Fitness of Nutrition Regulation in a Caterpillar Pest Mythimna separata (Walker): Insights from the Geometric Framework
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
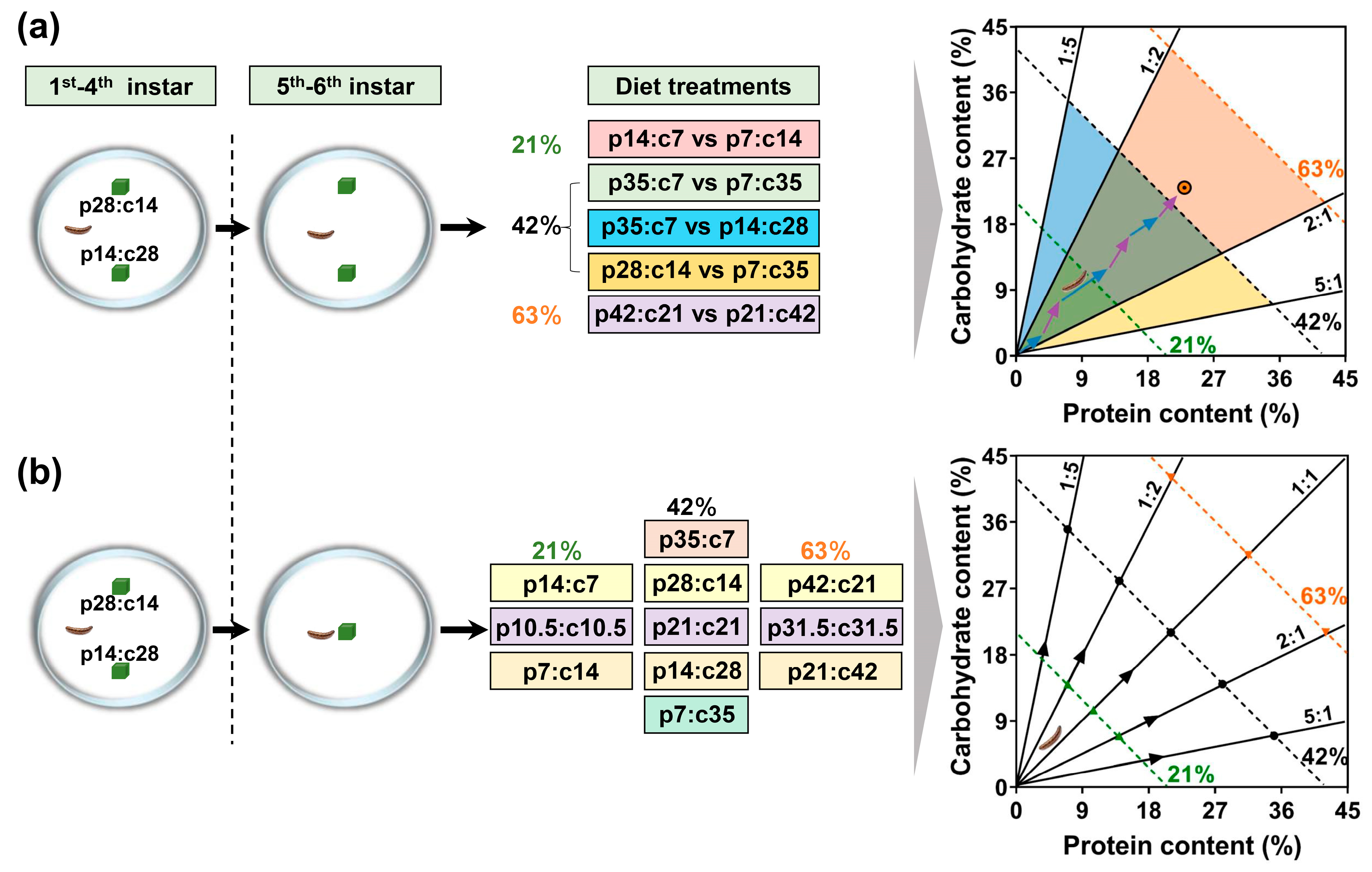
2.1. Insect Rearing and Experimental Chambers
2.2. Experimental Diets
2.3. Experimental Treatments
2.4. Experimental Protocol
2.5. Statistical Analysis
3. Results
3.1. Choice Experiment
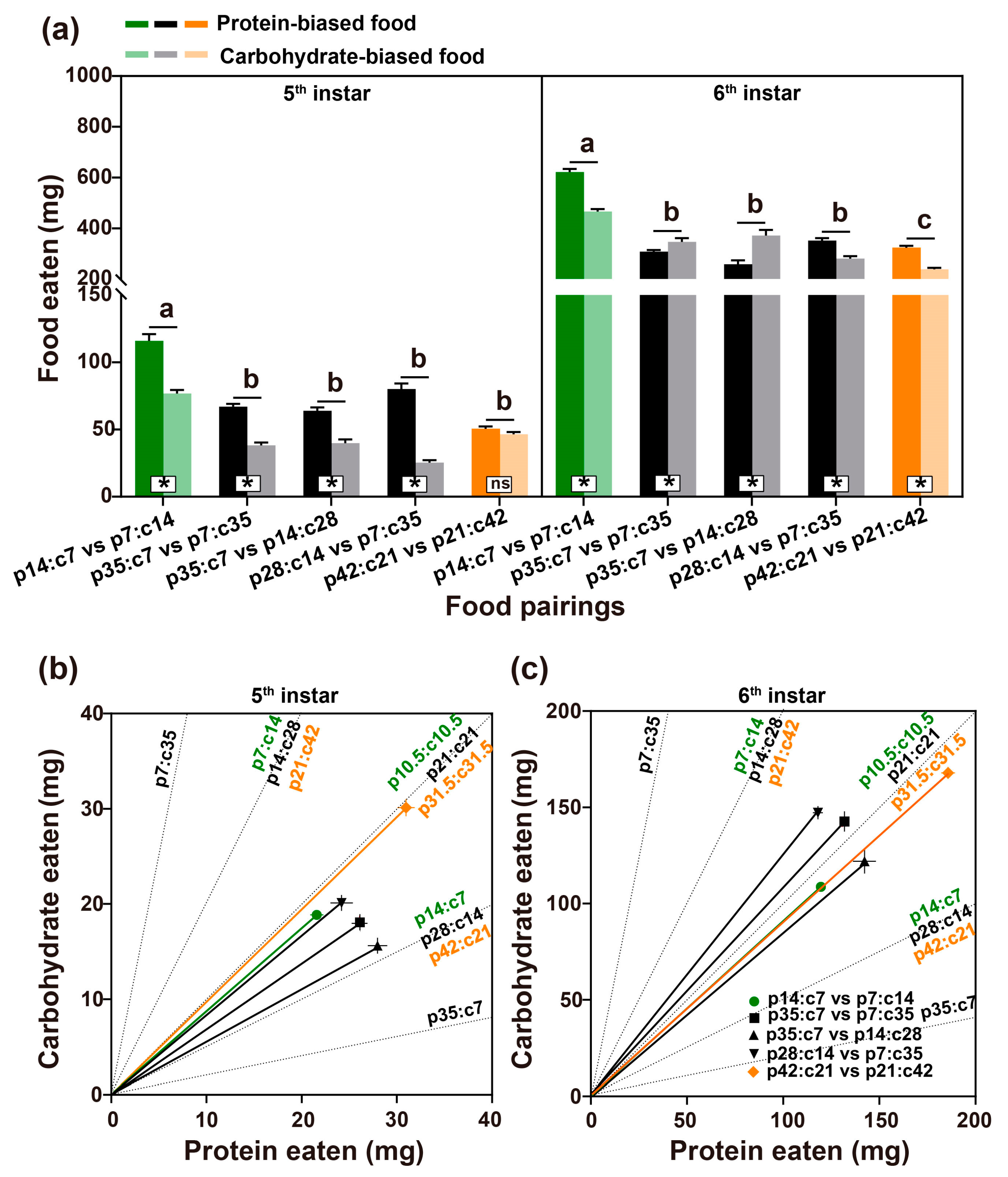
3.1.1. Food and Nutrients Consumed
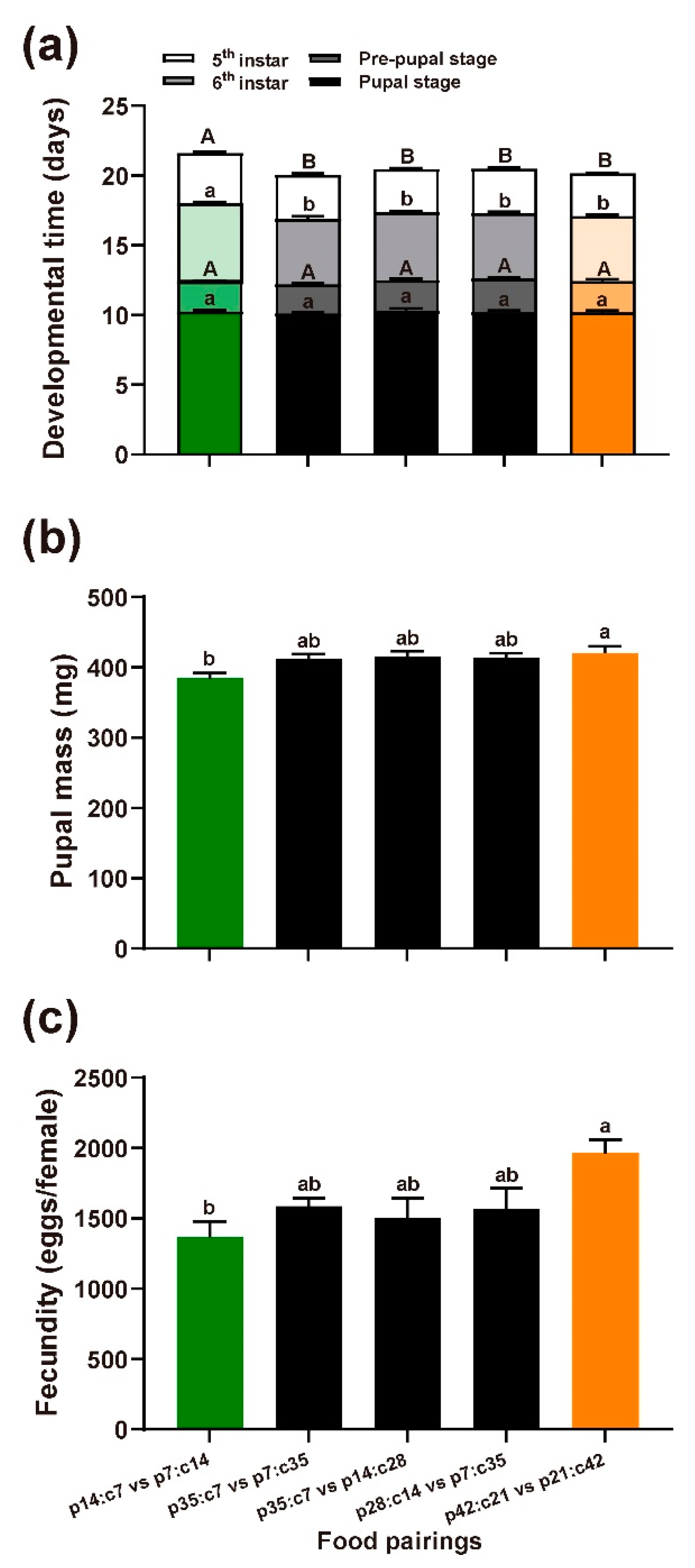
3.1.2. Performance
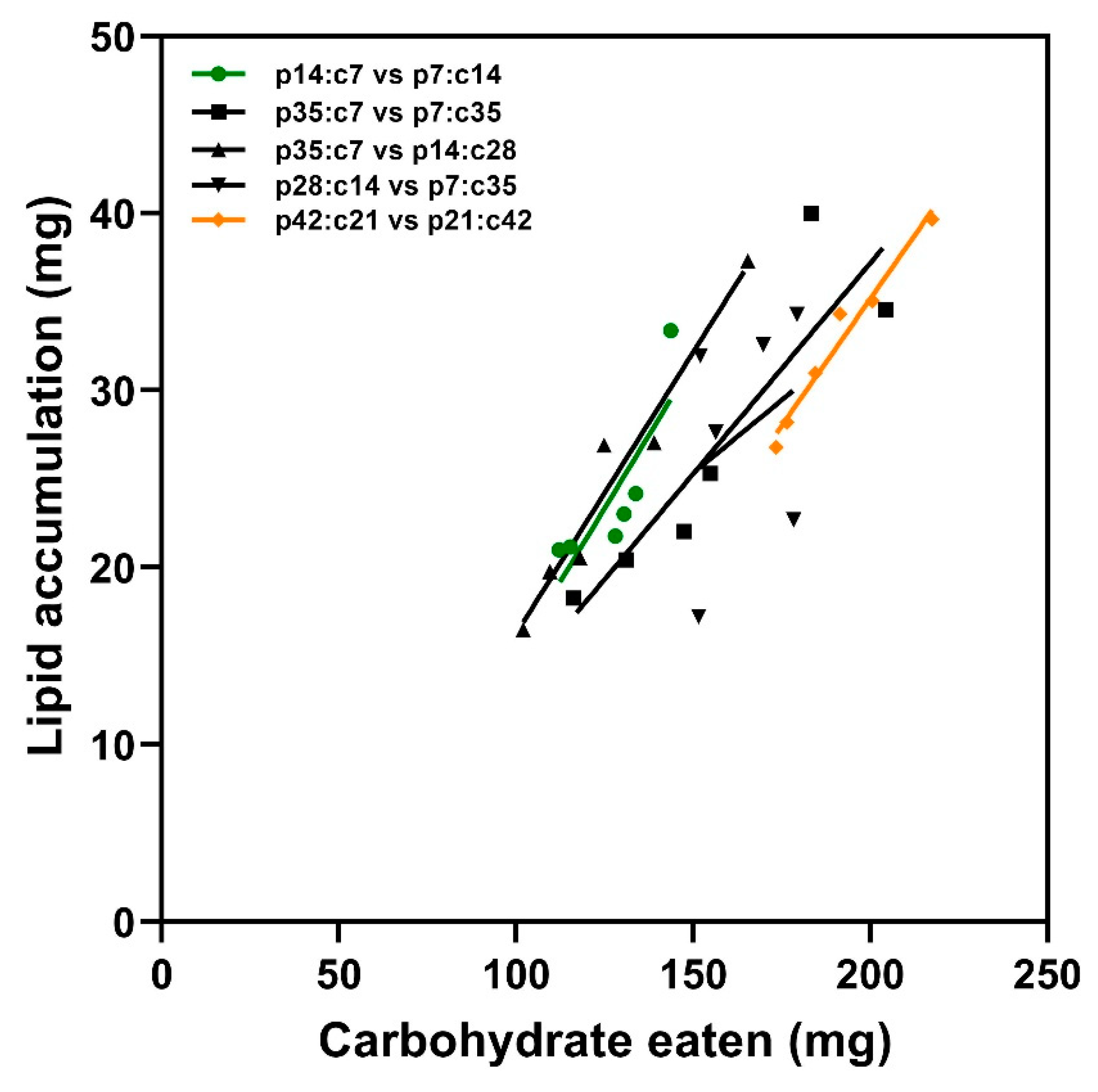
3.1.3. Lipid Accumulation
3.2. No-Choice Experiment
3.2.1. Food and Nutrient Consumed across the Nutrient Landscape
3.2.2. Performance across the Nutrient Landscape
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mcgroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C: N: P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Van Loon, B.; Van Loon, J.J.; Dicke, M. Insect-Plant Biology; Oxford University Press on Demand: Oxford, UK, 2005. [Google Scholar]
- Behmer, S.T.; Joern, A. Coexisting generalist herbivores occupy unique nutritional feeding niches. Proc. Natl. Acad. Sci. USA 2008, 105, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Le Couteur, D.G.; James, D.E.; George, J.; Gunton, J.E.; Solon-Biet, S.M.; Raubenheimer, D. The geometric framework for nutrition as a tool in precision medicine. Nutr. Healthy Aging 2017, 4, 217–226. [Google Scholar] [CrossRef]
- Behmer, S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef]
