Analysis of the Composition of Different Instars of Tenebrio molitor Larvae using Near-Infrared Reflectance Spectroscopy for Prediction of Amino and Fatty Acid Content
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Samples
2.2. Moisture and Protein Analysis
2.3. Amino Acid Analysis
2.3.1. Sample Preparation
2.3.2. Derivatization
2.3.3. HPLC Analysis
2.4. Lipid Content and Fatty Acid Analysis
2.4.1. Lipid Extraction
2.4.2. Derivatization
2.4.3. Gas Chromatography
2.5. Spectra Collection
2.6. Multivariate Analysis
- = indicates data to be fitted with a mean value;
- = predicted value of the ith observation;
- = measured value of ith observation;
- nc = number of observations in calibration set;
- np = number of observations in prediction set.
- SD = prediction set standard deviation.
2.7. Statistical Analysis
3. Results
3.1. Growth Parameter
3.2. Proximate Analysis
3.3. NIR Spectra
3.4. Prediction of Amino Acid Content
3.5. Prediction of Fatty Acid Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations (UN). World Population Prospects 2022. Summary of Results; United Nations: New York, NY, USA, 2022. [Google Scholar]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible Insects in a Food Safety and Nutritional Perspective: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Patel, S.; Suleria, H.A.R.; Rauf, A. Edible insects as innovative foods: Nutritional and functional assessments. Trends Food Sci. Technol. 2019, 86, 352–359. [Google Scholar] [CrossRef]
- van Huis, A.; Tomberlin, J.K. (Eds.) Insects as Food and Feed. From Production to Consumption; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017. [Google Scholar]
- Morales-Ramos, J.A.; Kay, S.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Morphometric Analysis of Instar Variation in Tenebrio molitor (Coleoptera: Tenebrionidae). Ann. Entomol. Soc. Am. 2015, 108, 146–159. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Developmental Plasticity in Tenebrio molitor (Coleoptera: Tenebrionidae): Analysis of Instar Variation in Number and Development Time under Different Diets. J. Entomol. Sci. 2010, 45, 75–90. [Google Scholar] [CrossRef]
- Esperk, T.; Tammaru, T.; Nylin, S. Intraspecific Variability in Number of Larval Instars in Insects. J. Econ. Entomol. 2007, 100, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Loudon, C. Development of Tenebrio molitor in low oxygen levels. J. Insect Physiol. 1988, 34, 97–103. [Google Scholar] [CrossRef]
- Urs, K.; Hopkins, T.L. Effect of moisture on growth rate and development of two strains of Tenebrio molitor L. (Coleoptera, Tenebrionidae). J. Stored Prod. Res. 1973, 8, 291–297. [Google Scholar] [CrossRef]
- Ludwig, D.; Fiore, C. Further Studies on the Relationship between Parental Age and the Life Cycle of the Mealworm, Tenebrio Molitor. Ann. Entomol. Soc. Am. 1960, 53, 595–600. [Google Scholar] [CrossRef]
- Connat, J.L.; Delbecque, J.P.; Glitho, I.; Delachambre, J. The onset of metamorphosis in Tenebrio molitor larvae (Insecta, Coleoptera) under grouped, isolated and starved conditions. J. Insect Physiol. 1991, 37, 653–662. [Google Scholar] [CrossRef]
