The Potential of Fluralaner as a Bait Toxicant to Control Pest Yellowjackets in California
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Field Sites
2.2. Monitoring
2.3. Bait Stations
2.4. Bait Preparation
2.5. Evaporation Controls
2.6. Choice Tests
2.7. Efficacy Studies
2.7.1. Field Trials Conducted in 2020
2.7.2. Field Trials Conducted in 2021
2.8. DNA Extraction and Microsatellite Genotyping
2.9. Data Analyses
3. Results
3.1. Choice Tests
3.2. Baiting Studies
3.2.1. Field Trials Conducted in 2020
3.2.2. Field Trials Conducted in 2021
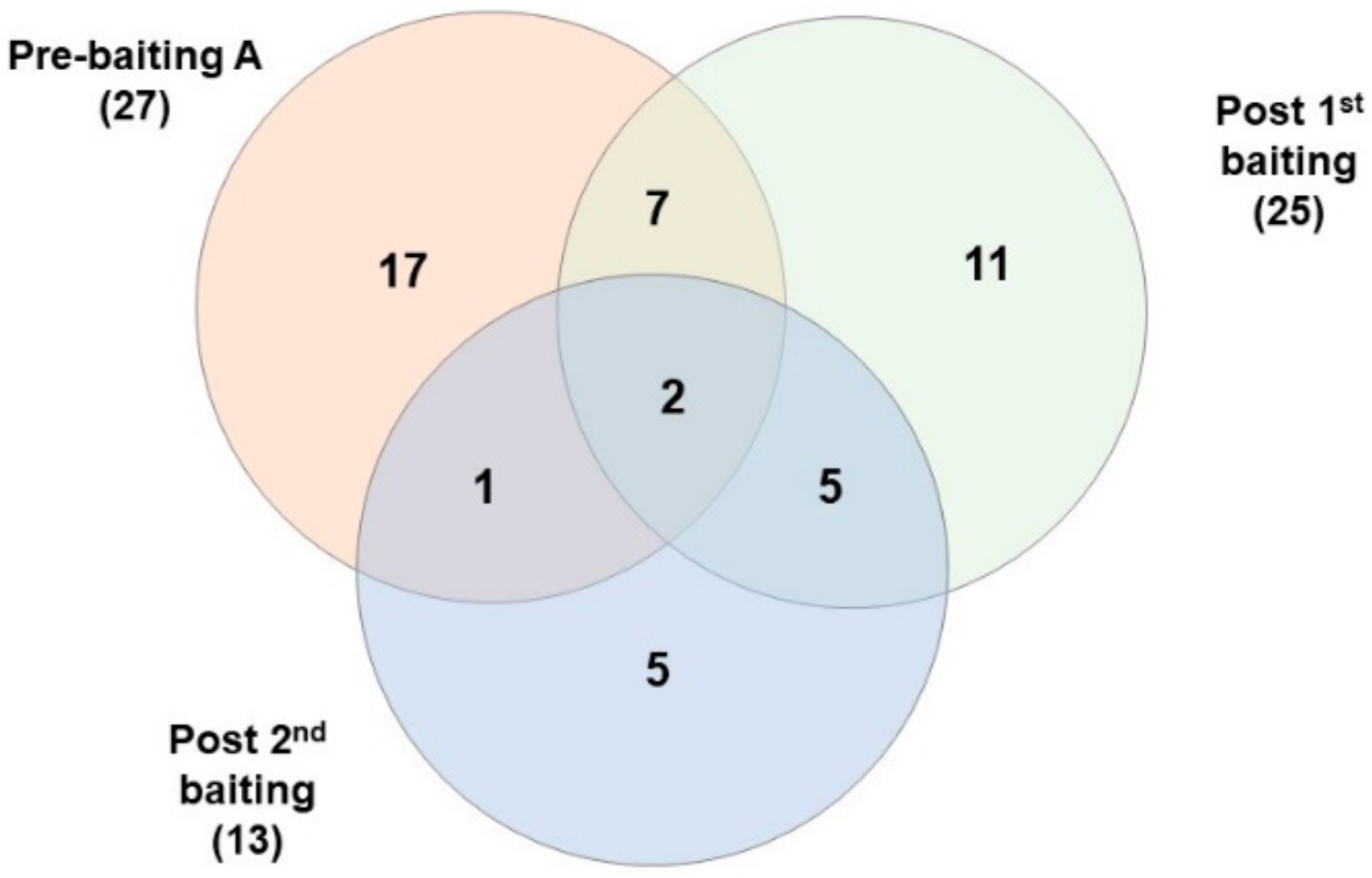
3.3. DNA Extraction and Microsatellite Genotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akre, R.D.; MacDonald, J.F. Biology, economic importance and control of yellow jackets. In Economic Impact and Control of Social Insects; Vinson, S.B., Ed.; Praeger: New York, NY, USA, 1986; pp. 353–412. [Google Scholar]
- Rust, M.K.; Reierson, D.A.; Vetter, R.S. Developing Baits for the Control of Yellowjackets in California. Final Report 2010 for Structural Pest Control Board Structural Pest Control Board, Grant No. 041–04. 2010; pp. 1–33. Available online: http://www.pestboard.ca.gov/howdoi/research/2009_yellowjacket.pdf (accessed on 5 March 2023).
