Parasitism Features of a Fig Wasp of Genus Apocrypta (Pteromalidae: Pteromalinae) Associated with a Host Belonging to Ficus Subgenus Ficus
Abstract
Simple Summary
Abstract
1. Introduction
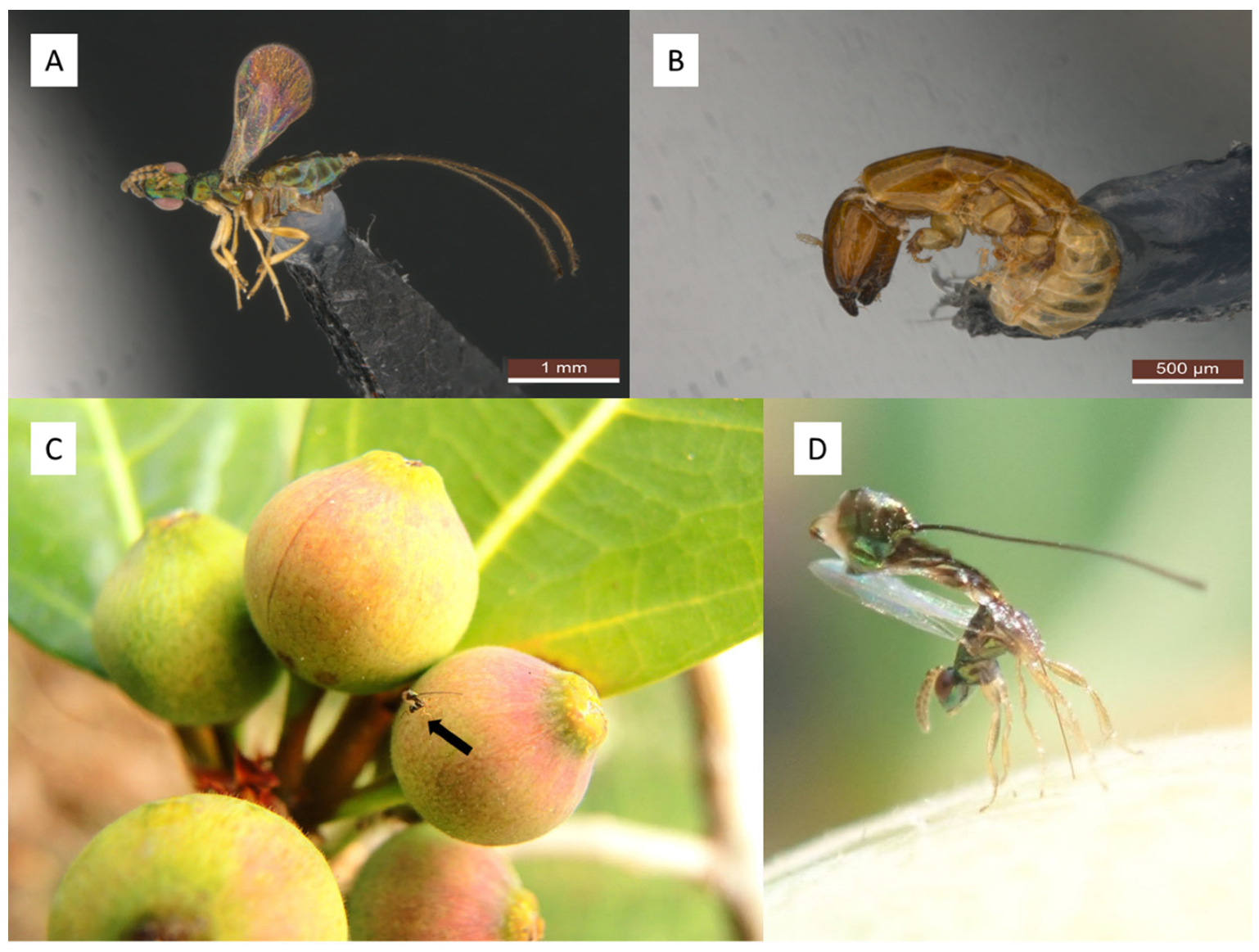
2. Materials and Methods
- -
- A phase denotes the initial period before the female florets are mature.
- -
- B phase is the short period when the female florets are mature, prompting the pollinator to enter the syconium to lay eggs and pollinate.