- Ludwig, D. Effects of Temperature and Parental Age on the Life Cycle of the Mealworm, Tenebrio Molitor Linnaeus (Coleoptera, Tenebrionidae). Ann. Entomol. Soc. Am. 1956, 49, 12–15. [Google Scholar] [CrossRef]
- Stull, V.J.; Kersten, M.; Bergmans, R.S.; Patz, J.A.; Paskewitz, S. Crude Protein, Amino Acid, and Iron Content of Tenebrio molitor (Coleoptera, Tenebrionidae) Reared on an Agricultural Byproduct from Maize Production: An Exploratory Study. Ann. Entomol. Soc. Am. 2019, 112, 533–543. [Google Scholar] [CrossRef]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an Alternative Protein Source for Monogastric Animal: A Review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of four species of feeder insects. Zoo Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef]
- Mariod, A.A. Nutrient Composition of Mealworm (Tenebrio molitor). In African Edible Insects as Alternative Source of Food, Oil, Protein and Bioactie Components; Springer: Cham, Switzerland, 2020; pp. 275–280. [Google Scholar]
- Bukkens, S.G. The nutritional value of edible insects. Ecol. Food Nutr. 1997, 36, 287–319. [Google Scholar] [CrossRef]
- Ravzanaadii, N.; Kim, S.-H.; Choi, W.-H.; Hong, S.-J.; Kim, N.-J. Nutritional Value of Mealworm, Tenebrio molitor as Food Source. Int. J. Ind. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia-Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Kröncke, N.; Neumeister, M.; Benning, R. Near-Infrared Reflectance Spectroscopy for Quantitative Analysis of Fat and Fatty Acid Content in Living Tenebrio molitor Larvae to Detect the Influence of Substrate on Larval Composition. Insects 2023, 14, 114. [Google Scholar] [CrossRef]
- Evans, A.C. Some Aspects of Chemical Changes During Insect Metamorphosis. J. Exp. Biol. 1932, 9, 314–321. [Google Scholar] [CrossRef]
- Kerr, B.J.; Easter, R.A. Effect of feeding reduced protein, amino acid-supplemented diets on nitrogen and energy balance in grower pigs. J. Anim. Sci. 1995, 73, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.J.P.; Sá, M.V.C.; Browdy, C.L.; Vazquez-Anon, M. Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aquaculture 2014, 431, 20–27. [Google Scholar] [CrossRef]
- Islam-Ud-Din, C.A.S.; Sharif, M.; Nisa, M.; Javaid, A.; Hashimi, N.; Sarwar, N. Supplementation of ruminally protected proteins and amino acids: Feed consumption, digestion and performance of cattle and sheep. Int. J. Agric. Biol. 2009, 4, 477–482. [Google Scholar]
- Kröncke, N.; Benning, R. Self-Selection of Feeding Substrates by Tenebrio molitor Larvae of Different Ages to Determine Optimal Macronutrient Intake and the Influence on Larval Growth and Protein Content. Insects 2022, 13, 657. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Determination of Moisture and Protein Content in Living Mealworm Larvae (Tenebrio molitor L.) Using Near-Infrared Reflectance Spectroscopy (NIRS). Insects 2022, 13, 560. [Google Scholar] [CrossRef]