- Rust, M.K.; Su, N.-Y. Managing social insects of urban importance. Annu. Rev. Entomol. 2012, 57, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Akre, R.D. Economics and control of yellowjackets (Vespula, Dolichovespula). In The Biology of Social Insects; Breed, M.D., Michener, C.D., Evans, H.E., Eds.; Westview Press: Boulder, CO, USA, 1982; pp. 109–113. [Google Scholar]
- Ebeling, W. Urban Entomology; Division of Agricultural Sciences, University of California: Berkeley, CA, USA, 1975. [Google Scholar]
- Davis, H.G.; Zwick, R.W.; Rogoff, W.M.; McGovern, T.P.; Berzoza, M. Perimeter traps baited with synthetic lures for suppression of yellowjackets in fruit orchards. Environ. Entomol. 1973, 2, 569–571. [Google Scholar] [CrossRef]
- Langley, R.L. Animal-related fatalities in the United States—An update. Wilderness Environ. Med. 2005, 16, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.; Mack, K.; Haileyesus, T.; Proescholdsbell, S.; Annest, J.L. National estimates of noncanine bite and sting injuries treated in US hospital emergency departments, 2001–2010. Wilderness Environ. Med. 2014, 255, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, M.G.; Holzer, K.J.; Carbone, J.T.; Salas-Wright, C.P. Arthropod bites and stings treated in emergency departments: Recent trends and correlates. Wilderness Environ. Med. 2019, 30, 394–400. [Google Scholar] [CrossRef]
- Reierson, D.A.; Wagner, R.E. Trapping yellowjackets with a new standard plastic wet trap. J. Econ. Entomol. 1975, 68, 395–398. [Google Scholar] [CrossRef]
- Gulmahamad, H. Yellowjacket control gets creative. Pest Control 2002, 70, 34–41. [Google Scholar]
- Reierson, D.A.; Rust, M.K.; Vetter, R.S. Traps and protein bait to suppress populations of yellowjackets (Hymenoptera: Vespidae). In Proceedings of the Sixth International Conference on Urban Pests, Budapest, Hungary, 13–16 July 2008; Available online: https://.www.icup.org.uk/media/bwonefa0/icup875.pdf (accessed on 5 March 2023).
- Mussen, E.C.; Rust, M.K. Yellowjackets and Other Social Wasps. 2012. Available online: http://ipm.ucanr.edu/PMG/PESTNOTES/pn7450.html (accessed on 21 March 2022).
- Choe, D.-H.; Campbell, K.; Hoddle, M.S.; Kabashima, J.; Dimson, M.; Rust, M.K. Evaluation of a hydrogel matrix for baiting western yellowjacket (Vespidae: Hymenoptera). J. Econ. Entomol. 2018, 111, 1799–1805. [Google Scholar] [CrossRef]
- Hanna, C.; Foote, D.; Kremen, C. Short-and long-term control of Vespula pensylvanica in Hawaii by fipronil baiting. Pest Manag. Sci. 2012, 68, 1026–1033. [Google Scholar] [CrossRef]