- -
- C phase is the growth period of the seeds or galls.
- -
- D phase denotes the period when the male florets are mature (only on syconia from male trees), at which time the wasp larvae hatch and leave the syconia.
- -
- E phase (only on female trees) is the period when the seeds become mature and attract frugivores.
3. Results
4. Discussion
- (1)
- Oviposition strategy: Apocrypta wasps have been shown to be synovigenic, meaning that they produce and mature eggs continuously over time [64]. This suggests that, no matter how strong the parasitism ability of “Apocrypta pedunculosa” is, it cannot overwhelm a single fig with its offspring at any one time. This concept was also directly supported by the two studies which confirmed that Sycoscapter wasps, closely related to Apocrypta wasps, usually lay only a few eggs in a single fig [65,66]. These physiological and behavioral adaptions are believed to guarantee that parasitoid NPFWs would never overexploit pollinators, as only male pollinators can liberate all the wasps inside by making holes in the enclosed inflorescence [8]. If NPFWs were too abundant in a fig, they would probably kill off all the males, resulting in all the wasps dying inside their natal fig and thus being counter-selected.
- (2)
- Severe habitat: Although Apocrypta wasps are confirmed to live long, surviving for up to 60 days when feeding in the laboratory [22,64], we inferred that “Apocrypta pedunculosa” would not live for such a long period in the field due to the severe climate conditions, such as high temperature and irradiance, strong seasonal monsoon winds, and sporadic typhoons [44,67]. These climate conditions detrimentally affect not only the fig wasps but also the fig trees [67,68,69]. Therefore, even if the F. pedunculosa var. mearnsii population could supply sufficient resources through continuous fig production and the formation of dense aggregations which might enable “Apocrypta pedunculosa” to display high searching efficacy, this wasp would still periodically encounter hazardous weather conditions, which would reduce its parasitism rate among male figs [44,70]. This implies that the studied wasp might have a long handling time with decreased efficiency in this hazardous habitat. Ants are also known to disturb the NPFW oviposition, and an ant species was observed in F. pedubculosa var. mearnsii figs [71]. However, we believe that this ant would not exert sufficient pressure to cause this parasitism phenomenon, so hazardous climate conditions may be the main influencing factor in this study.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wootton, J.T.; Emmerson, M. Measurement of interaction strength in nature. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 419–444. [Google Scholar] [CrossRef]
- Landi, P.; Minoarivelo, H.O.; Brännström, A.; Hui, C.; Dieckmann, U.J.P.E. Complexity and stability of ecological networks: A review of the theory. Popul. Ecol. 2018, 60, 319–345. [Google Scholar] [CrossRef]
- Haverkamp, A.; Smid, H.M. A neuronal arms race: The role of learning in parasitoid–host interactions. Curr. Opin. Insect Sci. 2020, 42, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F. Parsimony Analysis of coevolving species associations. In Tangled Trees: Phylogeny, Cospeciation, and Coevolution; University of Chicago Press: Chicago, IL, USA, 2002; pp. 22–64. [Google Scholar]
- Araujo, S.B.L.; Braga, M.P.; Brooks, D.R.; Agosta, S.J.; Hoberg, E.P.; von Hartenthal, F.W.; Boeger, W.A. Understanding host-switching by ecological fitting. PLoS ONE 2015, 10, e0139225. [Google Scholar] [CrossRef]