- Bhatia, H.; Mehdizadeh, H.; Drapeau, D.; Yoon, S. In-line monitoring of amino acids in mammalian cell cultures using raman spectroscopy and multivariate chemometrics models. Eng. Life Sci. 2018, 18, 55–61. [Google Scholar] [CrossRef]
- Farah, J.S.; Cavalcanti, R.N.; Guimarães, J.T.; Balthazar, C.F.; Coimbra, P.T.; Pimentel, T.C.; Esmerino, E.A.; Duarte, M.C.K.; Freitas, M.Q.; Granato, D.; et al. Differential scanning calorimetry coupled with machine learning technique: An effective approach to determine the milk authenticity. Food Control 2021, 121, 107585. [Google Scholar] [CrossRef]
- Noel, S.J.; Jørgensen, H.J.H.; Bach Knudsen, K.E. Prediction of protein and amino acid composition and digestibility in individual feedstuffs and mixed diets for pigs using near-infrared spectroscopy. Anim. Nutr. 2021, 7, 1242–1252. [Google Scholar] [CrossRef]
- Bruno-Soares, A.M.; Murray, I.; Paterson, R.M.; Abreu, J.M. Use of near infrared reflectance spectroscopy (NIRS) for the prediction of the chemical composition and nutritional attributes of green crop cereals. Anim. Feed Sci. Technol. 1998, 75, 15–25. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Near-Infrared spectroscopy and hyperspectral imaging for non-destructive quality assessment of cereal grains. Appl. Spectrosc. Rev. 2018, 53, 667–687. [Google Scholar] [CrossRef]
- Frank, J.F.; Birth, G.S. Application of Near Infrared Reflectance Spectroscopy to Cheese Analysis. J. Dairy Sci. 1982, 65, 1110–1116. [Google Scholar] [CrossRef]
- Margolies, B.J.; Barbano, D.M. Determination of fat, protein, moisture, and salt content of Cheddar cheese using mid-infrared transmittance spectroscopy. J. Dairy Sci. 2018, 101, 924–933. [Google Scholar] [CrossRef]
- Dong, W.; Ni, Y.; Kokot, S. A near-infrared reflectance spectroscopy method for direct analysis of several chemical components and properties of fruit, for example, Chinese hawthorn. J. Agric. Food Chem. 2013, 61, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, T.; Nilsen, B.N.; Tøgersen, G.; Hammond, R.P.; Hildrum, K.I. On-line, proximate analysis of ground beef directly at a meat grinder outlet. Meat Sci. 1996, 43, 245–253. [Google Scholar] [CrossRef]
- Prieto, N.; Andrés, S.; Giráldez, F.J.; Mantecón, A.R.; Lavín, P. Potential use of near infrared reflectance spectroscopy (NIRS) for the estimation of chemical composition of oxen meat samples. Meat Sci. 2006, 74, 487–496. [Google Scholar] [CrossRef]
- Prieto, N.; Roehe, R.; Lavín, P.; Batten, G.; Andrés, S. Application of near infrared reflectance spectroscopy to predict meat and meat products quality: A review. Meat Sci. 2009, 83, 175–186. [Google Scholar] [CrossRef]
- Dowell, F.E.; Throne, J.E.; Wang, D.; Baker, J.E. Identifying Stored-Grain Insects Using Near-Infrared Spectroscopy. J. Econ. Entomol. 1999, 92, 165–169. [Google Scholar] [CrossRef]
- Biancolillo, A.; Firmani, P.; Bucci, R.; Magrì, A.; Marini, F. Determination of insect infestation on stored rice by near infrared (NIR) spectroscopy. Microchem. J. 2019, 145, 252–258. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Plewka, N.; Huck, C.W. Insect Protein Content Analysis in Handcrafted Fitness Bars by NIR Spectroscopy. Gaussian Process Regression and Data Fusion for Performance Enhancement of Miniaturized Cost-Effective Consumer-Grade Sensors. Molecules 2021, 26, 6390. [Google Scholar] [CrossRef]
