Oviposition Preference and Age-Stage, Two-Sex Life Table Analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) on Different Maize Varieties
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Population Rearing
2.2. Plant Materials and Growth Conditions
2.3. Oviposition Selectivity of S. frugiperda to Different Maize Varieties
2.4. Effects of Maize Varieties on the Development and Population Parameters of S. frugiperda
2.5. Analysis of the Age-Stage, Two-Sex Life Table
2.6. Statistical Analysis
3. Results
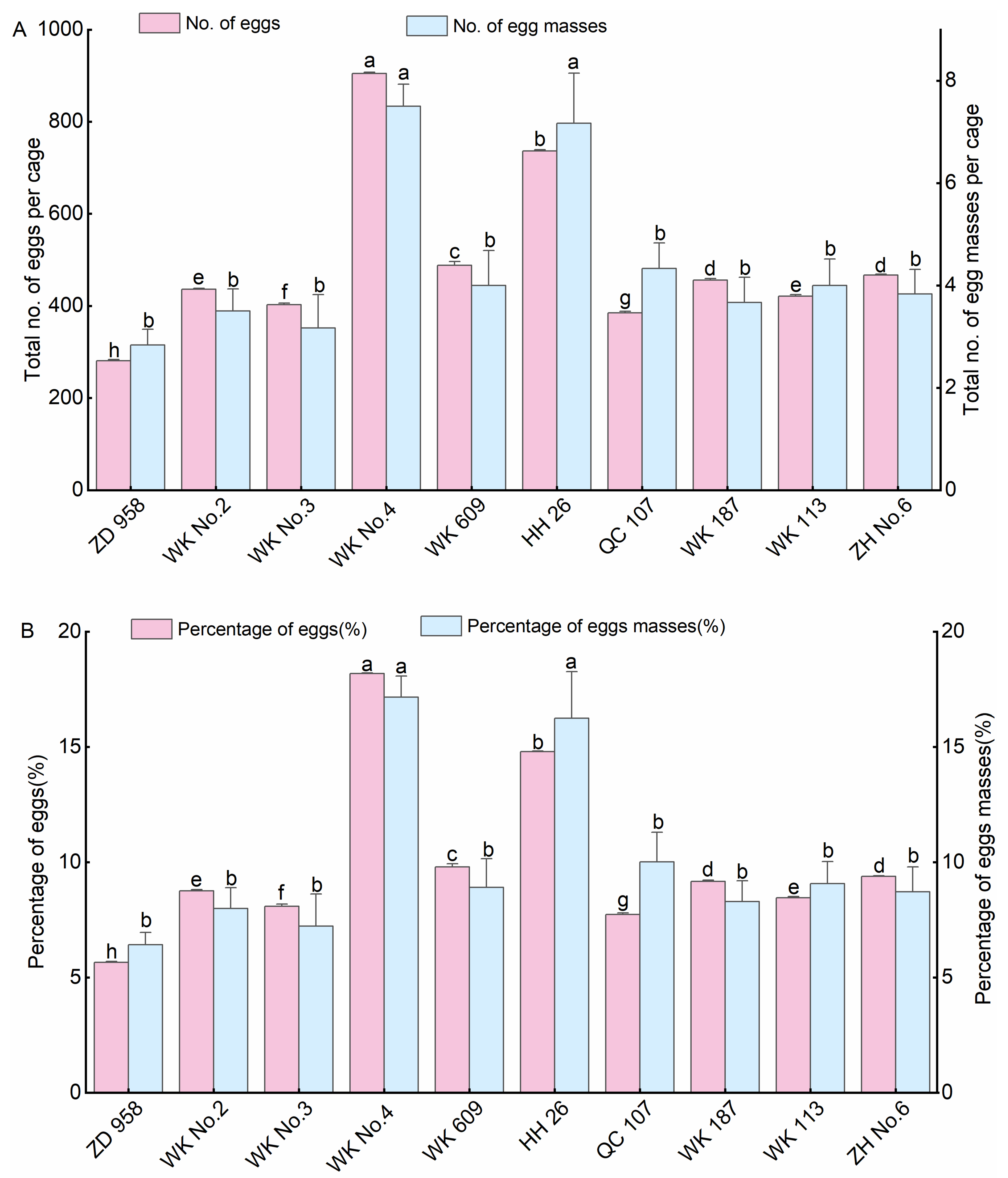
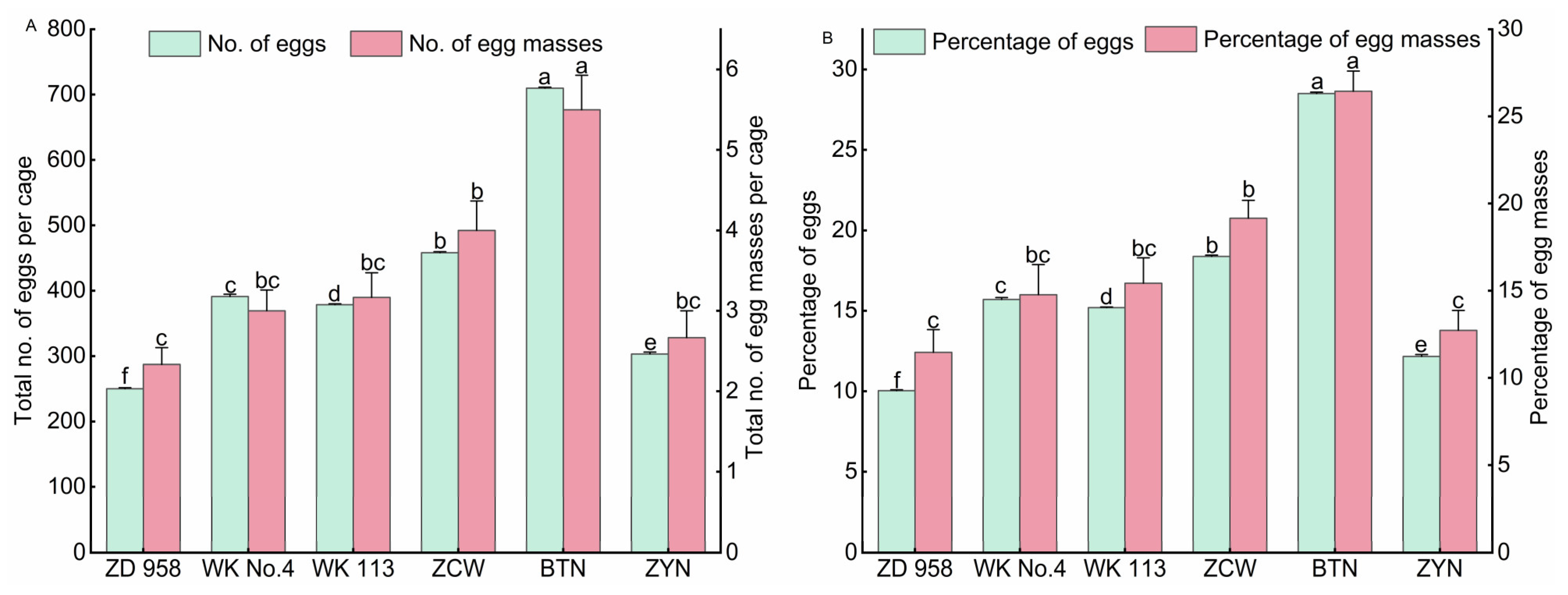
3.1. Oviposition Selectivity of S. frugiperda on Different Maize Varieties
3.1.1. Oviposition Selectivity of S. frugiperda on Common Maize Varieties
3.1.2. Oviposition Selectivity of S. frugiperda on Special Maize Varieties
3.1.3. Oviposition Selectivity of S. frugiperda on Different Maize Varieties
3.2. Developmental Duration of Each Stage of S. frugiperda Feeding on Different Maize Varieties
3.3. The Pupal Weight of S. frugiperda Reared on Different Maize Varieties
3.4. Effects of Different Maize Varieties on Biological Parameters of Adults of S. frugiperda
3.5. Population Parameters of S. frugiperda Fed on Six Maize Varieties
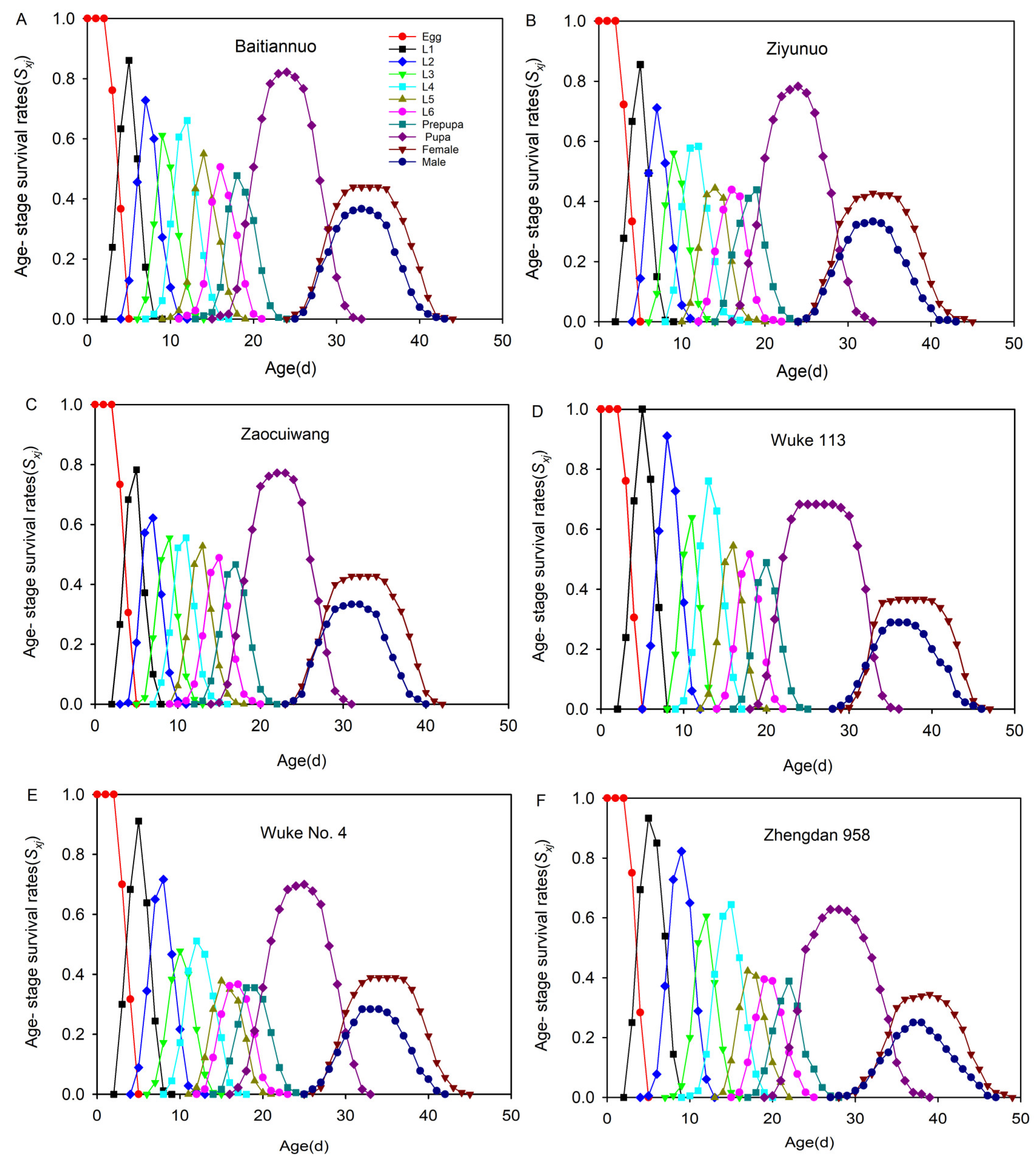
3.6. Age-Stage-Specific Survival Rate (Sxj)
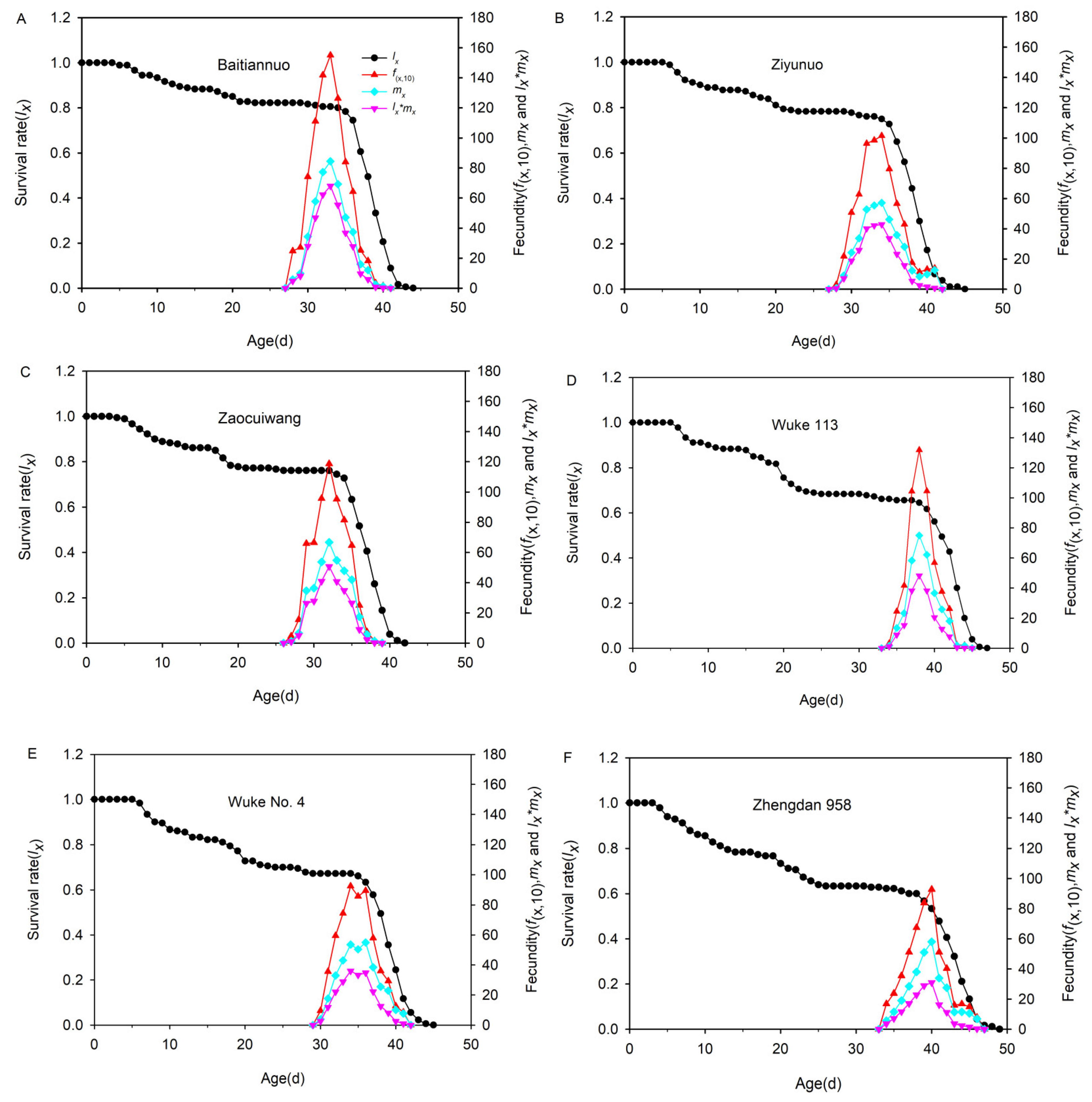
3.7. Population Survival Rate and Fecundity
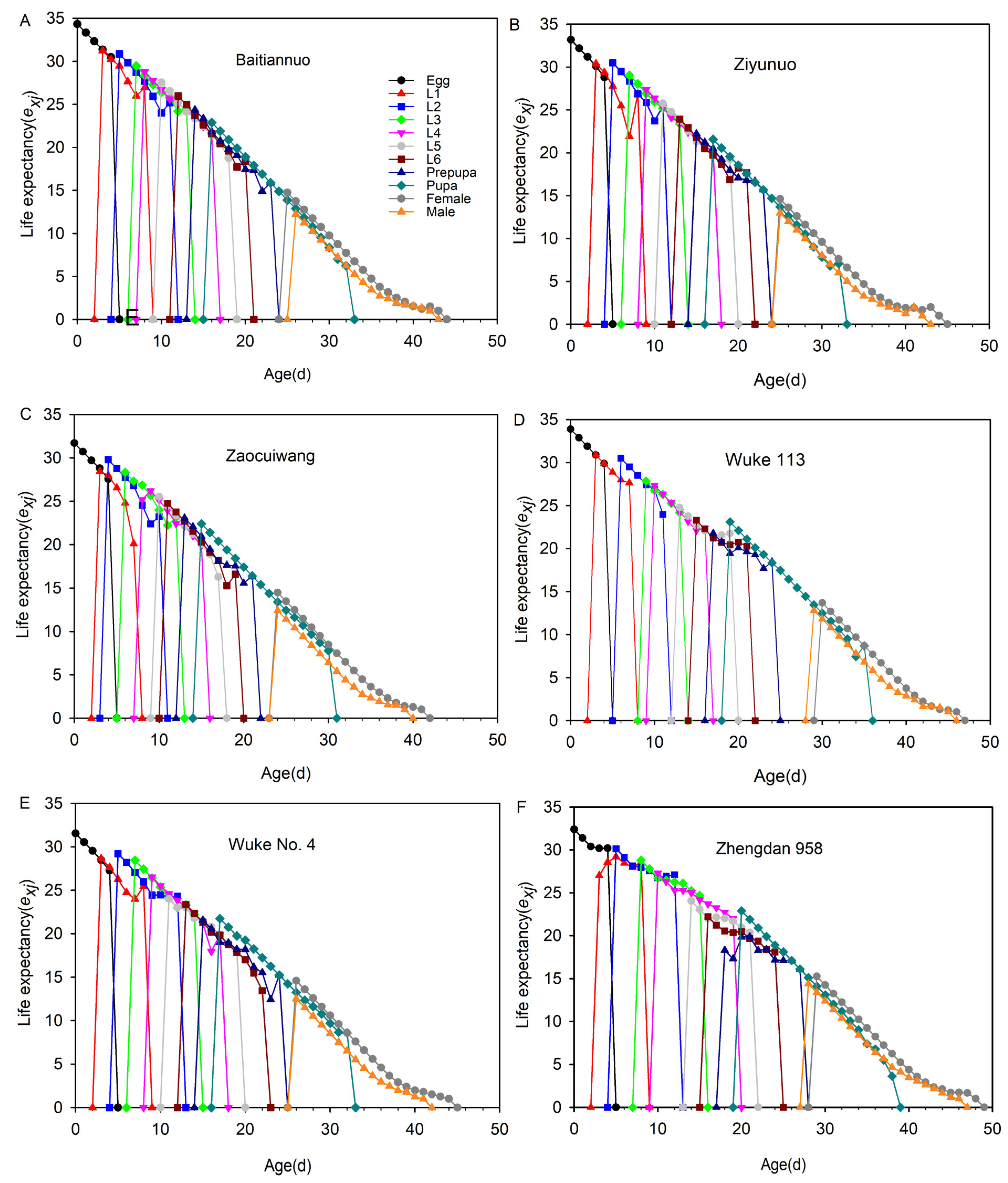
3.8. Life Expectancy (exj)
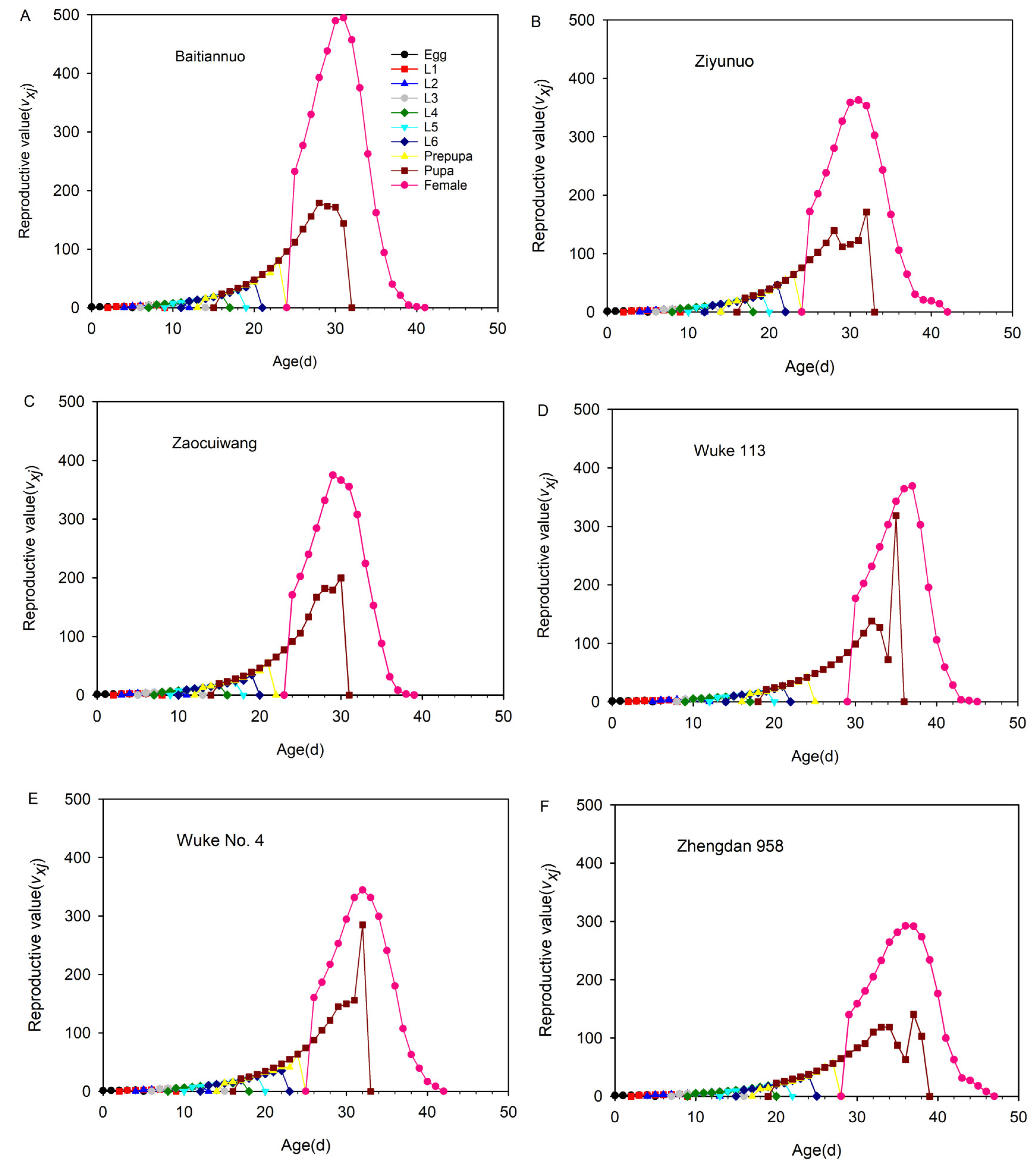