- Bala, K.; Sood, A.K.; Pathania, V.S.; Thakur, S. Effect of plant nutrition in insect pest management: A review. J. Pharmacogn. Phytochem. 2018, 7, 2737–2742. [Google Scholar]
- Simpson, S.J.; Clissold, F.J.; Lihoreau, M.; Ponton, F.; Wilder, S.M.; Raubenheimer, D. Recent advances in the integrative nutrition of arthropods. Annu. Rev. Entomol. 2015, 60, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, D.; Simpson, S.J.; Mayntz, D. Nutrition, ecology and nutritional ecology: Toward an integrated framework. Funct. Ecol. 2009, 23, 4–16. [Google Scholar] [CrossRef]
- Fischer, K.; Fiedler, K. Response of the copper butterfly Lycaena tityrus to increased leaf nitrogen in natural food plants: Evidence against the nitrogen limitation hypothesis. Oecologia 2000, 124, 235–241. [Google Scholar] [CrossRef]
- Jauset, A.M.; Sarasua, M.J.; Avilla, J.; Albajes, R. Effect of nitrogen fertilization level applied to tomato on the greenhouse whitefly. Crop Prot. 2000, 19, 255–261. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The nature of nutrition: A unifying framework. Aust. J. Zool. 2012, 59, 350–368. [Google Scholar] [CrossRef]
- Behmer, S.T.; Raubenheimer, D.; Simpson, S.J. Frequency-dependent food selection in locusts: A geometric analysis of the role of nutrient balancing. Anim. Behav. 2001, 61, 995–1005. [Google Scholar] [CrossRef]
- Lee, K.P.; Behmer, S.T.; Simpson, S.J. Nutrient regulation in relation to diet breadth: A comparison of Heliothis sister species and a hybrid. J. Exp. Biol. 2006, 209, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P. Sex-specific differences in nutrient regulation in a capital breeding caterpillar, Spodoptera litura (Fabricius). J. Insect Physiol. 