- Mellado-Carretero, J.; García-Gutiérrez, N.; Ferrando, M.; Güell, C.; García-Gonzalo, D.; de Lamo-Castellví, S. Rapid Discrimination and Classification of Edible Insect Powders Using ATR-FTIR Spectroscopy Combined with Multivariate Analysis; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020; Volume 6. [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten. VDLUFA Methodenbuch, Band III-Die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Bonn, Germany, 2013; p. 2190. [Google Scholar]
- Roth, M. Fluorescence reaction for amino acids. Anal. Chem. 1971, 43, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Beltrán, A.; Mellinas, C.; Jiménez, A.; Garrigós, M.C. Analytical methods combined with multivariate analysis for authentication of animal and vegetable food products with high fat content. Trends Food Sci. Technol. 2018, 77, 120–130. [Google Scholar] [CrossRef]
- Barlocco, N.; Vadell, A.; Ballesteros, F.; Galietta, G.; Cozzolino, D. Predicting intramuscular fat, moisture and Warner-Bratzler shear force in pork muscle using near infrared reflectance spectroscopy. Anim. Sci. 2006, 82, 111–116. [Google Scholar] [CrossRef]
- Prieto, N.; Andrés, S.; Giráldez, F.J.; Mantecón, A.R.; Lavín, P. Ability of near infrared reflectance spectroscopy (NIRS) to estimate physical parameters of adult steers (oxen) and young cattle meat samples. Meat Sci. 2008, 79, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wijewardane, N.K.; Ge, Y.; Xu, Z.; Wilkins, M.R. Visible/near infrared spectroscopy and machine learning for predicting polyhydroxybutyrate production cultured on alkaline pretreated liquor from corn stover. Bioresour. Technol. Rep. 2020, 9, 100386. [Google Scholar] [CrossRef]
- Gruen, L.C. Effect of other amino acids on recovery of tryptophan following acid hydrolysis. Aust. J. Biol. Sci. 1973, 26, 287–290. [Google Scholar]
- van Huis, A.; Rumpold, B.; Maya, C.; Roos, N. Nutritional Qualities and Enhancement of Edible Insects. Annu. Rev. Nutr. 2021, 41, 551–576. [Google Scholar] [CrossRef]
- Eberle, S.; Schaden, L.-M.; Tintner, J.; Stauffer, C.; Schebeck, M. Effect of Temperature and Photoperiod on Development, Survival, and Growth Rate of Mealworms, Tenebrio molitor. Insects 2022, 13, 321. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Toviho, O.A.; Bársony, P. Nutrient Composition and Growth of Yellow Mealworm (Tenebrio molitor) at Different Ages and Stages of the Life Cycle. Agriculture 2022, 12, 1924. [Google Scholar] [CrossRef]
- Yu, X.; He, Q.; Wang, D. Dynamic Analysis of Major Components in the Different Developmental Stages of Tenebrio molitor. Front. Nutr. 2021, 8, 689746. [Google Scholar] [CrossRef] [PubMed]
- Fast, P.G. Insect lipids. Prog. Chem. Fats Other Lipids 1970, 11, 181–242. [Google Scholar]