- Samková, A.; Raška, J.; Hadrava, J.; Skuhrovec, J. Effect of host switching simulation on the fitness of the gregarious parasitoid anaphes flavipes from a novel two-generation approach. Sci. Rep. 2021, 11, 19473. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.C.; Corner, E.J.H. Flora Malesiana Series I (Seed Plants); National Herbarium Nederkand: Leiden, The Netherlands, 2005; Volume 17, pp. 1–727. [Google Scholar]
- Weiblen, G.D. How to be a fig wasp. Annu. Rev. Entomol. 2002, 47, 299–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Zhang, S.; Chen, S.; Wang, Y.; Wen, P.; Ma, X.; Shi, Y.; Qi, R.; Yang, Y.; et al. Genomes of the banyan tree and pollinator wasp provide insights into fig-wasp coevolution. Cell 2020, 183, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, Y.; Jing, Y.; Segar, S.T.; Zhang, Y.; Wang, G.; Chen, J.; Liu, Q.F.; Chen, S.; Chen, Y.; et al. Molecular mechanisms of mutualistic and antagonistic interactions in a plant–pollinator association. Nat. Ecol. Evol. 2021, 5, 974–986. [Google Scholar] [CrossRef]
- Rodriguez, L.J.; Bain, A.; Chou, L.S.; Conchou, L.; Cruaud, A.; Gonzales, R.; Hossaert-McKey, M.; Rasplus, J.Y.; Tzeng, H.Y.; Kjellberg, F. Diversification and spatial structuring in the mutualism between Ficus septica and its pollinating wasps in insular south east Asia. BMC Evol. Biol. 2017, 17, 207. [Google Scholar] [CrossRef]
- Yu, H.; Tian, E.; Zheng, L.; Deng, X.; Cheng, Y.; Chen, L.; Wu, W.; Tanming, W.; Zhang, D.; Compton, S.G.; et al. Multiple parapatric pollinators have radiated across a continental fig tree displaying clinal genetic variation. Mol. Ecol. 2019, 28, 2391–2405. [Google Scholar] [CrossRef]
- Xie, H.; Yang, P.; Xia, Y.; Kjellberg, F.; Darwell, C.T.; Li, Z. Maintenance of specificity in sympatric host-specific fig/wasp pollination mutualisms. Peer J. 2022, 10, e13897. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.H.; Sasaki, A.; Kusumi, J.; Chou, P.A.; Tzeng, H.Y.; Li, H.Q.; Yu, H. Pollinator sharing, copollination, and speciation by host shifting among six closely related dioecious fig species. Commun. Biol. 2022, 5, 284. [Google Scholar] [CrossRef] [PubMed]
- Cruaud, A.; Jabbour-Zahab, R.; Genson, G.; Kjellberg, F.; Kobmoo, N.; van Noort, S.; Yang, D.R.; Peng, Y.Q.; Ubaidillah, R.; Hanson, P.E.; et al. Phylogeny and evolution of life-history strategies in the Sycophaginae non-pollinating fig wasps (Hymenoptera, Chalcidoidea). BMC Evol. Biol. 2011, 11, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.M. How to be a fig wasp parasite on the fig-fig wasp mutualism. Curr. Opin. Insect Sci. 2015, 8, 34–40. [Google Scholar] [CrossRef]
- Kerdelhué, C.; Rasplus, J.Y. Non-pollinating Afrotropical fig wasps affect the fig-pollinator mutualism in Ficus within the subgenus Sycomorus. Oikos 1996, 75, 3–14. [Google Scholar] [CrossRef]
- Kerdelhué, C.; Rossi, J.P.; Rasplus, J.Y. Comparative community ecology studies on Old World figs and fig wasps. Ecology 2000, 81, 2832–2849. [Google Scholar] [CrossRef]
- Tzeng, H.Y.; Tseng, L.J.; Ou, C.H.; Lu, K.C.; Lu, F.Y.; Chou, L.S. Confirmation of the parasitoid feeding habit in Sycoscapter, and their impact on pollinator abundance in Ficus formosana. Symbiosis 2008, 45, 129–134. [Google Scholar]
- Pereira, R.A.S.; Teixeira, S.D.P.; Kjellberg, F. An inquiline fig wasp using seeds as a resource for small male rroduction: A potential first step for the evolution of new feeding habits? Biol. J. Linn. Soc. 2007, 92, 9–17. [Google Scholar] [CrossRef]