3.9. Reproductive Value (vxj) of Each Age-Stage Group of Spodoptera frugiperda on Six Different Maize Varieties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Int. J. Trop. Insect. Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Montezano, D.G.; Sosa-Gómez, D.R.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, R.J.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Willse, A.; Martinelli, S.; Head, G.P. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest. Manag. Sci. 2016, 72, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2016, 60, 255–267. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rodrigues, J.V.C.; Santos-Amaya, O.F.; Paula-Moraes, S.V.; Pereira, E.J.G. The oviposition behavior of fall armyworm moths is unlikely to compromise the refuge strategy in genetically modified Bt crops. J. Pest. Sci. 2020, 93, 965–977. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agr. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Xie, M.M.; Li, H.H.; Yang, J.J.; Zhang, M.L.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Prot. 2019, 45, 10–19. [Google Scholar]
- Chen, X.Y.; Jiang, H.X.; Ma, Y.; Li, W.S.; Meng, Z.Y.; Wang, S.L.; Jia, X.P.; Chen, X.F.; Zhao, X.L.; Chen, X.J. Preliminary study on the monitoring and control measures of Spodoptera frugiperda in Longnan City. China Plant Prot. 2020, 40, 83–85. [Google Scholar]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.L.; Henri, H.; Vavre, F. Transporter frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa-Cancino, W.; Rojas, J.C.; Cruz-Lopez, L.; Castillo, A.; Malo, E.A. Attraction, feeding preference, and performance of Spodoptera frugiperda larvae (Lepidoptera: Noctuidae) reared on two varieties of maize. Environ. Entomol. 2016, 45, 384–389. [Google Scholar] [CrossRef]