- Finkel, A.J. The lipid composition of Tenebrio molitor larvae. Physiol. Zool. 1948, 21, 111–133. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, H.; Chen, G.; Qiao, L.; Li, J.; Liu, B.; Liu, Z.; Li, M.; Liu, X. Growth performance and nutritional profile of mealworms reared on corn stover, soybean meal, and distillers’ grains. Eur. Food Res. Technol. 2019, 245, 2631–2640. [Google Scholar] [CrossRef]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of Olive Pomace to Feeding Substrate Affects Growth Performance and Nutritional Value of Mealworm (Tenebrio molitor L.) Larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef]
- Adámková, A.; Mlček, J.; Adámek, M.; Borkovcová, M.; Bednářová, M.; Hlobilová, V.; Knížková, I.; Juríková, T. Tenebrio molitor (Coleoptera: Tenebrionidae)-Optimization of Rearing Conditions to Obtain Desired Nutritional Values. J. Insect Sci. Online 2020, 20. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.; van Boekel, M.A.; Lakemond, C.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Kulma, M.; Kouřimská, L.; Plachý, V.; Božik, M.; Adámková, A.; Vrabec, V. Effect of sex on the nutritional value of house cricket, Acheta domestica L. Food Chem. 2019, 272, 267–272. [Google Scholar] [CrossRef]
- Lease, H.M.; Wolf, B.O. Lipid content of terrestrial arthropods in relation to body size, phylogeny, ontogeny and sex. Physiol. Entomol. 2011, 36, 29–38. [Google Scholar] [CrossRef]
- Nestel, D.; Papadopoulos, N.T.; Liedo, P.; Gonzales-Ceron, L.; Carey, J.R. Trends in lipid and protein contents during medfly aging: An harmonic path to death. Arch. Insect Biochem. Physiol. 2005, 60, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, I.V.; Rippke, G.R.; Hurburgh, C.R. Determination of amino acid composition of soybeans (Glycine max) by near-infrared spectroscopy. J. Agric. Food Chem. 2006, 54, 3485–3491. [Google Scholar] [CrossRef]
- Ouyang, Q.; Chen, Q.; Zhao, J.; Lin, H. Determination of Amino Acid Nitrogen in Soy Sauce Using Near Infrared Spectroscopy Combined with Characteristic Variables Selection and Extreme Learning Machine. Food Bioprocess Technol. 2013, 6, 2486–2493. [Google Scholar] [CrossRef]
- Fontaine, J.; Hörr, J.; Schirmer, B. Near-infrared reflectance spectroscopy enables the fast and accurate prediction of the essential amino acid contents in soy, rapeseed meal, sunflower meal, peas, fishmeal, meat meal products, and poultry meal. J. Agric. Food Chem. 2001, 49, 57–66. [Google Scholar] [CrossRef]
- Shen, F.; Niu, X.; Yang, D.; Ying, Y.; Li, B.; Zhu, G.; Wu, J. Determination of amino acids in Chinese rice wine by fourier transform near-infrared spectroscopy. J. Agric. Food Chem. 2010, 58, 9809–9816. [Google Scholar] [CrossRef] [PubMed]
- Benes, E.; Biró, B.; Fodor, M.; Gere, A. Analysis of wheat flour-insect powder mixtures based on their near infrared spectra. Food Chem. X 2022, 13, 100266. [Google Scholar] [CrossRef]
- Cozzolino, D.; Murray, I. Effect of Sample Presentation and Animal Muscle Species on the Analysis of Meat by near Infrared Reflectance Spectroscopy. J. Near Infrared Spectrosc. 2002, 10, 37–44. [Google Scholar] [CrossRef]