- Wang, R.; Matthews, A.; Ratcliffe, J.; Berwell, L.J.; Peng, Y.Q.; Chou, L.S.; Yu, H.; Yang, H.W.; Compton, S.G. First Record of an apparently rare fig wasp feeding strategy: Obligate seed predation. Ecol. Entomol. 2014, 39, 492–500. [Google Scholar] [CrossRef]
- Compton, S.G.; Rasplus, J.Y.; Ware, A.B. African fig wasp parasitoid communities. In Parasitoid Community Ecology; Oxford University Press: Oxford, UK, 1994; pp. 346–368. [Google Scholar]
- Yadav, P.; Borges, R.M. Host-parasitoid development and survival strategies in a non-pollinating fig wasp community. Acta Oecologica 2018, 90, 60–68. [Google Scholar] [CrossRef]
- Jousselin, E.; van Noort, S.; Berry, V.; Rasplus, J.Y.; Rønsted, N.; Erasmus, J.C.; Greeff, J.M. One fig to bind them all: Host conservatism in a fig wasp community unraveled by cospeciation analyses among pollinating and nonpollinating fig wasps. Evolution 2008, 62, 1777–1797. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Peng, Y.Q.; Harder, L.D.; Huang, J.F.; Yang, D.R.; Zhang, D.Y.; Liao, W.J. The nature of interspecific interactions and co-diversification patterns, as illustrated by the fig microcosm. New Phytol. 2019, 224, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Weiblen, G.D. Correlated evolution in fig pollination. Syst. Biol. 2004, 53, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.Y.; Ou, C.H.; Lu, F.Y.; Bain, A.; Chou, L.S.; Kjellberg, F. The effect of fig wall thickness in Ficus erecta var. beecheyana on parasitism. Acta Oecologica 2014, 57, 38–43. [Google Scholar] [CrossRef]
- Silvieus, S.I.; Clement, W.L.; Weiblen, G.D. Cophylogeny of figs, polliantors, gallers and parasitoids. In Specialization, Speciation and Radiation: The Evolutionary Biology of Herbivorous Insects; University of California Press: Berkeley, CA, USA, 2008; pp. 225–239. [Google Scholar]
- McLeish, M.J.; van Noort, S.; Tolley, K.A. African parasitoid fig wasp diversification is a function of Ficus species ranges. Mol. Phylogenetics Evol. 2010, 57, 122–134. [Google Scholar] [CrossRef]
- McLeish, M.J.; Beukman, G.; van Noort, S.; Wossler, T.C. Host-plant species conservatism and ecology of a parasitoid fig Wasp genus (Chalcidoidea; Sycoryctinae; Arachonia). PLoS ONE 2010, 7, 44804. [Google Scholar] [CrossRef] [PubMed]
- Ulenberg, S.A.; van Pelt, W. A revision of the species of Apocrypta Coquerel: A taxonomic study incorporating uni- and multivariate analyses. Verh. K. Ned. Akad. Wet. Afd. Nat. 1985, 83, 41–148. [Google Scholar]
- Burks, R.; Mitroiu, M.D.; Fusu, L.; Heraty, J.M.; Janšta, P.; Heydon, S.; Papilloud, N.D.S.; Peters, R.S.; Tselikh, E.V.; Woolley, J.B.; et al. From Hell’s heart I stab at thee! A determined approach to rendering Pteromalidae (Hymenoptera) monophyletic. J. Hymenopt. Res. 2022, 94, 13–88. [Google Scholar] [CrossRef]
- Segar, S.T.; Lopez-Vaamonde, C.; Rasplus, J.Y.; Cook, J.M. The Global Phylogeny of the Subfamily Sycoryctinae (Pteromalidae): Parasites of an Obligate Mutualism. Mol. Phylogenetics Evol. 2012, 65, 116–125. [Google Scholar] [CrossRef]
- Tzeng, H.Y. Taxonomic Study of the Genus Ficus in Taiwan. Ph.D. Thesis, Department of Forestry, National Chung-Hsing University, Taichung, Taiwan, 2004; 396p. (In Chinese with English summary). [Google Scholar]
- Lu, Z.L.; Zhang, Z.; Zhou, Q.M.; Lu, J.; Tian, H.; Li, H.Q. Molecular analyses of Ficus erecta and its allies within the subsection Frutescentiae (Moraceae). Plant Syst. Evol. 2017, 303, 603–614. [Google Scholar] [CrossRef]