2010, 56, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Kwon, S.T.; Roh, C. Caterpillars use developmental plasticity and diet choice to overcome the early life experience of nutritional imbalance. Anim. Behav. 2012, 84, 785–793. [Google Scholar] [CrossRef]
- Clark, R.M.; Mcconnell, A.; Zera, A.J.; Behmer, S.T.; Lee, K.P. Nutrient regulation strategies differ between cricket morphs that trade-off dispersal and reproduction. Funct. Ecol. 2013, 27, 1126–1133. [Google Scholar] [CrossRef]
- Clark, R.M.; Zera, A.J.; Behmer, S.T. Nutritional physiology of life-history trade-offs: How food protein-carbohydrate content influences life-history traits in the wing-polymorphic cricket Gryllus firmus. J. Exp. Biol. 2015, 218, 298–308. [Google Scholar] [CrossRef]
- Clark, R.M.; Zera, A.J.; Behmer, S.T.; Sinclair, B. Metabolic rate is canalized in the face of variable life history and nutritional environment. Funct. Ecol. 2016, 30, 922–931. [Google Scholar] [CrossRef]
- Roeder, K.A.; Behmer, S.T.; Davidowitz, G. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 2014, 28, 1135–1143. [Google Scholar] [CrossRef]
- Clissold, F.J.; Kertesz, H.; Saul, A.M.; Sheehan, J.L.; Simpson, S.J. Regulation of water and macronutrients by the Australian plague locust, Chortoicetes terminifera. J. Insect Physiol. 2014, 69, 35–40. [Google Scholar] [CrossRef]
- Deans, C.A.; Sword, G.A.; Behmer, S.T. Revisiting macronutrient regulation in the polyphagous herbivore Helicoverpa zea (Lepidoptera: Noctuidae): New insights via nutritional geometry. J. Insect Physiol. 2015, 81, 21–27. [Google Scholar] [CrossRef]
