Can Macrolophus pygmaeus (Hemiptera: Miridae) Mitigate the Damage Caused to Plants by Bemisia tabaci (Hemiptera: Aleyrodidae)?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Experimental Design and Sampling
2.3. Data Analysis
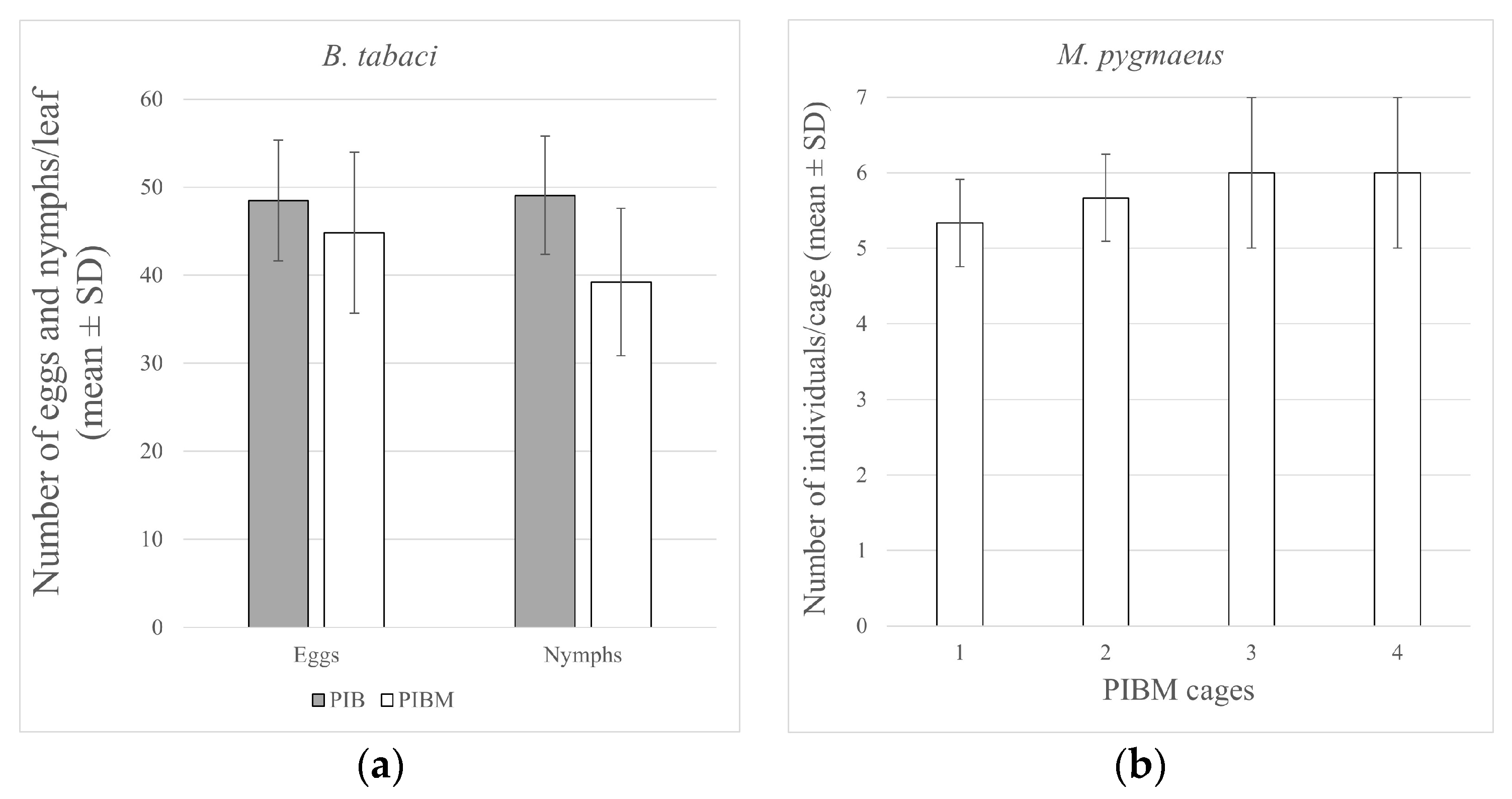
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gange, A.C.; Brown, V.K. Multitrophic Interactions in Terrestrial Systems; Blackwell Science Ltd.: Oxford, UK, 1997; p. 458. [Google Scholar]
- Tscharntke, T.; Hawkins, B.A. Multitrophic Level Interactions; Cambridge University Press: Cambridge, UK, 2002; pp. 1–7. [Google Scholar]
- Ohgushi, T. Indirect interaction webs: Herbivore-induced effects through trait change in plants. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 81–105. [Google Scholar] [CrossRef]
- Shikano, I. Evolutionary ecology of multitrophic interactions between plants, insect herbivores and entomopathogens. J. Chem. Ecol. 2017, 43, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.J.; Raguso, R.A. Lingering effects of herbivory and plant defenses on pollinators. Curr. Biol. 2018, 28, R1164–R1169. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Araj, S.E. Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol. Control 2018, 116, 53–61. [Google Scholar] [CrossRef]
- Zytynska, S.E.; Meyer, S.T. Effects of biodiversity in agricultural landscapes on the protective microbiome of insects—A review. Entomol. Exp. Et Appl. 2019, 167, 2–13. [Google Scholar]
- Schifani, E.; Castracani, C.; Giannetti, D.; Spotti, F.A.; Reggiani, R.; Leonardi, S.; Mori, A.; Grasso, D.A. New tools for conservation biological control: Testing ant-attracting artificial Nectaries to employ ants as plant defenders. Insects 2020, 11, 129. [Google Scholar] [CrossRef]
- Gerling, D. Whiteflies: Their Bionomics, Pest Status and Management; Intercept Ltd. Press: Andover, UK, 1990; p. 348. [Google Scholar]
- Martin, J.H.; Mifsud, D.; Rapisarda, C. The whiteflies (Hemiptera: Aleyrodidae) of Europe and the Mediterranean basin. Bull. Entomol. Res. 2000, 90, 407–448. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar]