- Bain, A.; Tzeng, H.Y.; Wu, W.J.; Chou, L.S. Ficus (Moraceae) and fig wasps (Hymenoptera: Chalcidoidea) in Taiwan. Bot. Stud. 2015, 56, 11–23. [Google Scholar] [CrossRef]
- Chen, C.H.; Chou, L.Y. The Blastophagini of Taiwan (Hymenoptera: Agaonidae: Agaoninae). J. Taiwan Mus. 1997, 50, 113–154. [Google Scholar]
- Weiblen, G.; Flick, B.; Spencer, H. Seed set and wasp predation in dioecious Ficus variegata from an Australian wet tropical forest. Biotropica 1995, 27, 391–394. [Google Scholar] [CrossRef]
- Weiblen, G.D.; Yu, D.W.; West, S.A. Pollination and parasitism in functionally dioecious figs. Biol. Sci. 2001, 268, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Q.; Yang, D.R.; Wang, Q.Y. Quantitative tests of interaction between pollinating and non-pollinating fig wasps on dioecious Ficus hispida. Ecol. Entomol. 2005, 30, 70–77. [Google Scholar] [CrossRef]
- Compton, S.G.; McLaren, F.A.C. Respiratory adaptations in some male fig wasps. Proc. K. Ned. Akad. Wet. Ser. C Biol. Med. Sci. 1989, 92, 57–71. [Google Scholar]
- Fan, K.Y.; Bain, A.; Tzeng, H.Y.; Chiang, Y.P.; Chou, L.S.; Kuo-Huang, L.L. Comparative anatomy of the fig wall (Ficus, Moraceae). Botany 2019, 97, 417–426. [Google Scholar] [CrossRef]
- Wang, R.W.; Zheng, Q. Structure of a fig wasp community: Temporal segregation of oviposition and larval diets. Symbiosis 2008, 45, 113–116. [Google Scholar]
- Kuo, C.C.; Bain, A.; Chiu, Y.T.; Ho, Y.C.; Chen, W.H.; Chou, L.S.; Tzeng, H.Y. Topographic effect on the phenology of Ficus pedunculosa var. mearnsii (Mearns fig) in its northern boundary distribution, Taiwan. Sci. Rep. 2017, 7, 14699. [Google Scholar] [CrossRef]
- Galil, J.; Eisikowitch, D. On the pollination ecology of Ficus sycomorus in East Africa. Ecology 1968, 49, 259–269. [Google Scholar] [CrossRef]
- Kruitwagen, A.; Beukeboom, L.W.; Wertheim, B. Optimization of native biocontrol agents, with parasitoids of the invasive pest Drosophila suzukii as an example. Evol. Appl. 2018, 11, 1473–1497. [Google Scholar] [CrossRef] [PubMed]
- Conchou, L.; Ciminera, M.; Hossaert-McKey, M.; Kjellberg, F. The non-pollinating fig wasps associated with Ficus guianensis: Community structure and impact of the large species on the fig/pollinator mutualism. Acta Oecologica 2014, 57, 28–37. [Google Scholar] [CrossRef]
- Wang, R.; Chen, X.Y.; Chen, Y.; Wang, G.; Dunn, D.W.; Quinnell, R.J.; Compton, S.G. Loss of top-down biotic interactions changes the relative benefits for obligate mutualists. Proc. R. Soc. B. 2019, 286, 20182501. [Google Scholar] [CrossRef]
- Zhang, X.W.; Li, L.H. An early gall-inducing parasitic wasp adversely affects the fitness of its host Ficus tree but not the pollinator. Sci. Rep. 2020, 10, 14941. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.S.; do Prado, A.P. Non-Pollinating Wasps Distort the Sex Ratio of Pollinating Fig Wasps. Oikos 2005, 110, 613–619. [Google Scholar] [CrossRef]
- Yan, X.; Peng, Y.Q.; Yang, D.R. Spatial distribution patterns of three fig wasps on Ficus semicordata: How non-pollinators affect pollinator’s sex ratio. Acta Ecol. Sin. 2012, 32, 99–103. [Google Scholar] [CrossRef]
- Yu, H.; Compton, S.G. Moving your sons to safety: Galls containing male fig wasps expand into the centre of figs, away from enemies. PLoS ONE 2012, 7, 30833. [Google Scholar] [CrossRef]