- Pannuti, L.E.R.; Baldin, E.L.L.; Hunt, T.E.; Paula-Moraes, S.V. On-plant larval movement and feeding behavior of fall armyworm (Lepidoptera: Noctuidae) on reproductive corn stages. Environ. Entomol. 2016, 45, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Costa, E.N.; Di Bello, M.M.; Diniz, J.F.S.; Ribeiro, Z.A.; Boiça Júnior, A.L. Oviposition preference and antibiosis to Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazilian maize landraces. J. Econ. Entomol. 2019, 112, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga Morales, X.; Tamiru, A.; Sobhy, I.S.; Bruce, T.J.A.; Midega, C.A.O.; Khan, Z. Evaluation of African maize cultivars for resistance to fall armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) larvae. Plants 2021, 10, 392. [Google Scholar] [CrossRef]
- Xu, C.F.; Sa, R.N.; He, J.Y.; Guo, X.; Lu, F.; Cheng, W.; Hu, G.; Chen, F.J.; Wan, G.J.; Zhao, J.Y. Comparative study on the adaptability of Spodoptera frugiperda to different corn varieties. J. Environ. Entomol. 2021, 43, 891–900. [Google Scholar]
- Yang, Y.; Tian, H.L.; Yi, H.M.; Liu, Y.W.; Ren, J.; Wang, R.; Wang, L.; Zhao, J.R.; Wang, F.G. Analysis of the status of protection of maize varieties in China. Chin. Agric. Sci. 2020, 53, 1095–1107. [Google Scholar]
- Yang, Y.; Wang, F.G.; Zhao, J.R.; Liu, Y.W. Analysis of the current situation of accredited maize varieties in China. Sci. Agric. Sin. 2014, 47, 4360–4370. [Google Scholar]