- Fiallo-Olivé, E.; Pan, L.L.; Liu, S.S.; Navas-Castillo, J. Transmission of begomoviruses and other whitefly-borne viruses: Dependence on the vector species. Phytopathology 2020, 110, 10–17. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Phytopathol. 2011, 56, 1–19. [Google Scholar]
- Liu, S.S.; Colvin, J.; De Barro, P.J. Species concepts as applied to the whitefly Bemisia tabaci systematics: How many species are there? J. Integr. Agric. 2012, 11, 176–186. [Google Scholar] [CrossRef]
- Lee, W.; Park, J.; Lee, G.S.; Lee, S.; Akimoto, S.I. Taxonomic status of the Bemisia tabaci complex (Hemiptera: Aleyrodidae) and reassessment of the number of its constituent species. PLoS ONE 2013, 8, e63817. [Google Scholar] [CrossRef] [PubMed]
- Boykin, L.M.; Savill, A.; De Barro, P. Updated mtCOI reference dataset for the Bemisia tabaci species complex. F1000Research 2017, 6, 1835. [Google Scholar] [CrossRef]
- Tan, X.L.; Wang, S.; Ridsdill-Smith, J.; Liu, T.X. Direct and indirect impacts of infestation of tomato plant by Myzus persicae (Hemiptera: Aphididae) on Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 2014, 9, e94310. [Google Scholar]
- Tan, X.L.; Chen, J.L.; Benelli, G.; Desneux, N.; Yang, X.Q.; Liu, T.X.; Ge, F. Pre-infestation of tomato plants by aphids modulates transmission-acquisition relationship among whiteflies, tomato yellow leaf curl virus (TYLCV) and plants. Front. Plant Sci. 2017, 8, 1597. [Google Scholar]
- Yang, F.; Tan, X.L.; Liu, F.H.; Wang, S.; Chen, J.L.; Yi, T.Y. A functional response evaluation of pre-infestation with Bemisia tabaci cryptic species MEAM1 on predation by Propylea japonica of Myzus persicae on host plant tomatoes. Arthropod Plant Interact. 2017, 11, 825–832. [Google Scholar]
- Moreno-Ripoll, R.; Gabarra, R.; Symondson, W.O.C.; King, R.A.; Agustí, N. Do the interactions among natural enemies compromise the biological control of the whitefly Bemisia tabaci? J. Pest Sci. 2014, 87, 133–141. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Bouagga, S.; Zhang, N.X.; Moerkens, R.; Messelink, G.; Jaques, J.A.; Flors, V.; Broufas, G.; Urbaneja, A.; Pappas, M.L. Induction of plant defenses: The added value of zoophytophagous predators. J. Pest Sci. 2022, 95, 1501–1517. [Google Scholar] [CrossRef]