- Leroy, B.; Lambotte, S.; Dotreppe, O.; Lecocq, H.; Istasse, L.; Clinquart, A. Prediction of technological and organoleptic properties of beef Longissimus thoracis from near-infrared reflectance and transmission spectra. Meat Sci. 2004, 66, 45–54. [Google Scholar] [CrossRef]
- Rødbotten, R.; Nilsen, B.; Hildrum, K. Prediction of beef quality attributes from early post mortem near infrared reflectance spectra. Food Chem. 2000, 69, 427–436. [Google Scholar] [CrossRef]
- Ripoll, G.; Failla, S.; Panea, B.; Hocquette, J.-F.; Dunner, S.; Olleta, J.L.; Christensen, M.; Ertbjerg, P.; Richardson, I.; Contò, M.; et al. Near-Infrared Reflectance Spectroscopy for Predicting the Phospholipid Fraction and the Total Fatty Acid Composition of Freeze-Dried Beef. Sensors 2021, 21, 4230. [Google Scholar] [CrossRef]
- Urbach, G. Relations between cheese flavour and chemical composition. Int. Dairy J. 1993, 3, 389–422. [Google Scholar] [CrossRef]
- Escuredo, O.; González Martín, M.I.; Wells Moncada, G.; Fischer, S.; Hernández Hierro, J.M. Amino acid profile of the quinoa (Chenopodium quinoa Willd.) using near infrared spectroscopy and chemometric techniques. J. Cereal Sci. 2014, 60, 67–74. [Google Scholar] [CrossRef]
- van Kempen, T.; Bodin, J.-C. Near-infrared reflectance spectroscopy (NIRS) appears to be superior to nitrogen-based regression as a rapid tool in predicting the poultry digestible amino acid content of commonly used feedstuffs. Anim. Feed Sci. Technol. 1998, 76, 139–147. [Google Scholar] [CrossRef]
- Zhang, B.; Rong, Z.Q.; Shi, Y.; Wu, J.G.; Shi, C.H. Prediction of the amino acid composition in brown rice using different sample status by near-infrared reflectance spectroscopy. Food Chem. 2011, 127, 275–281. [Google Scholar] [CrossRef]
- Leni, G.; Caligiani, A.; Sforza, S. Killing method affects the browning and the quality of the protein fraction of Black Soldier Fly (Hermetia illucens) prepupae: A metabolomics and proteomic insight. Food Res. Int. 2019, 115, 116–125. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Sorci, A.; Bonzanini, F.; Lolli, V.; Maistrello, L.; Sforza, S. Influence of the killing method of the black soldier fly on its lipid composition. Food Res. Int. 2019, 116, 276–282. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Protein and Amino Acid Requirements in Human Nutrition; Report of a Joint WHO/FAO/UNU Expert Consultation; World Health Organization: Albany, Australia, 2007. [Google Scholar]
- Kambhampati, S.; Li, J.; Evans, B.S.; Allen, D.K. Accurate and efficient amino acid analysis for protein quantification using hydrophilic interaction chromatography coupled tandem mass spectrometry. Plant Methods 2019, 15, 46. [Google Scholar] [CrossRef]
- Kovalenko, I.V.; Rippke, G.R.; Hurburgh, C.R. Measurement of soybean fatty acids by near-infrared spectroscopy: Linear and nonlinear calibration methods. J. Am. Oil Chem. Soc. 2006, 83, 421–427. [Google Scholar] [CrossRef]