- Zhang, D.D.; Xiao, Y.T.; Xu, P.J.; Yang, X.M.; Wu, Q.L.; Wu, K.M. Insecticide resistance monitoring for the invasive populations of fall armyworm, Spodoptera frugiperda in China. J. Integr. Agr. 2021, 20, 783–791. [Google Scholar] [CrossRef]
- Buntin, G.D. A review of plant response to fall armyworm, Spodoptera frugiperda (J E Smith), injury in selected field and forage crops. Fla. Entomol. 1986, 69, 549–559. [Google Scholar] [CrossRef]
- Fotso Kuate, A.; Hanna, R.; Doumtsop Fotio, A.R.P.; Abang, A.F.; Nanga, S.N.; Ngatat, S.; Tindo, M.; Masso, C.; Ndemah, R.; Suh, C. Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Cameroon: Case study on its distribution, damage, pesticide use, genetic differentiation and host plants. PLoS ONE 2019, 14, e0215749. [Google Scholar]
- Atlihan, R.; Kasap, İ.; Özgökçe, M.S.; Polat-Akköprü, E.; Chi, H. Population growth of Dysaphis pyri (Hemiptera: Aphididae) on different pear cultivars with discussion on curve fitting in life table studies. J. Econ. Entomol. 2017, 110, 1890–1898. [Google Scholar] [CrossRef]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Chi, H.; You, M.S.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhu, Q.Z.; Tan, Y.T.; Ma, Q.L.; Wang, R.F.; Zhang, M.F.; Xu, H.H.; Zhang, Z.X. Artificial diets and rearing technique of Spodoptera frugiperda (J. E. Smith) in laboratory. J. Environ. Entomol. 2019, 41, 742–747. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2020; Available online: http://140.120.197.173/Ecology/Download/Twosex-MSChart-B100000.rar (accessed on 19 June 2020).