- Ashra, H.; Nair, S. Trait plasticity during plant-insect interactions: From molecular mechanisms to impact on community dynamics. Plant Sci. 2022, 317, 111188. [Google Scholar]
- Dinant, S.; Kehr, J. Sampling and analysis of phloem sap. In Plant Mineral Nutrients; Humana Press: Totowa, NJ, USA, 2013; pp. 185–194. [Google Scholar]
- De Lima Toledo, C.A.; da Silva Ponce, F.; Oliveira, M.D.; Aires, E.S.; Seabra Júnior, S.; Lima, G.P.P.; de Oliveira, R.C. Change in the Physiological and Biochemical Aspects of Tomato Caused by Infestation by Cryptic Species of Bemisia tabaci MED and MEAM1. Insects 2021, 12, 1105. [Google Scholar]
- Buntin, D.G.; Gilbertz, D.A.; Oetting, R.D. Chlorophyll loss and gas exchange in tomato leaves after feeding injury by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 1993, 86, 517–522. [Google Scholar]
- Islam, M.T.; Qiu, B.; Ren, S. Host preference and influence of the sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) on eggplant (Solanum melongena L.). Acta Agric. Scand. Sect. B Soil Plant Sci. 2010, 60, 320–325. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Xue, M.; Zhao, H.; Wang, C. Dynamic changes in photosynthesis and chlorophyll fluorescence in Nicotiana tabacum infested by Bemisia tabaci (Middle East–Asia Minor 1) nymphs. Arthropod Plant Interact. 2013, 7, 431–443. [Google Scholar] [CrossRef]
- Chand, R.; Jokhan, A.; Prakash, R. Egg deposition by Spiralling whiteflies (Aleurodicus dispersus) reduces the stomatal conductance of cassava (Manihot esculenta). Wētā 2018, 52, 55–60. [Google Scholar]
- Yee, W.L.; Toscano, N.C.; Chu, C.C.; Henneberry, T.J.; Nichols, R.L. Bemisia argentifolii (Homoptera: Aleyrodidae) action thresholds and cotton photosynthesis. Environ. Entomol. 1996, 25, 1267–1273. [Google Scholar]
- Lin, T.B.; Wolf, S.; Schwartz, A.; Saranga, Y. Silverleaf whitefly stress impairs sugar export from cotton source leaves. Physiol. Plant. 2000, 109, 291–297. [Google Scholar]