- Sun, S.L.; Abudisilimu, N.; Yi, H.; Li, S.; Liu, T.X.; Jing, X. Understanding nutritive need in harmonia axyridis larvae:insights from nutritional geometry. Insect Sci. 2022, 29, 12. [Google Scholar] [CrossRef] [PubMed]
- Helm, B.R.; Slater, G.P.; Rajamohan, A.; Yocum, G.D.; Greenlee, K.J.; Bowsher, J.H. The geometric framework for nutrition reveals interactions between protein and carbohydrate during larval growth in honey bees. Biol. Open 2017, 6, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef]
- Lee, K.P. Dietary protein:carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: A test using a chemically defined diet. J. Insect Physiol. 2015, 75, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Mcclure, C.; Priest, N.K.; Hunt, J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosoph. Melanogaster. Aging Cell 2015, 14, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Boggs, C.L. Understanding insect life histories and senescence through a resource allocation lens. Funct. Ecol. 2009, 23, 27–37. [Google Scholar] [CrossRef]
- O’brien, D.M.; Fogel, M.L.; Boggs, C.L. Renewable and nonrenewable resources: Amino acid turnover and allocation to reproduction in Lepidoptera. Proc. Natl. Acad. Sci. USA 2002, 99, 4413–4418. [Google Scholar] [CrossRef]
- O’brien, D.M.; Boggs, C.L.; Fogel, M.L. The amino acids used in reproduction by butterflies: A comparative study of dietary sources using compound-specific stable isotope analysis. Physiol. Biochem. Zool. 2005, 78, 819–827. [Google Scholar] [CrossRef]