- Sackmann, P.; Rabinovich, M.; Corley, J.C. Successful removal of German yellowjackets (Hymenoptera: Vespidae) by toxic baiting. J. Econ. Entomol. 2001, 94, 811–816. [Google Scholar] [CrossRef]
- Harris, R.J.; Etheridge, N.D. Comparison of baits containing fipronil and sulfuramid for control of Vespula wasps. N. Z. J. Zool. 2001, 28, 39–48. [Google Scholar] [CrossRef]
- Rust, M.K. Recent advancements in the control of cat fleas. Insects 2020, 11, 668. [Google Scholar] [CrossRef]
- Asahi, M.; Kobayashi, M.; Matsui, H.; Nakahira, K. Differential mechanisms of action of the novel 𝜸-aminobutyric acid receptor antagonist ectoparasiticides fluralaner (A1443) and fipronil. Pest Manag. Sci. 2015, 71, 91–95. [Google Scholar] [CrossRef]
- Sheng, C.-W.; Jia, Z.-Q.; Liu, D.; Wu, H.-Z.; Luo, X.-M.; Song, P.-P.; Xu, L.; Peng, Y.-C.; Han, Z.-J.; Zhao, C.-Q. Insecticidal spectrum of fluralaner to agricultural and sanitary pests. J. Asia-Pac. Entomol. 2017, 20, 1213–1218. [Google Scholar] [CrossRef]
- Rust, M.K.; Choe, D.-H.; Wilson-Rankin, E.; Campbell, K.; Kabashima, J.; Dimson, M. Controlling yellow jackets with fipronil-based protein baits in urban recreational areas. Int. J. Pest Manag. 2017, 63, 234–241. [Google Scholar] [CrossRef]
- Rust, M.K.; Choe, D.-H.; Sutherland, A.; Sorensen, M.; Nobua-Behrmann, B.; Kabashima, J.; Campbell, K.; Hubble, C.; Park, H.E. Controlling yellowjackets in urban recreational areas. In Proceedings of the Tenth International Conference on Urban Pests, Barcelona, Spain, 27–29 June 2022; Bueno-Mari, R., Montalvo, T., Robinson, B., Eds.; Available online: https://www.icup.org.uk/media/humonick/3-rust-17-f-pp-45-48.pdf (accessed on 5 March 2023).
- Daly, D.; Archer, M.E.; Watts, P.C.; Speed, M.P.; Hughes, M.R.; Barker, F.S.; Jones, J.; Odgaard, K.; Kemp, S.J. Polymorphic microsatellite loci for eusocial wasps (Hymenoptera: Vespidae). Mol. Ecol. Notes 2002, 2, 273–275. [Google Scholar] [CrossRef]
- Hasegawa, E.; Takahashi, J. Microsatellite loci for genetic research in the hornet Vespa mandarinia and related species. Mol. Ecol. Notes 2002, 2, 306–308. [Google Scholar] [CrossRef]
- Thoren, P.A.; Paxton, R.J.; Estoup, A. Unusually high frequency of (CT)n and (GT)n microsatellite loci in yellow-jacket wasp, Vespula rufa (L.) (Hymenoptera: Vespidae). Insect Mol. Biol. 1995, 4, 141–148. [Google Scholar] [CrossRef]
- Jones, O.R.; Wang, J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Resour. 2010, 10, 551–555. [Google Scholar] [CrossRef]
- Statistix 10; Analytical Software: Tallahassee, FL, USA, 2013.