- Lin, T.B.; Schwartz, A.; Saranga, Y. Photosynthesis and productivity of cotton under silverleaf whitefly stress. Crop Sci. 1999, 39, 174–184. [Google Scholar] [CrossRef]
- Islam, T.; Shunxiang, R. Effect of sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) infestation on eggplant (Solanum melongena L.) leaf. J. Pest Sci. 2009, 82, 211–215. [Google Scholar] [CrossRef]
- Farina, A.; Barbera, A.C.; Leonardi, G.; Massimino Cocuzza, G.E.; Suma, P.; Rapisarda, C. Bemisia tabaci (Hemiptera: Aleyrodidae): What Relationships with and Morpho-Physiological Effects on the Plants It Develops on? Insects 2022, 13, 351. [Google Scholar] [CrossRef]
- Urbaneja, A.; González-Cabrera, J.; Arno, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 2012, 68, 1215–1222. [Google Scholar]
- De Backer, L.; Megido, R.C.; Haubruge, É.; Verheggen, F.J. Macrolophus pygmaeus (Rambur) as an efficient predator of the tomato leafminer Tuta absoluta (Meyrick) in Europe. A review. Biotechnol. Agron. Soc. Environ. 2014, 18, 536–543. [Google Scholar]
- Wheeler, A.G. Biology of the Plant Bugs (Hemiptera: Miridae): Pests, Predators, Opportunists; Cornell University Press: Ithaca, NY, USA, 2001. [Google Scholar]
- Ingegno, B.L.; Pansa, M.G.; Tavella, L. Plant preference in the zoophytophagous generalist predator Macrolophus pygmaeus (Heteroptera: Miridae). Biol. Control 2011, 58, 174–181. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lopez-Gallego, E.; Pérez-Marcos, M.; Perera-Fernandez, L.G.; Ramirez-Soria, M.J. How safe is it to rely on Macrolophus pygmaeus (Hemiptera: Miridae) as a biocontrol agent in tomato crops? Front. Ecol. Evol. 2018, 6, 132. [Google Scholar]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 1991, 10, 506–513. [Google Scholar] [PubMed]
- De Barro, P.J.; Driver, F. Use of RAPD PCR to distinguish the B biotype from other biotypes of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Aust. J. Entomol. 1997, 36, 149–152. [Google Scholar] [CrossRef]
- Calvo, J.; Urbaneja, A. Nesidiocoris tenuis, un aliado para el control biológico de la mosca blanca. Hortic. Int. 2004, 44, 20–25. [Google Scholar]