- Huang, Y.B.; Chi, H. Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): With an invalidation of the jackknife technique. J. Appl. Entomol. 2013, 137, 327–339. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Erratum: Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813, Erratum in Pest Manag. Sci. 2014, 70, 1936. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
- Li, D.Y.; Zhi, J.R.; Zhang, T.; Ye, J.Q.; Yu, Y.C.; Hu, C.X. Preference of Spodoptera frugiperda to four host plants. Plant Prot. 2019, 45, 50–54. [Google Scholar]
- He, L.M.; Zhao, S.Y.; Gao, X.W.; Wu, K.M. Ovipositional responses of Spodoptera frugiperda on host plants provide a basis for using Bt-transgenic maize as trap crop in China. J. Integr. Agr. 2021, 20, 804–814. [Google Scholar] [CrossRef]
- Keerthi, M.C.; Mahesha, H.S.; Manjunatha, N.; Gupta, A.; Saini, R.P.; Shivakumara, K.T.; Bhargavi, H.A.; Gupta, G.; Kulkarni, N.S. Biology and oviposition preference of fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) on fodder crops and its natural enemies from Central India. Int. J. Pest Manag. 2021, 67, 1–10. [Google Scholar] [CrossRef]
- Sotelo-Cardona, P.; Chuang, W.P.; Lin, M.Y.; Chiang, M.Y.; Ramasamy, S. Oviposition preference not necessarily predicts offspring performance in the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) on vegetable crops. Sci. Rep. 2021, 11, 15885. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, Y.M.; Wang, Q.; Liu, X.B.; Fu, Y.; Zhang, Y.; Chen, J.L. Push-pull plants in wheat intercropping system to manage Spodoptera frugiperda. J. Pest. Sci. 2022, 95, 1–15. [Google Scholar] [CrossRef]
- Xu, D.; Li, W.J.; Wang, L.; Yin, H.C.; Cong, S.B.; Yang, N.N.; Xie, Y.L.; Wan, P. Feeding and oviposition preference and adaptability of the fall armyworm, Spodoptera frugiperda (Lepidoptera Noctuidae), on two leguminous vegetables. J. Environ. Entomol. 2022, 44, 800–807. [Google Scholar]
- Wang, Y.H.; Chen, W.H.; Yi, C.H.; Xiao, W.X.; Yin, X.A.; Liu, Q.; Zhang, X.M. characteristics of oviposition and feeding selectivity of Spodoptera frugiperda to four diffirent maize cultivars. Jiangsu Agric. Sci. 2022, 50, 26–31. [Google Scholar]
- Nascimento, P.T.; Von Pinho, R.G.; Fadini, M.A.M.; Souza, C.S.F.; Valicente, F.H. Does singular and stacked corn affect choice behavior for oviposition and feed in Spodoptera frugiperda (Lepidoptera: Noctuidae)? Neotrop. Entomol. 2020, 49, 302–310. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Block, A.K.; Hunter, C.T.; Rering, C.; Christensen, S.A.; Meagher, R.L. Contrasting insect attraction and herbivore-induced plant volatile production in maize. Planta 2018, 248, 105–116. [Google Scholar] [CrossRef]
- Yactayo-Chang, J.P.; Mendoza, J.; Willms, S.D.; Rering, C.C.; Beck, J.J.; Block, A.K. Zea mays volatiles that influence oviposition and feeding behaviors of Spodoptera frugiperda. J. Chem. Ecol. 2021, 47, 799–809. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, D.D.; Xiu, X.J.; Yu, J.; Hou, M.L. Corn and rice volatile components eliciting olfactory responses in Spodoptera frugiperda. Plant Prot. 2022, 48, 90–97+110. [Google Scholar]
- Takahashi, C.G.; Kalns, L.L.; Bernal, J.S. Plant defense against fall armyworm in micro-sympatric maize (Zea mays ssp. mays) and Balsas teosinte (Zea mays ssp. parviglumis). Entomol. Exp. Appl. 2012, 145, 191–200. [Google Scholar] [CrossRef]
- Qin, J.D. Host specificity and nutrition of phytophagous insects. Acta Entomol. Sin. 1962, 11, 169–185. [Google Scholar]
- Wang, W.W.; He, P.Y.; Zhang, Y.Y.; Liu, T.X.; Jing, X.; Zhang, S.Z. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef]
- Dai, Q.X.; Li, Z.Y.; Tian, Y.J.; Zhang, Z.F.; Wang, L.; Lu, Y.Y.; Li, Y.Z.; Chen, K.W. Effects of different corn varieties on development and reproduction of Spodoptera frugiperda. Chin. J. Appl. Ecol. 2020, 31, 3273–3281. [Google Scholar]
- Sun, Y.; Liu, X.G.; Lv, G.Q.; Hao, X.Z.; Li, S.H.; Li, G.P.; Feng, H.Q. Comparison of population fitness of Spodoptera frugiperda (Lepidoptera: Noctuidae) feeding on wheat and different varieties of maize. Plant Prot. 2020, 46, 126–131. [Google Scholar]
- Behmer, S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef]
- Silva, D.M.; Bueno, A.F.; Andrade, K.; Stecca, C.S.; Neves, P.M.O.J.; Oliveira, M.C.N. Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Sci. Agric. 2017, 74, 18–31. [Google Scholar] [CrossRef]
- Schuh, M.K.; Bahlai, C.A.; Malmstrom, C.M.; Landis, D.A. Effect of switchgrass ecotype and cultivar on establishment, feeding, and development of fall armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 440–449. [Google Scholar] [CrossRef] [PubMed]