- Barros, E.M.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 2010, 137, 237–245. [Google Scholar] [CrossRef]
- Darragh, K.; Byers, K.J.; Merrill, R.M.; Mcmillan, W.O.; Schulz, S.; Jiggins, C.D. Male pheromone composition depends on larval but not adult diet in Heliconius melpomene. Ecol. Entomol. 2019, 44, 397–405. [Google Scholar] [CrossRef]
- Colasurdo, N.; Gélinas, Y.; Despland, E. Larval nutrition affects life history traits in a capital breeding moth. J. Exp. Biol. 2009, 212, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.E.; Tuchinskaya, I.; Buck, N.A.; Tabashnik, B.E. Hexameric storage proteins during metamorphosis and egg production in the diamondback moth, Plutella xylostella (Lepidoptera). J. Insect Physiol. 2000, 46, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Telang, A.; Buck, N.; Wheeler, D. Response of storage protein levels to variation in dietary protein levels. J. Insect Physiol. 2002, 48, 1021–1029. [Google Scholar] [CrossRef]
- Sharma, H.; Sullivan, D.J.; Bhatnagar, V. Population dynamics and natural mortality factors of the Oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae), in South-Central India. Crop Prot. 2002, 21, 721–732. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, L.; Zhang, L.; Sappington, T.W.; Hu, Y. Regulation of migration in Mythimna separata (Walker) in China: A review integrating environmental, physiological, hormonal, genetic, and molecular factors. Environ. Entomol. 2011, 40, 516–533. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Zeng, J.; Liu, J. Population dynamics of the armyworm in China: A review of the past 60 years’ research. Chin. J. Appl. Entomol. 2014, 51, 890–898. [Google Scholar]