- Grant, C.D.; Rogers, C.J.; Lauret, T.H. Control of ground-nesting yellow jackets with toxic baits—A five-year testing program. J. Econ. Entomol. 1968, 61, 1653–1656. [Google Scholar] [CrossRef]
- Rogers, C.J. Field testing of “Yellow Jackets Stoppers”® and population depletion trapping for the control of ground-nesting yellowjackets. Proc. Pap. Conf. Calif. Mosq. Vector Control Assoc. 1972, 40, 132–134. [Google Scholar]
- MacDonald, J.F.; Akre, R.D.; Matthews, R.W. Evaluation of yellowjacket abatement in the United States. Bull. Entomol. Soc. Am. 1976, 22, 397–401. [Google Scholar] [CrossRef]
- Loope, K.J.; Millar, J.G.; Wilson Rankin, E.E. Weak nestmate discrimination behavior in native and invasive populations of a yellowjacket wasp (Vespula pensylvanica). Biol. Invasions 2018, 20, 3431–3444. [Google Scholar] [CrossRef]
- Akre, R.D.; Hill, W.B.; MacDonald, J.F.; Garnett, W.B. Foraging distances of Vespula pensylvanica workers (Hymenoptera: Vespidae). J. Kansas Entomol. Soc. 1975, 48, 12–16. [Google Scholar]
- Visscher, P.K.; Vetter, R.S. Annual and multi-year nests of the western yellowjacket, Vespula pensylvanica, in California. Insectes Soc. 2002, 50, 160–166. [Google Scholar] [CrossRef]

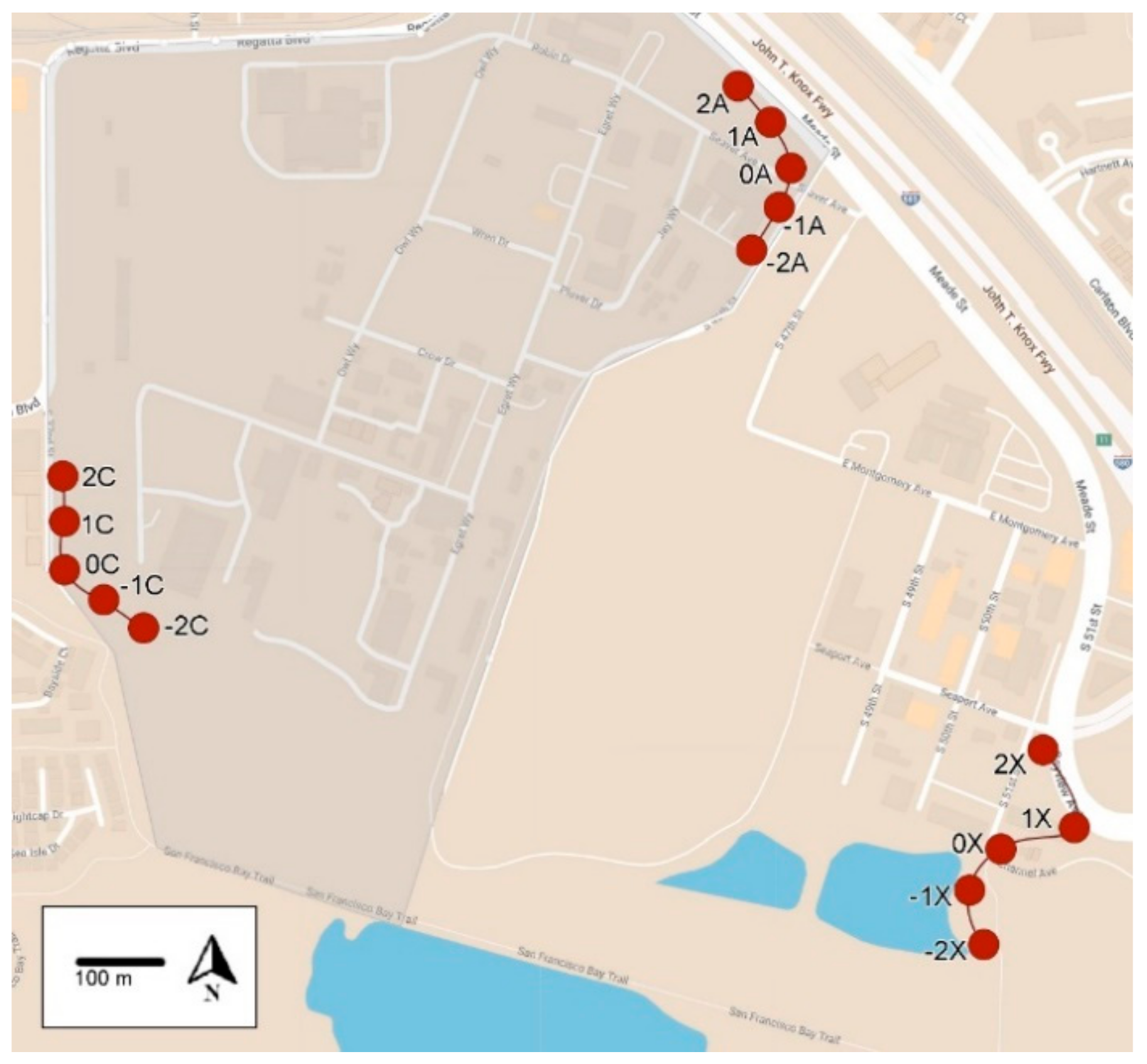



| Locations | No. Monitoring Traps | Average YJ/T/D (% Reduction) Days after Baiting | |||
|---|---|---|---|---|---|
| Pre-Baiting | Day 20 | Day 27 | Day 34 | ||
| 0A, −1A, −2A | 3 | 26.9 | 1.5 (94) | 3.4 (84) | 1.4 (93) |
| Transect A (all 9 traps) | 9 | 20.2 | 3.0 (85) | 5.6 (72) | 5.0 (75) |
| Transect B (untreated) | 7 | 6.1 | 16.2 (0) | 11.9 (0) | 2.3 (62) |
| Transect C (untreated) | 7 | 4.4 | 11.9 (0) | 9.2 (0) | 4.0 (9) |
| Locations | No. Monitoring Traps | Average YJ/T/D (% Reduction) Days after Baiting | ||
|---|---|---|---|---|
| Pre-Baiting | Day 11 | Day 20 | ||
| −2A, −3A, −4A | 3 | 27.4 | 0.5 (96) | 1.3 (84) |
| Transect A (all 9 traps) | 9 | 5.0 | 0.1 (96) | 0.2 (94) |
| Transect B (untreated) | 7 | 2.3 | 0.4 (68) | 0.2 (88) |
| Transect C (untreated) | 7 | 4.0 | 0.8 (60) | 0.4 (87) |
| Locations | No. Monitoring Traps | Average YJ/T/D (% Reduction) | ||
|---|---|---|---|---|
| Pre-Baiting | Day 14 | Day 35 | ||
| Treated area | 6 | 14.87 | 1.88 (87) | 1.80 (88) |
| Untreated area | 10 | 4.61 | 0.79 (83) | 5.06 (0) |
| Locations | Average YJ/T/D (% Reduction) Days after Baiting | |||||
|---|---|---|---|---|---|---|
| Pre-Bait | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | |
| Transect A (treated) | 14.40 | 9.74 (32) | 7.83 (46) | 12.83 (11) | 10.31 (28) | 20.83 (0) |