- He, L.M.; Wang, T.L.; Chen, Y.C.; Ge, S.S.; Wyckhuys, K.A.G.; Wu, K.M. Larval diet affects development and reproduction of East Asian strain of the fall armyworm, Spodoptera frugiperda. J. Integr. Agr. 2021, 20, 736–744. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, K.X.; Ma, Y.; Zhang, Q.Y.; Liu, W.H.; Jiang, H.X.; Yang, L.K.; Liu, C.Z. Feeding selectivity of Spodoptera frugiperda on different maize cultivars and its relationship with chemical substances in maize leaves. China Plant Prot. 2021, 41, 10–16+39. [Google Scholar]
- Cabezas, M.F.; Nava, D.E.; Geissler, L.O.; Melo, M.; Garcia, M.S.; Krüger, R. Development and leaf consumption by Spodoptera cosmioides (Walker) (Lepidoptera: Noctuidae) reared on leaves of agroenergy crops. Neotrop. Entomol. 2013, 42, 588–594. [Google Scholar] [CrossRef]
- da Silva, D.M.; de Freitas Bueno, A.; dos Santos Stecca, C.; Andrade, K.; Neves, P.M.O.J.; de Oliveira, M.C.N. Biology of Spodoptera eridania and Spodoptera cosmioides (Lepidoptera: Noctuidae) on different host plants. Fla. Entomol. 2017, 100, 752–760. [Google Scholar] [CrossRef]
- Throop, H.L.; Lerdau, M.T. Effects of nitrogen deposition on insect herbivory: Implications for community and ecosystem processes. Ecosystems 2004, 7, 109–133. [Google Scholar] [CrossRef]
- Soto, E.M.; Padró, J.; Milla Carmona, P.; Tuero, D.T.; Carreira, V.P.; Soto, I.M. Pupal emergence pattern in cactophilic Drosophila and the effect of host plants. Insect. Sci. 2018, 25, 1108–1118. [Google Scholar] [CrossRef]
- Leuck, D.B.; Perkins, W.D. A method of estimating fall armyworm progeny reduction when evaluating control achieved by host-plant resistance. J. Econ. Entomol. 1972, 65, 482–483. [Google Scholar] [CrossRef]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef]
- Yang, G.; Wiseman, B.R.; Isenhour, D.J.; Espelie, K.E. Chemical and ultrastructural analysis of corn cuticular lipids and their effect on feeding by fall armyworm larvae. J. Chem. Ecol. 1993, 19, 2055–2074. [Google Scholar] [CrossRef]
- Kumar, H. Resistance in maize to Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae): An overview. Crop Prot. 1997, 16, 243–250. [Google Scholar] [CrossRef]
- Wu, F.A.; Zhou, J.X.; Yu, M.D.; Wang, Q.L.; Xu, L.; Lu, C.; Jing, C.J. Statistical inference on the intrinsic rate of increase of the carmine spider mite, Tetranychus cinnabarinus on different mulberry cultivars (Morus L.) under laboratory conditions. Acta Entomol. Sin. 2006, 49, 287–294. [Google Scholar]
- Wu, L.H.; Zhou, C.; Long, G.Y.; Yang, X.B.; Wei, Z.Y.; Liao, Y.J.; Yang, H.; Hu, C.X. Fitness of fall armyworm, Spodoptera frugiperda to three solanaceous vegetables. J. Integr. Agr. 2021, 20, 755–763. [Google Scholar] [CrossRef]
- Acharya, R.; Malekera, M.J.; Dhungana, S.K.; Sharma, S.R.; Lee, K.Y. Impact of Rice and Potato Host Plants Is Higher on the Reproduction than Growth of Corn Strain Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 256. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Espelie, K.E.; Shelton, A.M. Behavior of neonate diamondback moth larvae [Plutella xylostella (L.)] on leaves and on extracted leaf waxes of resistant and susceptible cabbages. J. Chem. Ecol. 1991, 17, 1691–1704. [Google Scholar] [CrossRef]
- Bergvinson, D.J.; Arnason, J.T.; Hamilton, R.L.; Mihm, J.A.; Jewell, D.C. Determining leaf toughness and its role in maize resistance to the european com borer (lepidoptera: Pyralidae). J. Econ. Entomol. 1994, 87, 1743–1748. [Google Scholar] [CrossRef]
- Chuang, W.P.; Ray, S.; Acevedo, F.E.; Peiffer, M.; Felton, G.W.; Luthe, D.S. Herbivore cues from the fall armyworm (Spodoptera frugiperda) larvae trigger direct defenses in maize. Mol. Plant Microbe Interact. 2014, 27, 461–470. [Google Scholar] [CrossRef]
- Tsai, J.H.; Wang, J.J. Effects of host plants on biology and life table parameters of Aphis spiraecola (Homoptera: Aphididae). Environ. Entomol. 2001, 30, 44–50. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna, L.; Kim, K.M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Xie, W.; Zhi, J.R.; Ye, J.Q.; Zhou, Y.M.; Li, C.; Liang, Y.J.; Yue, W.B.; Li, D.Y.; Zeng, G.; Hu, C.X. Age-stage, two-sex life table analysis of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) reared on maize and kidney bean. Chem. Biol. Technol. Agric. 2021, 8, 44. [Google Scholar] [CrossRef]


| Stage (Days) | n | Baitiannuo | n | Zaocuiwang | n | Ziyunuo | n | Wuke No.4 | n | Wuke 113 | n | Zhengdan 958 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg | 180 | 4.13 ± 0.06 a | 180 | 4.04 ± 0.56 a | 180 | 4.06 ± 0.06 a | 180 | 4.02 ± 0.06 a | 180 | 4.07 ± 0.05 a | 180 | 4.03 ± 0.05 a |