- Tong, D.; Zhang, L.; Wu, N.; Xie, D.; Fang, G.; Coates, B.S.; Sappington, T.W.; Liu, Y.; Cheng, Y.; Xia, J.; et al. The oriental armyworm genome yields insights into the long-distance migration of noctuid moths. Cell Rep. 2022, 41, 111843. [Google Scholar] [CrossRef]
- Jia, J.; Sun, S.L.; Kuang, W.; Tang, R.; Zhang, Z.F.; Song, C.; Liu, T.X.; Jing, X. A Semi-Synthetic Diet and the Potential Important Chemicals for Mythimna separata (Lepidoptera: Noctuidae). J. Insect Sci. 2019, 19, 4. [Google Scholar] [CrossRef]
- Deans, C.A.; Sword, G.A.; Lenhart, P.A.; Burkness, E.; Hutchison, W.D.; Behmer, S.T. Quantifying plant soluble protein and digestible carbohydrate content, using corn (Zea mays) as an exemplar. J. Vis. Exp. 2018, 138, e58164. [Google Scholar]
- Behmer, S.T.; Cox, E.; Raubenheimer, D.; Simpson, S.J. Food distance and its effect on nutrient balancing in a mobile insect herbivore. Anim. Behav. 2003, 66, 665–675. [Google Scholar] [CrossRef]
- Lee, K.P.; Behmer, S.T.; Simpson, S.J.; Raubenheimer, D. A geometric analysis of nutrient regulation in the generalist caterpillar Spodoptera littoralis (Boisduval). J. Insect Physiol. 2002, 48, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Behmer, S.T.; Simpson, S.J.; Raubenheimer, D. Herbivore foraging in chemically heterogeneous environments: Nutrients and secondary metabolites. Ecology 2002, 83, 2489–2501. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Analysis of covariance: An alternative to nutritional indices. Entomol. Exp. Appl. 1992, 62, 221–231. [Google Scholar] [CrossRef]
