | 2.436 |
| OBP16 | 0.214 | 0.3 |
| Dynein | 0.214 | 7.61 |
| OBP18 | 0.250 | 2.11 |
| OBP3 | 0.260 | 5.521 |
| Emp24 | 0.563 | 0.096 |
| Cop-gamma | 0.563 | 0.826 |
| OBP1 | 0.563 | 1.274 |
| Target | DWV Load | Mean | N | Ratio (High/Low) | 95% Value ci Low | 95% Value ci High | p-Value |
|---|---|---|---|---|---|---|---|
| Dicer-Drosha | High | 1.863 | 9 | 3.101 | 1.408 | 6.827 | 0.034 |
| Low | 0.601 | 11 | |||||
| TRBP2 | High | 1.453 | 9 | 1.973 | 1.212 | 3.212 | 0.034 |
| Low | 0.737 | 11 | |||||
| Argonaute 2 | High | 1.599 | 9 | 2.348 | 1.258 | 4.381 | 0.034 |
| Low | 0.681 | 11 | |||||
| SRC-C | High | 1.406 | 9 | 1.858 | 1.046 | 3.299 | 0.074 |
| Low | 0.757 | 11 | |||||
| Argonaute 3 | High | 1.479 | 9 | 2.038 | 1.033 | 4.021 | 0.074 |
| Low | 0.726 | 11 | |||||
| Aubergine | High | 1.465 | 9 | 2.002 | 1.1020 | 3.929 | 0.074 |
| Low | 0.732 | 11 | |||||
| Dicer 1 | High | 1.349 | 9 | 1.725 | 0.951 | 3.129 | 0.093 |
| Low | 0.783 | 11 | |||||
| Dicer | High | 2.101 | 9 | 3.857 | 0.857 | 17.355 | 0.093 |
| Low | 0.545 | 11 | |||||
| Tarbp2 | High | 1.304 | 9 | 1.62 | 0.846 | 3.105 | 0.144 |
| Low | 0.805 | 11 | |||||
| Argonaute 1 | High | 1.262 | 9 | 1.527 | 0.852 | 2.735 | 0.144 |
| Low | 0.827 | 12 |
| Argonaute1 | Argonaute2 | Argonaute3 | Aubergine | Dicer | Dicer1 | Dicer-Drosha | SRCC | Tarbp2 | TRBP2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Argonaute1 | 1 | |||||||||
| Argonaute2 | 0.095 | 1 | ||||||||
| Argonaute3 | 0.617 ** | 0.193 | 1 | |||||||
| Aubergine | 0.6328 * | 0.212 | 0.919 ** | 1 | ||||||
| Dicer | 0.485 * | 0.595 ** | 0.293 | 0.243 | 1 | |||||
| Dicer1 | 0.728 ** | 0.103 | 0.657 ** | 0.678 ** | 0.422 | 1 | ||||
| Dicer-Drosha | 0.536 * | 0.387 | 0.002 | 0.114 | 0.471 * | 0.411 | 1 | |||
| SRCC | 0.489 * | 0.583 ** | 0.505 * | 0.518 * | 0.611 * | 0.602 * | 0.607 ** | 1 | ||
| Tarbp2 | 0.812 ** | 0.338 | 0.601 ** | 0.616 ** | 0.481 * | 0.700 ** | 0.627 ** | 0.696 ** | 1 | |
| TRBP2 | 0.345 | 0.614 ** | 0.106 | 0.193 | 0.431 | 0.160 | 0.352 | 0.229 | 0.300 | 1 |
| Argonaute1 | Argonaute2 | Argonaute3 | Aubergine | Dicer | Dicer1 | Dicer-Drosha | SRCC | Tarbp2 | TRBP2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Argonaute1 | 1.00 | |||||||||
| Argonaute2 | −0.588 ** | 1.00 | ||||||||
| Argonaute3 | −0.240 | 0.798 ** | 1.00 | |||||||
| Aubergine | 0.573 ** | −0.231 | 0.224 | 1.00 | ||||||
| Dicer | −0.449 ** | 0.602 ** | 0.568 ** | 0.119 | 1.00 | |||||
| Dicer1 | −0.499 ** | 0.195 | 0.026 | −0.397 ** | 0.070 | 1.00 | ||||
| Dicer- Drosha | −0.584 ** | 0.734 ** | 0.496 ** | −0.307 * | 0.656 ** | 0.249 | 1.00 | |||
| SRCC | 0.132 | 0.447 * | 0.743 ** | 0.511 ** | 0.372 * | −0.180 | 0.136 | 1.00 | ||
| Tarbp2 | 0.749 ** | −0.506 ** | −0.043 | 0.747 * | −0.007 | −0.462 ** | −0.437 ** | 0.259 | 1.00 | |
| TRBP2 | −0.543 ** | 0.614 ** | 0.187 | −0.608 ** | 0.224 | 0.307 * | 0.629 ** | −0.213 | −0.754 ** | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebremedhn, H.; Claeys Bouuaert, D.; Asperges, M.; Amssalu, B.; De Smet, L.; de Graaf, D.C. Expression of Molecular Markers of Resilience against Varroa destructor and Bee Viruses in Ethiopian Honey Bees (Apis mellifera simensis) Focussing on Olfactory Sensing and the RNA Interference Machinery. Insects 2023, 14, 436. https://doi.org/10.3390/insects14050436
Gebremedhn H, Claeys Bouuaert D, Asperges M, Amssalu B, De Smet L, de Graaf DC. Expression of Molecular Markers of Resilience against Varroa destructor and Bee Viruses in Ethiopian Honey Bees (Apis mellifera simensis) Focussing on Olfactory Sensing and the RNA Interference Machinery. Insects. 2023; 14(5):436. https://doi.org/10.3390/insects14050436
Chicago/Turabian StyleGebremedhn, Haftom, David Claeys Bouuaert, Michel Asperges, Bezabeh Amssalu, Lina De Smet, and Dirk C. de Graaf. 2023. "Expression of Molecular Markers of Resilience against Varroa destructor and Bee Viruses in Ethiopian Honey Bees (Apis mellifera simensis) Focussing on Olfactory Sensing and the RNA Interference Machinery" Insects 14, no. 5: 436. https://doi.org/10.3390/insects14050436
APA StyleGebremedhn, H., Claeys Bouuaert, D., Asperges, M., Amssalu, B., De Smet, L., & de Graaf, D. C. (2023). Expression of Molecular Markers of Resilience against Varroa destructor and Bee Viruses in Ethiopian Honey Bees (Apis mellifera simensis) Focussing on Olfactory Sensing and the RNA Interference Machinery. Insects, 14(5), 436. https://doi.org/10.3390/insects14050436