- Alomar, O.; Riudavets, J.; Castañé, C. Macrolophus caliginosus in the biological control of Bemisia tabaci on greenhouse melons. Biol. Control 2006, 36, 154–162. [Google Scholar]
- Margaritopoulos, J.T.; Tsitsipis, J.A.; Perdikis, D.C. Biological characteristics of the mirids Macrolophus costalis and Macrolophus pygmaeus preying on the tobacco form of Myzus persicae (Hemiptera: Aphididae). Bull. Entomol. Res. 2003, 93, 39–45. [Google Scholar]
- Moerkens, R.; Berckmoes, E.; Van Damme, V.; Wittemans, L.; Tirry, L.; Casteels, H.; De Clercq, P.; De Vis, R. Inoculative release strategies of Macrolophus pygmaeus Rambur (Hemiptera: Miridae) in tomato crops: Population dynamics and dispersal. J. Plant Dis. Prot. 2017, 124, 295–303. [Google Scholar] [CrossRef]
- Sanchez, J.A.; López-Gallego, E.; Pérez-Marcos, M.; Perera-Fernández, L. The effect of banker plants and pre-plant release on the establishment and pest control of Macrolophus pygmaeus in tomato greenhouses. J. Pest Sci. 2021, 94, 297–307. [Google Scholar] [CrossRef]
- Bresch, C.; Ottenwalder, L.; Poncet, C.; Parolin, P. Tobacco as banker plant for Macrolophus pygmaeus to control Trialeurodes vaporariorum in tomato crops. Univers. J. Agric. Res. 2014, 2, 297–304. [Google Scholar] [CrossRef]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1997. [Google Scholar]
- Schutze, I.X.; Yamamoto, P.T.; Malaquias, J.B.; Herritt, M.; Thompson, A.; Merten, P.; Naranjo, S.E. Correlation-Based Network Analysis of the Influence of Bemisia tabaci Feeding on Photosynthesis and Foliar Sugar and Starch Composition in Soybean. Insects 2022, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Ibaraki, Y.; Murakami, J. Distribution of chlorophyll fluorescence parameter Fv/Fm within individual plants under various stress conditions. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Advances in Environmental Control, Automation, Madrid, Spain, 21–23 June 2006; Volume 761, pp. 255–260. [Google Scholar]
- Fernández-Marín, B.; Gago, J.; Clemente-Moreno, M.J.; Flexas, J.; Gulías, J.; García-Plazaola, J.I. Plant pigment cycles in the high-Arctic Spitsbergen. Polar Biol. 2019, 42, 675–684. [Google Scholar] [CrossRef]