| 1st instar | 171 | 2.44 ± 0.04 d | 167 | 2.40 ± 0.04 e | 166 | 2.19 ± 0.03 d | 163 | 2.77 ± 0.04 c | 164 | 3.04 ± 0.15 b | 160 | 3.61 ± 0.04 a |
| 2nd instar | 167 | 2.41 ± 0.04 d | 161 | 2.01 ± 0.03 e | 162 | 2.36 ± 0.04 d | 155 | 2.77 ± 0.05 c | 161 | 3.14 ± 0.03 b | 149 | 3.45 ± 0.04 a |
| 3rd instar | 164 | 2.05 ± 0.03 b | 159 | 1.89 ± 0.03 d | 160 | 2.02 ± 0.03 bc | 152 | 2.05 ± 0.03 b | 159 | 1.96 ± 0.03 cd | 144 | 2.37 ± 0.04 a |
| 4th instar | 161 | 2.62 ± 0.04 c | 156 | 2.10 ± 0.03 d | 158 | 2.56 ± 0.04 c | 150 | 2.56 ± 0.04 c | 155 | 2.98 ± 0.04 b | 141 | 3.33 ± 0.04 a |
| 5th instar | 159 | 2.13 ± 0.04 ab | 155 | 2.04 ± 0.04 b | 157 | 2.10 ± 0.03 ab | 147 | 2.05 ± 0.02 b | 150 | 2.10 ± 0.03 ab | 138 | 2.20 ± 0.04 a |
| 6th instar | 157 | 2.12 ± 0.03 b | 154 | 2.04 ± 0.03 c | 153 | 2.11 ± 0.03 bc | 143 | 2.11 ± 0.03 bc | 144 | 2.13 ± 0.03 b | 134 | 2.24 ± 0.04 a |
| Egg + larva | 157 | 17.87 ± 0.11 d | 154 | 16.25 ± 0.11 e | 153 | 17.59 ± 0.12 d | 143 | 18.29 ± 0.13 c | 144 | 19.42 ± 0.10 b | 134 | 21.31 ± 0.13 a |
| Prepupa | 148 | 2.25 ± 0.04 a | 139 | 2.18 ± 0.04 a | 142 | 2.23 ± 0.04 a | 127 | 2.17 ± 0.03 a | 125 | 2.26 ± 0.04 a | 115 | 2.23 ± 0.04 a |
| Pupa | 145 | 8.77 ± 0.05 d | 137 | 8.82 ± 0.04 d | 137 | 8.84 ± 0.04 d | 121 | 9.08 ± 0.03 c | 118 | 10.90 ± 0.06 a | 109 | 10.13 ± 0.12 b |
| Preadult | 145 | 28.81 ± 0.14 d | 137 | 27.24 ± 0.13 e | 137 | 28.58 ± 0.15 d | 121 | 29.63 ± 0.15 c | 118 | 32.63 ± 0.12 b | 109 | 33.72 ± 0.20 a |
| Adult | 145 | 10.26 ± 0.09 a | 137 | 10.34 ± 0.10 a | 137 | 10.33 ± 0.10 a | 121 | 10.08 ± 0.10 a | 118 | 10.24 ± 0.11 a | 109 | 9.74 ± 0.12 b |
| Total longevity | 145 | 39.06 ± 0.16 d | 137 | 37.58 ± 0.17 e | 137 | 38.91 ± 0.18 d | 121 | 39.71 ± 0.18 c | 118 | 42.86 ± 0.18 b | 109 | 43.46 ± 0.23 a |
| Stage (Days) | Sex | n | Baitiannuo | n | Zaocuiwang | n | Ziyunuo | n | Wuke No.4 | n | Wuke 113 | n | Zhengdan 958 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg | ♀ | 79 | 4.01 ± 0.09 Aab | 77 | 4.00 ± 0.08 Aab | 77 | 3.92 ± 0.09 Ab | 70 | 3.99 ± 0.09 Aab | 66 | 4.18 ± 0.09 Aa | 62 | 3.98 ± 0.10 Aab |
| ♂ | 66 | 4.11 ± 0.09 Aab | 60 | 3.93 ± 0.10 Ab | 60 | 3.95 ± 0.10 Ab | 51 | 4.00 ± 0.11 Aab | 52 | 3.98 ± 0.10 Aab | 47 | 4.21 ± 0.09 Aa | |
| 1st instar | ♀ | 79 | 2.43 ± 0.06 Ad | 77 | 2.21 ± 0.06 Ae | 77 | 2.43 ± 0.06 Ad | 70 | 2.86 ± 0.06 Ac | 66 | 3.06 ± 0.03 Ab | 62 | 3.68 ± 0.07 Aa |
| ♂ | 66 | 2.44 ± 0.07 Ad | 60 | 2.18 ± 0.05 Ae | 60 | 2.40 ± 0.07 Ad | 51 | 2.75 ± 0.07 Ac | 52 | 3.00 ± 0 Bb | 47 | 3.70 ± 0.07 Aa | |
| 2nd instar | ♀ | 79 | 2.43 ± 0.06 Ad | 77 | 2.08 ± 0.04 Ae | 77 | 2.31 ± 0.06 Ad | 70 | 2.84 ± 0.07 Ac | 66 | 3.11 ± 0.04 Ab | 62 | 3.48 ± 0.07 Aa |
| ♂ | 66 | 2.38 ± 0.07 Ad | 60 | 1.95 ± 0.04 Be | 60 | 2.43 ± 0.07 Ad | 51 | 2.67 ± 0.08 Ac | 52 | 3.13 ± 0.05 Ab | 47 | 3.34 ± 0.07 Aa | |
| 3rd instar | ♀ | 79 | 2.00 ± 0.04 Ab | 77 | 1.86 ± 0.05 Ac | 77 | 2.00 ± 0.03 Ab | 70 | 2.03 ± 0.05 Ab | 66 | 1.86 ± 0.04 Bc | 62 | 2.31 ± 0.06 Aa |
| ♂ | 66 | 2.06 ± 0.04 Ab | 60 | 1.92 ± 0.04 Ac | 60 | 2.08 ± 0.05 Ab | 51 | 2.04 ± 0.05 Abc | 52 | 2.06 ± 0.05 Ab | 47 | 2.40 ± 0.08 Aa | |
| 4th instar | ♀ | 79 | 2.70 ± 0.06 Ac | 77 | 2.08 ± 0.04 Ad | 77 | 2.53 ± 0.06 Ac | 70 | 2.53 ± 0.07 Ac | 66 | 3.02 ± 0.05 Ab | 62 | 3.39 ± 0.07 Aa |
| ♂ | 66 | 2.52 ± 0.06 Ac | 60 | 2.10 ± 0.06 Ad | 60 | 2.63 ± 0.07 Ac | 51 | 2.67 ± 0.08 Ac | 52 | 2.98 ± 0.07 Ab | 47 | 3.30 ± 0.07 Aa | |
| 5th instar | ♀ | 79 | 2.09 ± 0.04 Ab | 77 | 2.04 ± 0.06 Ab | 77 | 2.12 ± 0.05 Ab | 70 | 2.09 ± 0.03 Ab | 66 | 2.15 ± 0.05 Aab | 62 | 2.27 ± 0.06 Aa |
| ♂ | 66 | 2.18 ± 0.06 Aab | 60 | 2.05 ± 0.05 Ab | 60 | 2.05 ± 0.06 Ab | 51 | 2.06 ± 0.03 Ab | 52 | 2.12 ± 0.06 Aab | 47 | 2.26 ± 0.06 Aa | |
| 6th instar | ♀ | 79 | 2.14 ± 0.04 Aab | 77 | 2.01 ± 0.05 Ac | 77 | 2.10 ± 0.03 Ab | 70 | 2.10 ± 0.04 Abc | 66 | 2.12 ± 0.04 Aabc | 62 | 2.24 ± 0.05 Aa |
| ♂ | 66 | 2.11 ± 0.04 Aa | 60 | 2.07 ± 0.05 Aa | 60 | 2.08 ± 0.04 Aa | 51 | 2.14 ± 0.05 Aa | 52 | 2.12 ± 0.04 Aa | 47 | 2.15 ± 0.05 Aa | |
| Egg + larva | ♀ | 79 | 17.80 ± 0.16 Ad | 77 | 16.27 ± 0.16 Ae | 77 | 17.42 ± 0.17 Ad | 70 | 18.43 ± 0.18 Ac | 66 | 19.50 ± 0.12 Ab | 62 | 21.35 ± 0.19 Aa |
| ♂ | 66 | 17.79 ± 0.15 Acd | 60 | 16.20 ± 0.17 Ae | 60 | 17.63 ± 0.18 Ad | 51 | 18.31 ± 0.23 Ac | 52 | 19.38 ± 0.20 Ab | 47 | 21.36 ± 0.24 Aa | |
| Prepupa | ♀ | 79 | 2.18 ± 0.05 Bb | 77 | 2.22 ± 0.05 Aab | 77 | 2.23 ± 0.05 Aab | 70 | 2.20 ± 0.05 Aab | 66 | 2.35 ± 0.06 Aa | 62 | 2.21 ± 0.05 Aab |