- Scheiner, S.M. Design and Analysis of Ecological Experiments; Chapman and Hall, CRC Press: New York, NY, USA, 1998. [Google Scholar]
- Le Gall, M.; Behmer, S.T. Effects of protein and carbohydrate on an insect herbivore: The vista from a fitness landscape. Integr. Comp. Biol. 2014, 54, 942–954. [Google Scholar] [CrossRef]
- Lee, K.P.; Simpson, S.J.; Raubenheimer, D. A comparison of nutrient regulation between solitarious and gregarious phases of the specialist caterpillar, Spodoptera exempta (Walker). J. Insect Physiol. 2004, 50, 1171–1180. [Google Scholar] [CrossRef]
- Simpson, S.J.; Sibly, R.M.; Lee, K.P.; Behmer, S.T.; Raubenheimer, D. Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 2004, 68, 1299–1311. [Google Scholar] [CrossRef]
- Mayntz, D.; Raubenheimer, D.; Salomon, M.; Toft, S.; Simpson, S.J. Nutrient-specific foraging in invertebrate predators. Science 2005, 307, 111–113. [Google Scholar] [CrossRef]
- Behmer, S.T.; Joern, A. Insect herbivore outbreaks viewed through a physiological framework: Insights from Orthoptera. In Insect Outbreaks Revisit; Barbosa, P., Letourneau, D.K., Agrawal, A.A., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 1–29. [Google Scholar]
- Thompson, S.N.; Redak, R.A. Feeding behaviour and nutrient selection in an insect Manduca sexta L. and alterations induced by parasitism. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005, 191, 909–923. [Google Scholar] [CrossRef]