- Magagnoli, S.; Masetti, A.; Depalo, L.; Sommaggio, D.; Campanelli, G.; Leteo, F.; Lövei, G.L.; Burgio, G. Cover crop termination techniques affect ground predation within an organic vegetable rotation system: A test with artificial caterpillars. Biol. Control 2018, 117, 109–114. [Google Scholar]
- Stout, M.J.; Zehnder, G.W.; Baur, M.E. Potential for the use of elicitors of plant resistance in arthropod management programs. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 2002, 51, 222–235. [Google Scholar]
- Pappas, M.L.; Steppuhn, A.; Geuss, D.; Topalidou, N.; Zografou, A.; Sabelis, M.W.; Broufas, G.D. Beyond predation: The zoophytophagous predator Macrolophus pygmaeus induces tomato resistance against spider mites. PLoS ONE 2015, 10, e0127251. [Google Scholar]
- Perdikis, D.C.; Lykouressis, D.P. Macrolophus pygmaeus (Hemiptera: Miridae) population parameters and biological characteristics when feeding on eggplant and tomato without prey. J. Econ. Entomol. 2004, 97, 1291–1298. [Google Scholar] [CrossRef]
- Rasdi, M.Z.; Fauziah, I.; Mohamad, W.W.; Rahman, S.A.S.; Salmah, C.M.; Kamaruzaman, J. Biology of Macrolophus caliginosus (Heteroptera: Miridae) Predator of Trialeurodes vaporariorum (Homoptera: Aleyrodidae). Int. J. Biol. 2009, 1, 63. [Google Scholar]
- Sylla, S.; Brévault, T.; Diarra, K.; Bearez, P.; Desneux, N. Life-history traits of Macrolophus pygmaeus with different prey foods. PLoS ONE 2016, 11, e0166610. [Google Scholar] [CrossRef]
- Usha Rani, P.; Jyothsna, Y. Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol. Plant. 2010, 32, 695–701. [Google Scholar]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Jasmonic acid-mediated-induced resistance in groundnut (Arachis hypogaea L.) against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Plant Growth Regul. 2011, 30, 512–523. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Herbivore-and elicitor-induced resistance in groundnut to Asian armyworm, Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Plant Signal. Behav. 2011, 6, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.B.; Jiménez, A.; Urbaneja, A.; Pérez-Hedo, M.; Bento, J.M. Changes in plant responses induced by an arthropod influence the colonization behavior of a subsequent herbivore. Pest. Manag. Sci. 2021, 77, 4168–4180. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.R.; Cardoso, A.C.; Kant, M.R.; Mencalha, J.; Bernardo, A.M.G.; da Silveira, M.C.A.C.; Sarmento, R.A.; Venzon, M.; Pallini, A.; Janssen, A. Plant defences and spider-mite web affect host plant choice and performance of the whitefly Bemisia tabaci. J. Pest Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef]
- Schulze, A.; Zimmer, M.; Mielke, S.; Stellmach, H.; Melnyk, C.W.; Hause, B.; Gasperini, D. Wound-induced shoot-to-root relocation of JA-Ile precursors coordinates Arabidopsis growth. Mol. Plant 2019, 12, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, I.; Hause, B.; Maucher, H.; Pitzschke, A.; Miersch, O.; Ziegler, J.; Ryan, C.A.; Wasternack, C. Allene oxide cyclase dependence of the wound response and vascular bundle-specific generation of jasmonates in tomato–amplification in wound signalling. Plant J. 2003, 33, 577–589. [Google Scholar]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar]
- Chauvin, A.; Caldelari, D.; Wolfender, J.L.; Farmer, E.E. Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: A role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol. 2013, 197, 566–575. [Google Scholar]
- Zhang, N.X.; Messelink, G.J.; Alba, J.M.; Schuurink, R.; Kant, M.R.; Janssen, A. Phytophagy of omnivorous predator Macrolophus pygmaeus affects performance of herbivores through induced plant defences. Oecologia 2018, 186, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Schaefer, C.W.; Panizzi, A.R. Possible causes of disease symptoms resulting from the feeding of phytophagous Heteroptera. In Heteroptera of Economic Importance; CRC Press: Boca Raton, FL, USA, 2000; pp. 11–35. [Google Scholar]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
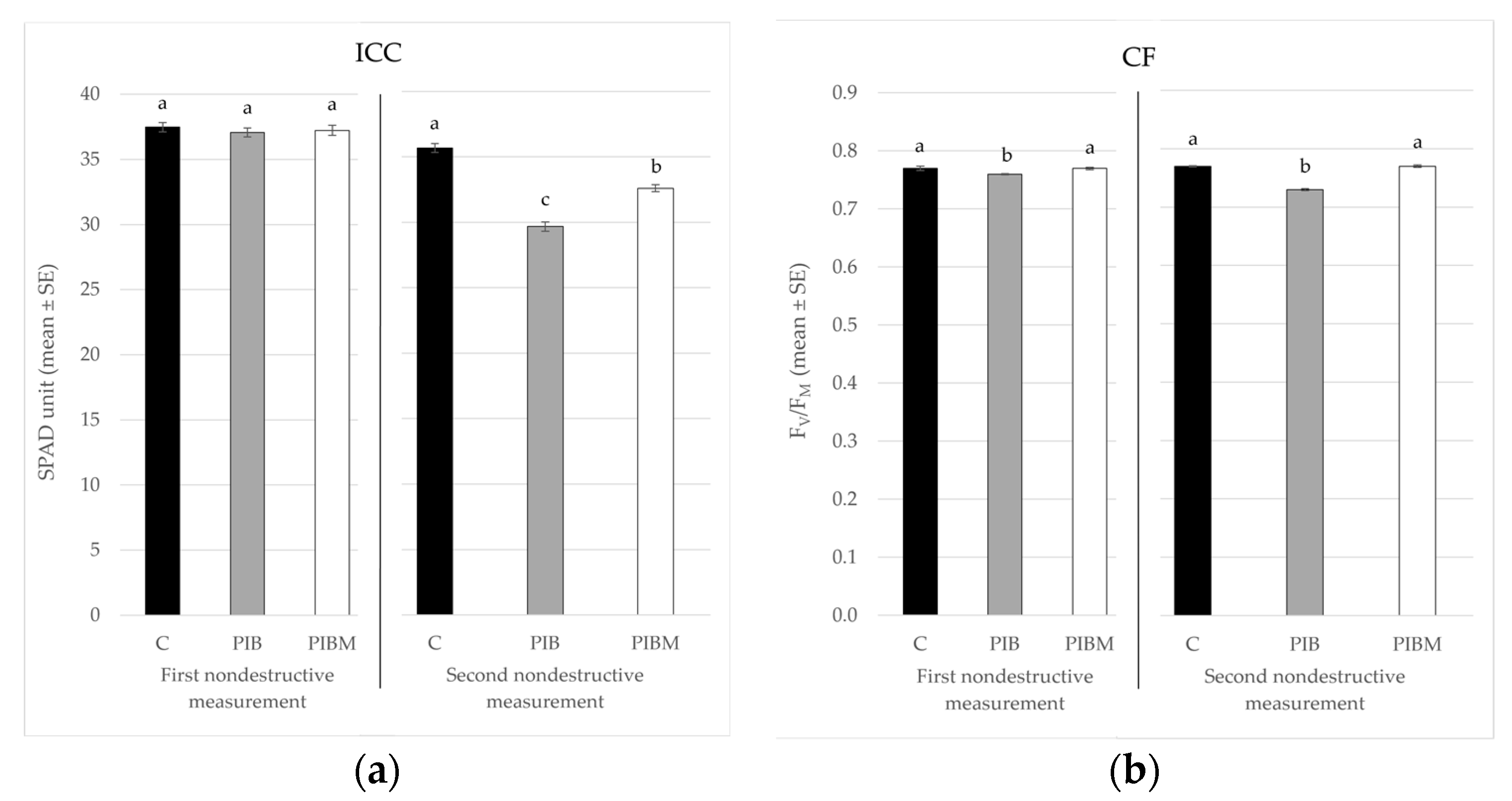

| Treatment | 1st ICC (SPAD Unit ± SE) | 2nd ICC (SPAD Unit ± SE) | 1st CF (FV/FM ± SE) | 2nd CF (FV/FM ± SE) |
|---|---|---|---|---|
| C | 37.48 ± 0.36 a | 35.68 ± 0.34 a | 0.77 ± 0.004 a | 0.77 ± 0.001 a |