- Ng, W.; Minasny, B.; Mendes, W.d.S.; Demattê, J.A.M. The influence of training sample size on the accuracy of deep learning models for the prediction of soil properties with near-infrared spectroscopy data. Soil 2020, 6, 565–578. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, P.; Zhang, F.; Yang, S.; Rady, A.; Wijewardane, N.K.; Huang, J.; Li, M. Fourier-transform infrared spectroscopy and machine learning to predict amino acid content of nine commercial insects. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Nestel, D.; Tolmasky, D.; Rabossi, A.; Quesada-Allué, L.A. Lipid, Carbohydrates and Protein Patterns During Metamorphosis of the Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2003, 96, 237–244. [Google Scholar] [CrossRef]


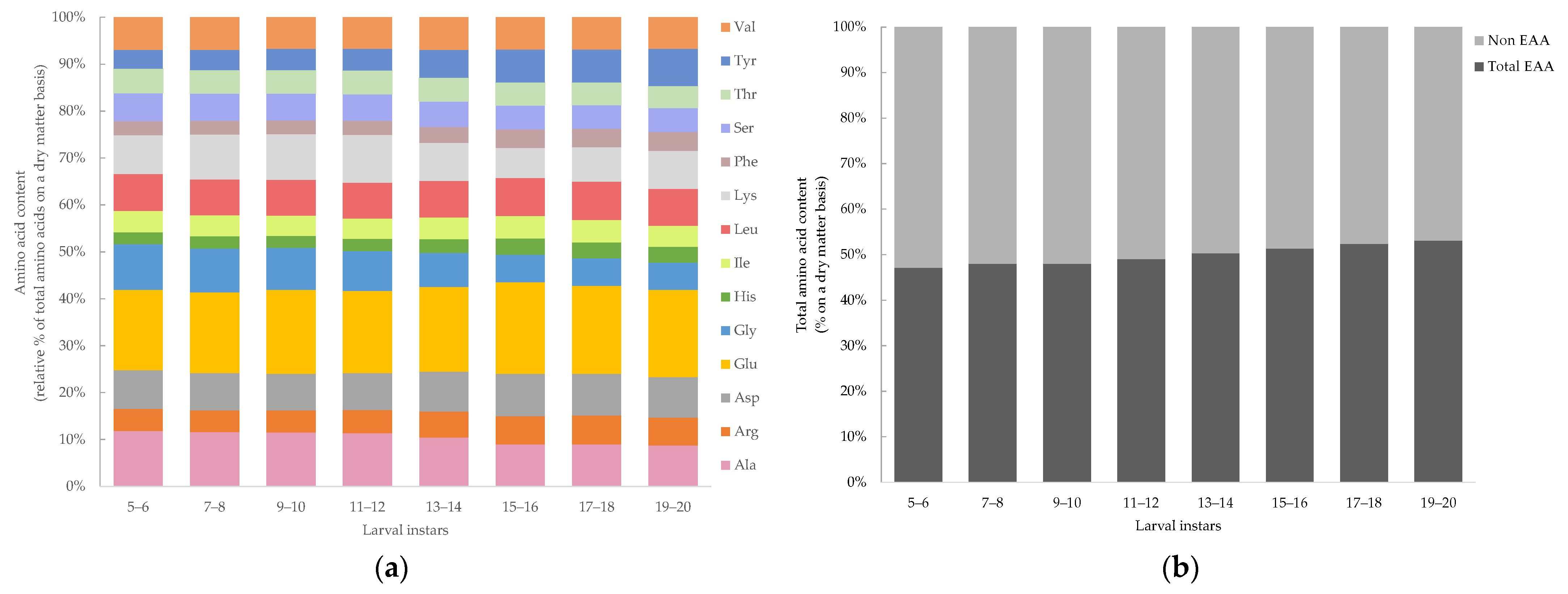
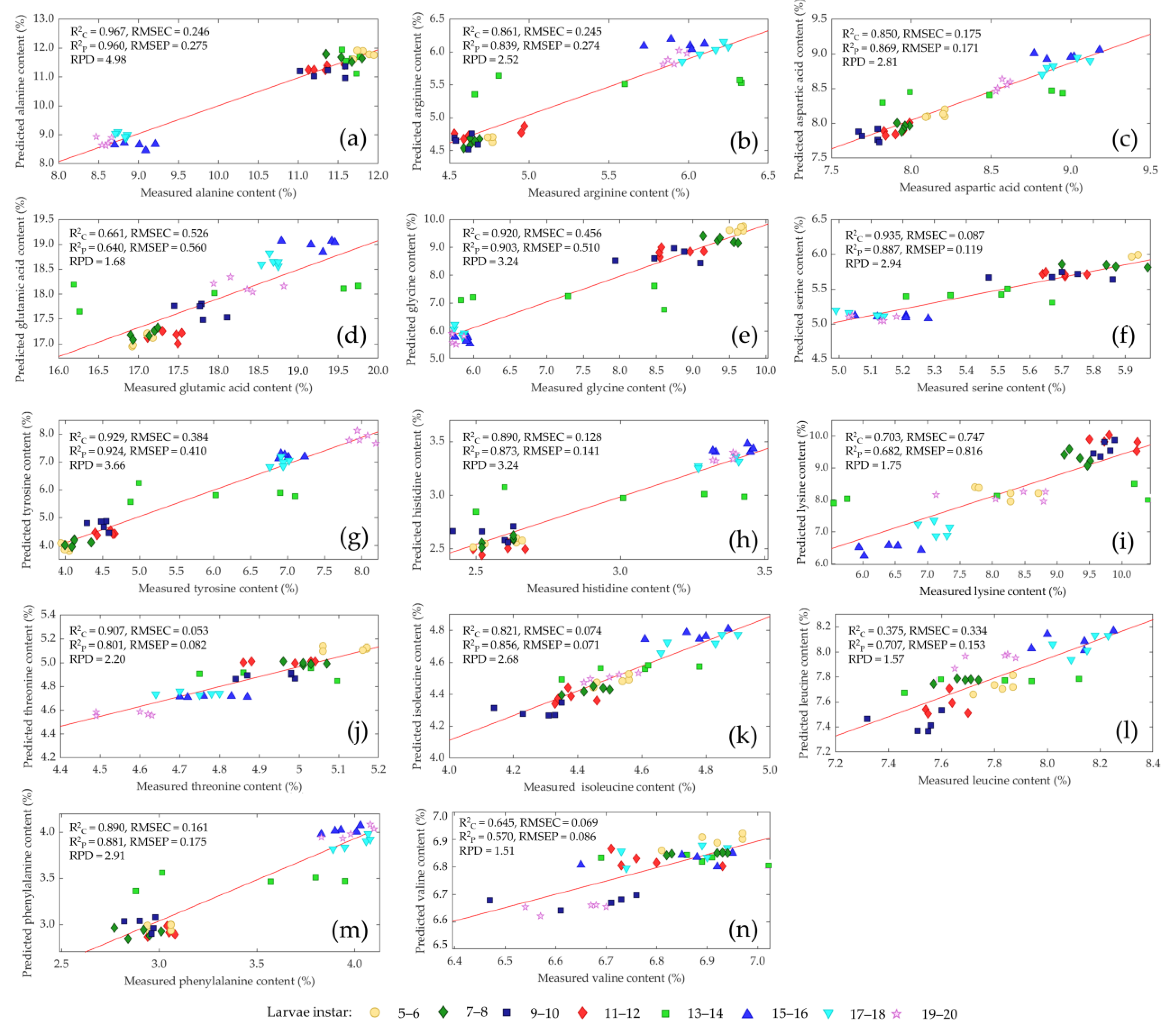

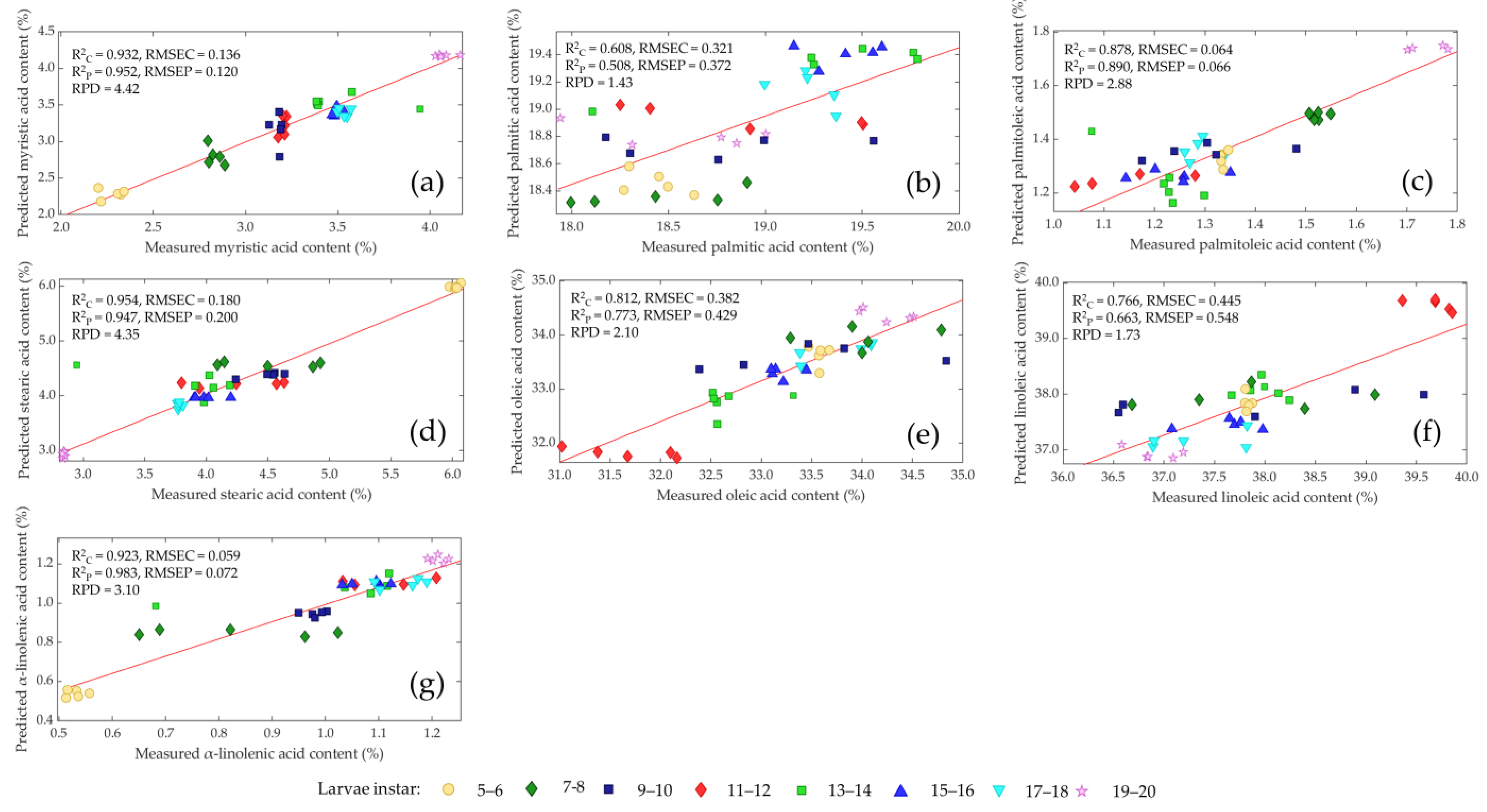
| Nutrient Component | Larvae Instars | |||||||
|---|---|---|---|---|---|---|---|---|
| 5–6 | 7–8 | 9–10 | 11–12 | 13–14 | 15–16 | 17–18 | 19–20 | |
| Moisture (%) * | 67.3 ± 0.2 a | 65.8 ± 1.5 a | 66.1 ± 1.1 a | 65.1 ± 2.1 a | 65.6 ± 0.4 a | 64.4 ± 0.9 a | 63.9 ± 0.8 a | 57.7 ± 1.5 b |
| Crude Protein (% FW) * | 25.7 ± 0.6 a | 23.7 ± 0.5 ab | 22.9 ± 0.1 ab | 22.6 ± 0.3 b | 22.6 ± 0.7 b | 22.7 ± 0.4 b | 22.7 ± 0.6 b | 21.3 ± 0.2 b |
| Crude Fat (% FW) ** | 4.9 ± 0.0 b | 7.1 ± 1.8 b | 7.0 ± 1.8 b | 6.7 ± 2.8 b | 11.7 ± 1.0 a | 12.3 ± 0.7 a | 11.8 ± 1.7 a | 15.7 ± 0.7 a |
| Amino Acid | Calibration Set (%) | Validation Set (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | SD | Minimum | Maximum | Mean | SD | |
| Ala | 8.74 | 11.82 | 10.36 | 1.36 | 8.47 | 11.95 | 10.50 | 1.37 |
| Arg | 4.56 | 6.38 | 5.27 | 0.66 | 4.53 | 6.33 | 5.28 | 0.69 |
| Asp | 7.71 | 9.18 | 8.30 | 0.46 | 7.67 | 9.18 | 8.33 | 0.48 |
| Glu | 15.94 | 19.86 | 17.95 | 0.91 | 16.19 | 19.75 | 17.94 | 0.94 |
| Gly | 5.77 | 9.69 | 7.62 | 1.62 | 5.70 | 9.69 | 7.64 | 1.65 |
| His | 2.48 | 3.40 | 2.91 | 0.39 | 2.42 | 3.46 | 2.92 | 0.40 |
| Ile | 4.20 | 4.84 | 4.51 | 0.18 | 4.14 | 4.90 | 4.53 | 0.19 |
| Leu | 7.38 | 8.20 | 7.75 | 0.42 | 7.32 | 8.25 | 7.80 | 0.24 |
| Lys | 5.61 | 10.46 | 8.36 | 1.38 | 5.55 | 10.41 | 8.36 | 1.43 |
| Phe | 2.71 | 4.12 | 3.39 | 0.49 | 2.77 | 4.10 | 3.40 | 0.51 |
| Ser | 5.02 | 6.00 | 5.49 | 0.34 | 4.99 | 6.03 | 5.51 | 0.35 |
| Thr | 4.54 | 5.12 | 4.87 | 0.17 | 4.49 | 5.17 | 4.88 | 0.18 |
| Tyr | 4.02 | 7.98 | 5.64 | 1.45 | 3.94 | 8.19 | 5.63 | 1.50 |
| Val | 6.53 | 6.98 | 6.79 | 0.12 | 6.47 | 7.02 | 6.80 | 0.13 |
| Amino Acid | Mathematical Treatment | No. of Latent Variables | Calibration Set | Validation Set | |||
|---|---|---|---|---|---|---|---|
| R2c | RMSEC | R2p | RMSEP | RPD | |||
| Ala | SNV | 8 | 0.967 | 0.246 | 0.960 | 0.275 | 4.98 |
| Arg | SNV | 8 | 0.861 | 0.245 | 0.839 | 0.274 | 2.52 |
| Asp | SNV | 8 | 0.850 | 0.175 | 0.869 | 0.171 | 2.81 |
| Glu | 1D | 8 | 0.661 | 0.526 | 0.640 | 0.560 | 1.68 |
| Gly | SNV | 8 | 0.920 | 0.456 | 0.903 | 0.510 | 3.24 |
| His | SNV | 8 | 0.890 | 0.128 | 0.873 | 0.141 | 2.84 |
| Ile | SNV | 8 | 0.821 | 0.074 | 0.856 | 0.071 | 2.68 |
| Leu | 1D | 8 | 0.775 | 0.334 | 0.707 | 0.153 | 1.57 |