- Lessells, C.M. Parasitoid foraging: Should parasitism be density dependent? J. Anim. Ecol. 1985, 54, 27–41. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Cornell, H.V. Maximum parasitism rates and successful biological control. Science 1994, 266, 1886. [Google Scholar] [CrossRef]
- Zhen, W.Q.; Huang, D.W.; Xiao, J.H.; Yang, D.R.; Zhu, C.D.; Xiao, H. Ovipositor length of three Apocrypta species: Effect on oviposition behavior and correlation with syconial thickness. Phytoparasitica 2005, 33, 113–120. [Google Scholar] [CrossRef]
- Diaz, M.F.; Ramirez, A.; Poveda, K. Efficiency of different egg parasitoids and increased floral diversity for the biological control of noctuid pests. Biol. Control. 2012, 60, 182–191. [Google Scholar] [CrossRef]
- Wang, X.Y.; Jennings, D.E.; Duan, J.J. Trade-offs in parasitism efficiency and brood size mediate parasitoid coexistence, with implications for biological control of the invasive emerald ash borer. J. Appl. Ecol. 2015, 52, 1255–1263. [Google Scholar] [CrossRef]
- Luo, S.; Xia, S.; Lu, Y.; Wu, K. Parasitism efficiency and progeny fitness of Peristenus spretus Chen et van Achterberg vary with nymphal instar of host, Apolygus lucorum (Meyer-Dür). Biol. Control 2022, 167, 104839. [Google Scholar] [CrossRef]
- Arhex, F.V.; Corley, J. The functional response of parasitoids and its implications for biological control. Biocontrol Sci. Technol. 2003, 13, 403–413. [Google Scholar] [CrossRef]
- Pritchard, D.W.; Paterson, R.A.; Bovy, H.C.; Barrios-O’Neill, D. Frair: An R package for fitting and comparing consumer functional responses. Methods Ecol. Evol. 2017, 8, 1528–1534. [Google Scholar] [CrossRef]
- Cook, J.M.; Segar, S.T. Speciation in Fig Wasps. Ecol. Entomol. 2010, 35, 54–66. [Google Scholar] [CrossRef]
- Suleman, N.; Raja, S.; Compton, S.G. Parasitism of a pollinator fig wasp: Mortalities are higher in figs with more pollinators, but are not related to local densities of figs. Ecol. Entomol. 2013, 38, 478–484. [Google Scholar] [CrossRef]
- Ives, A.R. Density-dependent and density-independent parasitoid aggregation in model host-parasitoid systems. Am. Nat. 1992, 140, 912–937. [Google Scholar] [CrossRef]
- Ghara, M.; Borges, R.M. Comparative life-history traits in a fig wasp community: Implications for community structure. Ecol. Entomol. 2010, 35, 139–148. [Google Scholar] [CrossRef]
- Cook, J.M.; Reuter, C.; Moore, J.C.; West, S.A. Fighting in fig wasps: Do males avoid killing brothers or do they never meet them? Ecol. Entomol. 2015, 40, 741–747. [Google Scholar] [CrossRef]
- Cook, J.M.; Reuter, C.; Moore, J.C.; West, S.A. Molecular markers reveal reproductive strategies of non-pollinating fig wasps. Ecol. Entomol. 2017, 42, 689–696. [Google Scholar] [CrossRef]
- Chiu, Y.T.; Bain, A.; Deng, S.L.; Ho, Y.C.; Chen, W.H.; Tzeng, H.Y. Effects of climate change on a mutualistic coastal species: Recovery from typhoon damages and risks of population erosion. PLoS ONE 2017, 12, 0186763. [Google Scholar] [CrossRef] [PubMed]
- Jevanandam, N.; Goh, A.G.R.; Corlett, R.T. Climate warming and the potential extinction of fig wasps, the obligate pollinators of figs. Biol. Lett. 2013, 9, 20130041. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.L.; DeGabriel, J.L.; Riegler, M.; Cook, J.M. A temperate pollinator with high thermal tolerance is still susceptible to heat events predicted under future climate change. Ecol. Entomol. 2018, 43, 506–512. [Google Scholar] [CrossRef]
- Bain, A.; Chou, L.S.; Tzeng, H.Y.; Ho, Y.C.; Chiang, Y.P.; Chen, W.H.; Chio, Y.T.; Li, G.Y.; Yang, H.W.; Kjellberg, F.; et al. Plasticity and diversity of the phenology of dioecious Ficus species in Taiwan. Acta Oecologica 2014, 57, 124–134. [Google Scholar] [CrossRef]