| Transect C (untreated) | 2.40 | 5.86 (0) | 4.08 (0) | 13.5 (0) | 8.49 (0) | 20.80 (0) |
| Transect X (untreated) | 0.57 | 0.23 (60) | 0.43 (25) | 0.54 (5) | 0.51 (10) | 0.57 (0) |
| Bait | No. Monitoring Traps | Average YJ/T/D (% Reduction) | ||
|---|---|---|---|---|
| Pre-Baiting | Day 21 | Day 42 | ||
| 0.045% fluralaner | 12 | 3.28 | 1.87 (43) | 2.92 (11) |
| 0.022% fluralaner | 6 | 5.86 | 1.83 (69) | 0.84 (86) |
| Untreated | 22 | 0.94 | 0.76 (19) | 0.38 (60) |
| Transect a | Time of Sample Collection | No. Specimens Collected | No. Specimens Genotyped | Proportion of Genotyped Individuals | Minimum No. Colonies Detected |
|---|---|---|---|---|---|
| A | Pre-baiting | 1273 | 45 | 0.04 | 27 |
| 20 days after 1st baiting | 630 | 37 | 0.06 | 19 | |
| 34 days after 1st baiting | 314 | 33 | 0.11 | 15 | |
| 11 days after 2nd baiting | 13 | 10 | 0.77 | 8 | |
| 20 days after 2nd baiting | 19 | 11 | 0.58 | 7 | |
| B | Pre-Baiting | 298 | 34 | 0.11 | 10 |
| 20 days after 1st baiting | 792 | 30 | 0.04 | 9 | |
| 11 days after 2nd baiting | 36 | 29 | 0.81 | 14 | |
| C | Pre-Baiting | 214 | 26 | 0.12 | 12 |
| 20 days after 1st baiting | 585 | 30 | 0.05 | 11 | |
| 11 days after 2nd baiting | 79 | 24 | 0.30 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rust, M.K.; Lee, C.-Y.; Park, H.E.; Campbell, K.; Choe, D.-H.; Sorensen, M.; Sutherland, A.; Hubble, C.; Nobua-Behrmann, B.; Kabashima, J.; et al. The Potential of Fluralaner as a Bait Toxicant to Control Pest Yellowjackets in California. Insects 2023, 14, 311. https://doi.org/10.3390/insects14040311
Rust MK, Lee C-Y, Park HE, Campbell K, Choe D-H, Sorensen M, Sutherland A, Hubble C, Nobua-Behrmann B, Kabashima J, et al. The Potential of Fluralaner as a Bait Toxicant to Control Pest Yellowjackets in California. Insects. 2023; 14(4):311. https://doi.org/10.3390/insects14040311
Chicago/Turabian StyleRust, Michael K., Chow-Yang Lee, Ho Eun Park, Kathleen Campbell, Dong-Hwan Choe, Mary Sorensen, Andrew Sutherland, Casey Hubble, Beatriz Nobua-Behrmann, John Kabashima, and et al. 2023. "The Potential of Fluralaner as a Bait Toxicant to Control Pest Yellowjackets in California" Insects 14, no. 4: 311. https://doi.org/10.3390/insects14040311
APA StyleRust, M. K., Lee, C.-Y., Park, H. E., Campbell, K., Choe, D.-H., Sorensen, M., Sutherland, A., Hubble, C., Nobua-Behrmann, B., Kabashima, J., Tseng, S.-P., & Post, L. (2023). The Potential of Fluralaner as a Bait Toxicant to Control Pest Yellowjackets in California. Insects, 14(4), 311. https://doi.org/10.3390/insects14040311