| Lys | 1D | 8 | 0.703 | 0.747 | 0.682 | 0.816 | 1.75 |
| Phe | None | 8 | 0.890 | 0.161 | 0.881 | 0.175 | 2.91 |
| Ser | SNV | 8 | 0.935 | 0.087 | 0.887 | 0.119 | 2.94 |
| Thr | SNV | 8 | 0.907 | 0.053 | 0.801 | 0.082 | 2.20 |
| Tyr | SNV | 8 | 0.929 | 0.384 | 0.924 | 0.410 | 3.66 |
| Val | SNV | 8 | 0.645 | 0.069 | 0.570 | 0.086 | 1.51 |
| Fatty Acid | Calibration Set (%) | Validation Set (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | SD | Minimum | Maximum | Mean | SD | |
| Myristic acid (C14:0) | 2.18 | 4.22 | 3.27 | 0.52 | 2.18 | 4.19 | 3.25 | 0.53 |
| Palmitic acid (16:0) | 17.83 | 19.79 | 18.89 | 0.52 | 17.94 | 19.78 | 18.91 | 0.53 |
| Palmitoleic acid (C16:1) | 1.04 | 1.80 | 1.36 | 0.18 | 1.04 | 1.78 | 1.36 | 0.19 |
| Stearic acid (C18:0) | 2.82 | 6.07 | 4.25 | 0.85 | 2.83 | 6.07 | 4.24 | 0.87 |
| Oleic acid (C18:1 n-9) | 31.01 | 34.99 | 33.29 | 0.89 | 31.02 | 34.83 | 33.32 | 0.90 |
| α-Linoleic acid (C18:2 n-6) | 36.44 | 39.84 | 37.95 | 0.93 | 36.55 | 39.85 | 37.89 | 0.95 |
| α-Linolenic acid (C18:3 n-3) | 0.51 | 1.25 | 1.01 | 0.22 | 0.51 | 1.26 | 1.00 | 0.22 |
| Fatty Acid | Mathematical Treatment | No. of Latent Variables | Calibration Set | Validation Set | |||
|---|---|---|---|---|---|---|---|
| R2C | RMSEC | R2P | RMSEP | RPD | |||
| Myristic acid (C14:0) | SNV | 8 | 0.932 | 0.136 | 0.952 | 0.120 | 4.42 |
| Palmitic acid (C16:0) | 1D | 8 | 0.608 | 0.321 | 0.508 | 0.372 | 1.43 |
| Palmitoleic acid (C16:1) | SNV | 8 | 0.878 | 0.064 | 0.890 | 0.066 | 2.88 |
| Stearic acid (C18:0) | None | 8 | 0.954 | 0.180 | 0.947 | 0.200 | 4.35 |
| Oleic acid (C18:1 n-9) | SNV | 8 | 0.812 | 0.382 | 0.773 | 0.429 | 2.10 |
| Linoleic acid (C18:2 n-6) | 1D | 8 | 0.766 | 0.445 | 0.663 | 0.548 | 1.73 |
| α-Linolenic acid (C18:3 n-3) | 1D | 8 | 0.923 | 0.059 | 0.893 | 0.072 | 3.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kröncke, N.; Wittke, S.; Steinmann, N.; Benning, R. Analysis of the Composition of Different Instars of Tenebrio molitor Larvae using Near-Infrared Reflectance Spectroscopy for Prediction of Amino and Fatty Acid Content. Insects 2023, 14, 310. https://doi.org/10.3390/insects14040310
Kröncke N, Wittke S, Steinmann N, Benning R. Analysis of the Composition of Different Instars of Tenebrio molitor Larvae using Near-Infrared Reflectance Spectroscopy for Prediction of Amino and Fatty Acid Content. Insects. 2023; 14(4):310. https://doi.org/10.3390/insects14040310
Chicago/Turabian StyleKröncke, Nina, Stefan Wittke, Nico Steinmann, and Rainer Benning. 2023. "Analysis of the Composition of Different Instars of Tenebrio molitor Larvae using Near-Infrared Reflectance Spectroscopy for Prediction of Amino and Fatty Acid Content" Insects 14, no. 4: 310. https://doi.org/10.3390/insects14040310
APA StyleKröncke, N., Wittke, S., Steinmann, N., & Benning, R. (2023). Analysis of the Composition of Different Instars of Tenebrio molitor Larvae using Near-Infrared Reflectance Spectroscopy for Prediction of Amino and Fatty Acid Content. Insects, 14(4), 310. https://doi.org/10.3390/insects14040310