| ♂ | 66 | 2.33 ± 0.06 Aa | 60 | 2.13 ± 0.06 Ab | 60 | 2.22 ± 0.05 Aab | 51 | 2.12 ± 0.05 Ab | 52 | 2.19 ± 0.06 Aab | 47 | 2.26 ± 0.07 Aab | |
| Pupa | ♀ | 79 | 8.73 ± 0.06 Ad | 77 | 8.84 ± 0.05 Ad | 77 | 8.86 ± 0.05 Ad | 70 | 9.10 ± 0.05 Ac | 66 | 10.92 ± 0.09 Aa | 62 | 10.16 ± 0.15 Ab |
| ♂ | 66 | 8.80 ± 0.07 Ad | 60 | 8.78 ± 0.07 Ad | 60 | 8.82 ± 0.08 Ad | 51 | 9.06 ± 0.05 Ac | 52 | 10.87 ± 0.08 Aa | 47 | 10.09 ± 0.18 Ab | |
| Preadult | ♀ | 79 | 28.71 ± 0.18 Ad | 77 | 27.34 ± 0.18 Ae | 77 | 28.51 ± 0.20 Ad | 70 | 29.73 ± 0.19 Ac | 66 | 32.77 ± 0.13 Ab | 62 | 33.73 ± 0.25 Aa |
| ♂ | 66 | 28.92 ± 0.20 Ac | 60 | 27.12 ± 0.19 Ae | 60 | 28.67 ± 0.23 Acd | 51 | 29.49 ± 0.24 Ac | 52 | 32.44 ± 0.22 Ab | 47 | 33.70 ± 0.32 Aa | |
| Adult | ♀ | 79 | 11.04 ± 0.08 Aab | 77 | 11.14 ± 0.08 Aa | 77 | 11.10 ± 0.09 Aa | 70 | 10.86 ± 0.07 Ab | 66 | 10.92 ± 0.09 Aab | 62 | 10.52 ± 0.09 Ac |
| ♂ | 66 | 9.32 ± 0.08 Ba | 60 | 9.30 ± 0.09 Ba | 60 | 9.33 ± 0.12 Ba | 51 | 9.02 ± 0.11 Bb | 52 | 9.37 ± 0.14 Ba | 47 | 8.72 ± 0.15 Bc | |
| Total longevity | ♀ | 79 | 39.75 ± 0.19 Ac | 77 | 38.48 ± 0.19 Ad | 77 | 39.61 ± 0.23 Ac | 70 | 40.59 ± 0.20 Ab | 66 | 43.70 ± 0.18 Aa | 62 | 44.24 ± 0.25 Aa |
| ♂ | 66 | 38.24 ± 0.23 Bb | 60 | 36.42 ± 0.22 Bc | 60 | 38.00 ± 0.25 Bb | 51 | 38.51 ± 0.25 Bb | 52 | 41.81 ± 0.28 Ba | 47 | 42.43 ± 0.37 Ba |
| Parameters | n | Baitiannuo | n | Zaocuiwang | n | Ziyunuo | n | Wuke No.4 | n | Wuke 113 | n | Zhengdan 958 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APOP(d) | 79 | 2.76 ± 0.07 d | 77 | 3.61 ± 0.06 c | 77 | 3.53 ± 0.06 c | 70 | 3.99 ± 0.08 b | 66 | 4.35 ± 0.07 a | 62 | 4.37 ± 0.08 a |
| TPOP(d) | 79 | 31.47 ± 0.19 e | 77 | 30.95 ± 0.19 e | 77 | 32.04 ± 0.21 d | 70 | 33.72 ± 0.23 c | 66 | 37.12 ± 0.15 b | 62 | 38.10 ± 0.26 a |
| Oviposition days (d) | 79 | 4.43 ± 0.07 a | 77 | 3.83 ± 0.05 bc | 77 | 3.92 ± 0.07 b | 70 | 3.71 ± 0.07 c | 66 | 3.38 ± 0.06 d | 62 | 3.00 ± 0.06 e |
| Fecundity (eggs/female) | 79 | 808.87 ± 6.54 a | 77 | 621.03 ± 6.40 b | 77 | 609.64 ± 8.48 b | 70 | 555.14 ± 12.95 c | 66 | 523.50 ± 12.01 c | 62 | 456.44 ± 7.80 d |
| Female proportion (Nf/N) | 180 | 0.44 ± 0.04 a | 180 | 0.43 ± 0.04 a | 180 | 0.43 ± 0.04 a | 180 | 0.39 ± 0.04 a | 180 | 0.37 ± 0.04 a | 180 | 0.34 ± 0.04 a |
| Male proportion (Nm/N) | 180 | 0.37 ± 0.04 a | 180 | 0.33 ± 0.04 ab | 180 | 0.33 ± 0.04 ab | 180 | 0.28 ± 0.04 ab | 180 | 0.29 ± 0.03 ab | 180 | 0.26 ± 0.03 b |
| Hatching rate | 79 | 0.9419 ± 0.0018 a | 77 | 0.9455 ± 0.0011 a | 77 | 0.9339 ± 0.0016 b | 70 | 0.9228 ± 0.0014 c | 66 | 0.9327 ± 0.0016 b | 62 | 0.9202 ± 0.0011 c |
| Hosts | Net Reproductive Rate (R0) (Offspring/Individual) | Intrinsic Rate of Increase (r) (d−1) | Finite Rate of Increase (λ) (d−1) | Mean Generation Time (T) (d) |
|---|---|---|---|---|
| Baitiannuo | 355.01 ± 30.04 a | 0.1748 ± 0.0027 a | 1.1910 ± 0.0033 a | 33.60 ± 0.19 c |
| Zaocuiwang | 265.66 ± 22.98 b | 0.1702 ± 0.0029 ab | 1.1856 ± 0.0034 ab | 32.79 ± 0.20 d |
| Ziyunuo | 260.79 ± 22.75 b | 0.1634 ± 0.0028 b | 1.1775 ± 0.0033 b | 34.06 ± 0.21 c |
| Wuke No.4 | 215.89 ± 20.79 bc | 0.1519 ± 0.0030 c | 1.1638 ± 0.0035 c | 35.39 ± 0.24 b |
| Wuke 113 | 191.95 ± 19.27 cd | 0.1345 ± 0.0027 d | 1.1440 ± 0.0030 d | 39.08 ± 0.16 a |
| Zhengdan 958 | 157.22 ± 16.38 d | 0.1275 ± 0.0028 d | 1.1360 ± 0.0032 d | 39.66 ± 0.27 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.-Y.; Zhang, Y.-L.; Quandahor, P.; Gou, Y.-P.; Li, C.-C.; Zhang, K.-X.; Liu, C.-Z. Oviposition Preference and Age-Stage, Two-Sex Life Table Analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) on Different Maize Varieties. Insects 2023, 14, 413. https://doi.org/10.3390/insects14050413
Zhang Q-Y, Zhang Y-L, Quandahor P, Gou Y-P, Li C-C, Zhang K-X, Liu C-Z. Oviposition Preference and Age-Stage, Two-Sex Life Table Analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) on Different Maize Varieties. Insects. 2023; 14(5):413. https://doi.org/10.3390/insects14050413
Chicago/Turabian StyleZhang, Qiang-Yan, Yan-Lei Zhang, Peter Quandahor, Yu-Ping Gou, Chun-Chun Li, Ke-Xin Zhang, and Chang-Zhong Liu. 2023. "Oviposition Preference and Age-Stage, Two-Sex Life Table Analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) on Different Maize Varieties" Insects 14, no. 5: 413. https://doi.org/10.3390/insects14050413
APA StyleZhang, Q.-Y., Zhang, Y.-L., Quandahor, P., Gou, Y.-P., Li, C.-C., Zhang, K.-X., & Liu, C.-Z. (2023). Oviposition Preference and Age-Stage, Two-Sex Life Table Analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) on Different Maize Varieties. Insects, 14(5), 413. https://doi.org/10.3390/insects14050413