- Bain, A.; Harrison, R.; Schatz, B. How to be an ant on figs. Acta Oecologica 2014, 57, 97–108. [Google Scholar] [CrossRef]
- Mackay, K.D.; Gross, G.L.; Rosseto, M. Small populations of fig trees offer a keystone food resource and conservation benefits for declining insectivorous birds. Glob. Ecol. Conserv. 2018, 14, e00403. [Google Scholar] [CrossRef]
- Wang, R.; Aylwin, R.; Cobb, J.; Craine, L.; Ghana, S.; Reyes-Betancort, J.A.; Quinnell, R.J.; Compton, S.G. The impact of fig wasps (Chalcidoidea), new to the Mediterranean, on reproduction of an invasive fig tree Ficus microcarpa (Moraceae) and their potential for its biological control. Biol. Control 2015, 81, 21–30. [Google Scholar] [CrossRef]




| Respond Variable | Fixed Effect | N | Estimate | Standard Error | LR Test | |
|---|---|---|---|---|---|---|
| LR | p Value | |||||
| Abundance of pollinator | Parasitism rate | 205 | −0.003 | 0.00002 | 279.66 | <0.01 * |
| Parasitism rate | Fig wall | 117 | −0.420 | 0.310 | 2.038 | 0.153 |
| Parasitism rate | Sex ratio of pollinator | 205 | 0.729 | 0.979 | 0.533 | 0.465 |
| Ficus spp. | Subgen. Ficus | Subgen. Sycomorus | ||||
|---|---|---|---|---|---|---|
| F. pedunculsoa var. mearnsii | F. fistulosa | F. hispida | F. semicordata | F. variegata | ||
| Diameter (mm) | Mean ± SD | 14.37 ± 2.74 | 20.63 ± 1.97 | 32.56 ± 2.12 * | 25.63 ± 2.54 * | 31.33 ± 1.15 * |
| Dunn’s test | - | 6.500 | 22.000 | 11.500 | 20.000 | |
| p-value | - | 0.201 | <0.001 | 0.024 | 0.001 | |
| Wall (mm) | Mean ± SD | 1.18 ± 0.12 | 3.25 ± 0.56 * | 4.85 ± 0.74 * | 2.54 ± 0.40 | 3.91 ± 0.45 * |
| Dunn’s test | - | 24.375 | 46.000 | 13.625 | 36.000 | |
| p-value | - | 0.001 | <0.001 | 0.056 | <0.001 | |
| Wall+galls (mm) | Mean ± SD | 3.54 ± 0.21 | 5.99 ± 0.29 * | 8.06 ± 0.56 * | 6.25 ± 0.84 * | 6.69 ± 0.38 * |
| Dunn’s test | - | 17.667 | −47.792 | −23.208 | −31.333 | |
| p-value | - | 0.013 | <0.001 | 0.001 | <0.001 | |
| Ovipositor sheath length of Apocrypta spp. (mm) | Mean ± SD | 2.26 ± 0.35 | 3.13 ± 0.23 | 4.02 ± 0.09 * | 2.12 ± 0.05 | 3.90 ± 0.19 * |
| Dunn’s test | - | 6.400 | 15.200 | −2.200 | 12.600 | |
| p-value | - | 0.169 | 0.001 | 0.636 | 0.007 | |
| Ovipositor sheath/Wall+galls | 0.64 | 0.52 | 0.50 | 0.34 | 0.58 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, P.-A.; Bain, A.; Chantarasuwan, B.; Tzeng, H.-Y. Parasitism Features of a Fig Wasp of Genus Apocrypta (Pteromalidae: Pteromalinae) Associated with a Host Belonging to Ficus Subgenus Ficus. Insects 2023, 14, 437. https://doi.org/10.3390/insects14050437
Chou P-A, Bain A, Chantarasuwan B, Tzeng H-Y. Parasitism Features of a Fig Wasp of Genus Apocrypta (Pteromalidae: Pteromalinae) Associated with a Host Belonging to Ficus Subgenus Ficus. Insects. 2023; 14(5):437. https://doi.org/10.3390/insects14050437
Chicago/Turabian StyleChou, Po-An, Anthony Bain, Bhanumas Chantarasuwan, and Hsy-Yu Tzeng. 2023. "Parasitism Features of a Fig Wasp of Genus Apocrypta (Pteromalidae: Pteromalinae) Associated with a Host Belonging to Ficus Subgenus Ficus" Insects 14, no. 5: 437. https://doi.org/10.3390/insects14050437
APA StyleChou, P.-A., Bain, A., Chantarasuwan, B., & Tzeng, H.-Y. (2023). Parasitism Features of a Fig Wasp of Genus Apocrypta (Pteromalidae: Pteromalinae) Associated with a Host Belonging to Ficus Subgenus Ficus. Insects, 14(5), 437. https://doi.org/10.3390/insects14050437