| PIB | 37.07 ± 0.33 a | 29.68 ± 0.35 c | 0.76 ± 0.001 b | 0.73 ± 0.002 b |
| PIBM | 37.23 ± 0.39 a | 32.61 ± 0.27 b | 0.77 ± 0.002 a | 0.77 ± 0.002 a |
| F; df; p | 0.32; 2, 33; <0.727 | 86.31; 2, 33; <0.001 | 4.89; 2, 33; <0.0138 | 91.9; 2, 33; <0.001 |
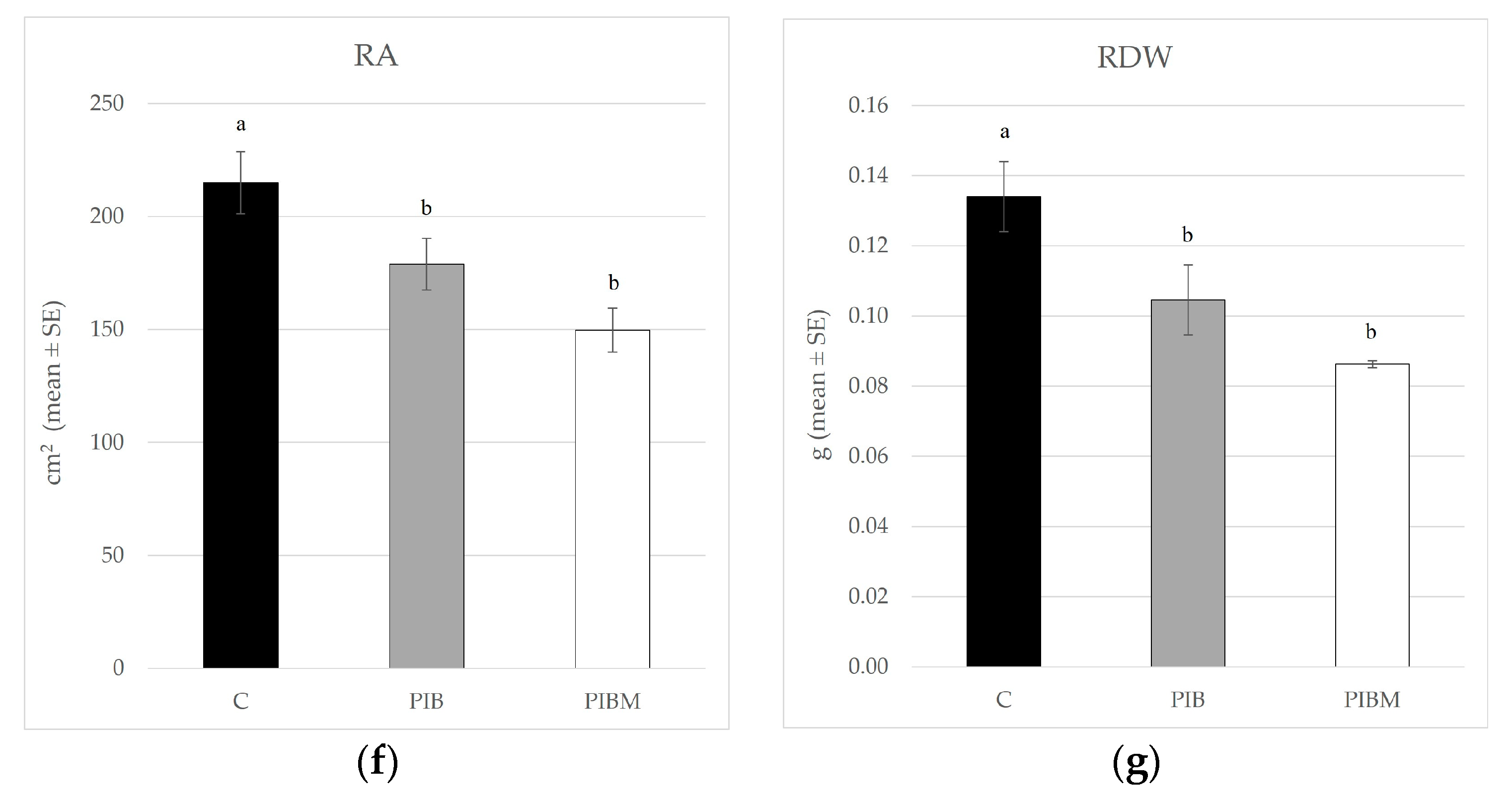
| Treatment | PH (cm ± SE) | ICC (SPAD Unit ± SE) | CF (FV/FM ± SE) | LA (cm2 ± SE) | SDW (g ± SE) | RA (cm2 ± SE) | RDW (g ± SE) |
|---|---|---|---|---|---|---|---|
| C | 26.07 ± 0.57 a | 35.60 ± 0.64 a | 0.78 ± 0.003 a | 1141.49 ± 35.67 a | 0.74 ± 0.03 a | 214.90 ± 13.69 a | 0.13 ± 0.01 a |
| PIB | 20.35 ± 1.03 b | 26.23 ± 1.23 c | 0.74 ± 0.007 b | 765.17 ± 74.21 c | 0.40 ± 0.06 c | 178.91 ± 11.38 b | 0.11 ± 0.01 b |
| PIBM | 21.73± 0.51 b | 31.91 ± 0.56 b | 0.77 ± 0.051 a | 939.17 ± 31.81 b | 0.57 ± 0.03 b | 149.83 ± 9.73 b | 0.08 ± 0.001 b |
| F; df; p | 16.297; 2, 33; <0.001 | 29.728; 2, 33; <0.001 | 16.159; 2, 33; <0.001 | 13.45; 2, 33; <0.001 | 17.12; 2, 33; <0.001 | 7.74; 2, 33; <0.0017 | 6.42; 2, 33; <0.0044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, A.; Massimino Cocuzza, G.E.; Suma, P.; Rapisarda, C. Can Macrolophus pygmaeus (Hemiptera: Miridae) Mitigate the Damage Caused to Plants by Bemisia tabaci (Hemiptera: Aleyrodidae)? Insects 2023, 14, 164. https://doi.org/10.3390/insects14020164
Farina A, Massimino Cocuzza GE, Suma P, Rapisarda C. Can Macrolophus pygmaeus (Hemiptera: Miridae) Mitigate the Damage Caused to Plants by Bemisia tabaci (Hemiptera: Aleyrodidae)? Insects. 2023; 14(2):164. https://doi.org/10.3390/insects14020164
Chicago/Turabian StyleFarina, Alessia, Giuseppe Eros Massimino Cocuzza, Pompeo Suma, and Carmelo Rapisarda. 2023. "Can Macrolophus pygmaeus (Hemiptera: Miridae) Mitigate the Damage Caused to Plants by Bemisia tabaci (Hemiptera: Aleyrodidae)?" Insects 14, no. 2: 164. https://doi.org/10.3390/insects14020164
APA StyleFarina, A., Massimino Cocuzza, G. E., Suma, P., & Rapisarda, C. (2023). Can Macrolophus pygmaeus (Hemiptera: Miridae) Mitigate the Damage Caused to Plants by Bemisia tabaci (Hemiptera: Aleyrodidae)? Insects, 14(2), 164. https://doi.org/10.3390/insects14020164