- Deans, C.A.; Sword, G.A.; Behmer, S.T. First evidence of protein-carbohydrate regulation in a plant bug (Lygus hesperus). J. Insect Physiol. 2019, 116, 118–124. [Google Scholar] [CrossRef]
- Austin, A.J.; Gilbert, J.D.J.; Priest, N. Solitary bee larvae prioritize carbohydrate over protein in parentally provided pollen. Funct. Ecol. 2021, 35, 1069–1080. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Integrating nutrition: A geometrical approach. Entomol. Exp. Appl. 1999, 91, 67–82. [Google Scholar] [CrossRef]
- Bernays, E. Feeding by lepidopteran larvae is dangerous. Ecol. Entomol. 1997, 22, 121–123. [Google Scholar] [CrossRef]
- Bernays, E.; Minkenberg, O. Insect herbivores: Different reasons for being a generalist. Ecology 1997, 78, 1157–1169. [Google Scholar] [CrossRef]
- Lima, S.L. Stress and decision-making under the risk of predation: Recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study Behav. 1998, 27, 215–290. [Google Scholar]
- Lin, X.; Xu, Y.; Jiang, J.; Lavine, M.; Lavine, L.C. Host quality induces phenotypic plasticity in a wing polyphenic insect. Proc. Natl. Acad. Sci. USA 2018, 115, 7563–7568. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Cao, Y.Z.; Li, K.B. Effect of supplementary nutrition on energy reserves in the adult Mythimna separata(Walker). Acta Entomol. Sinia 2000, 43, 207–210. [Google Scholar]
- Zhang, L. The Sensitive Period and Regulatory Mechanisms for the Shifting of Migrant into Resident in the Oriental Armyworm, Mythimna Separata (Walker) (Lepidoptera: Noctuidae). Ph.D. Dissertation, Chinese Academy of Agricultural Sciences, Beijing, China, 2006. [Google Scholar]
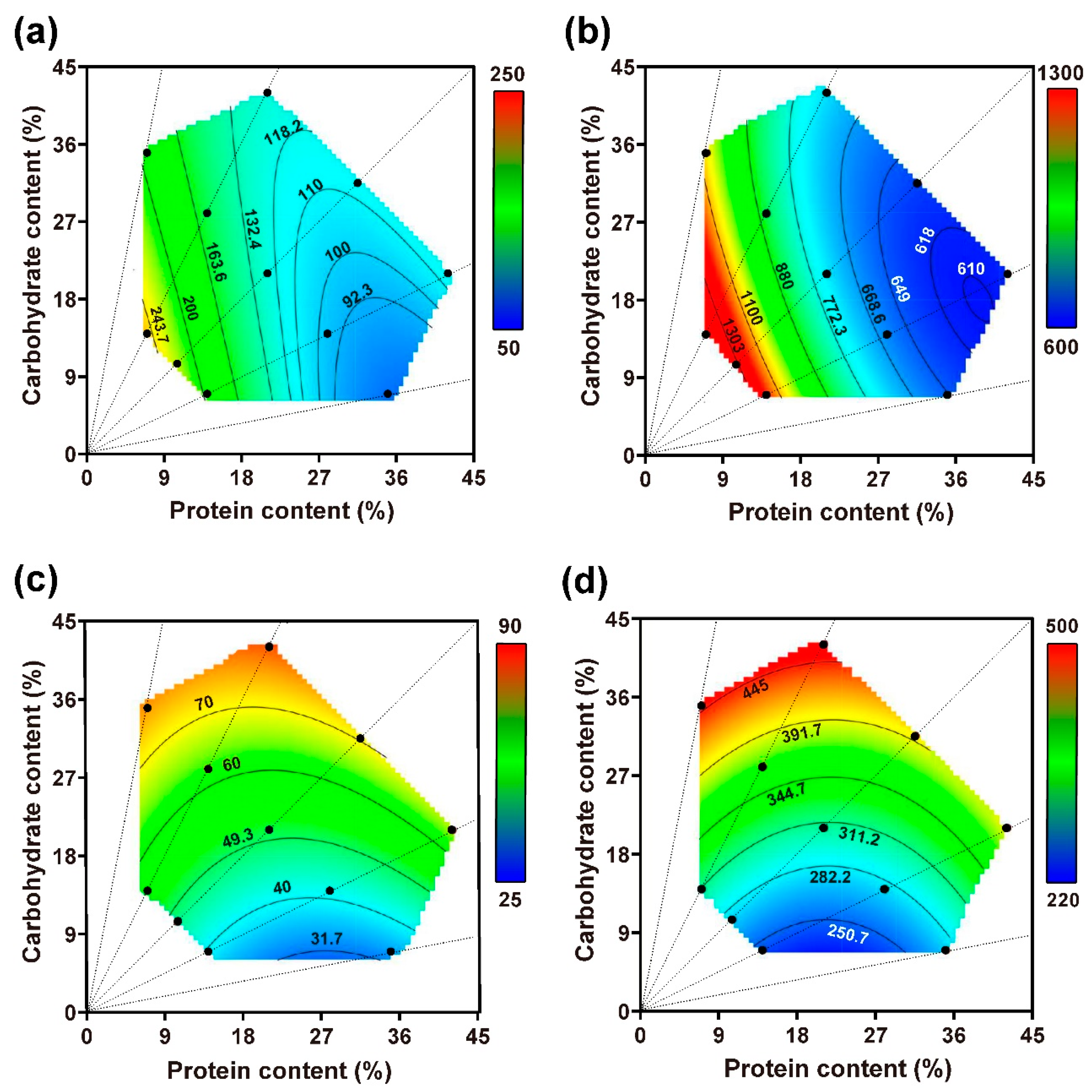
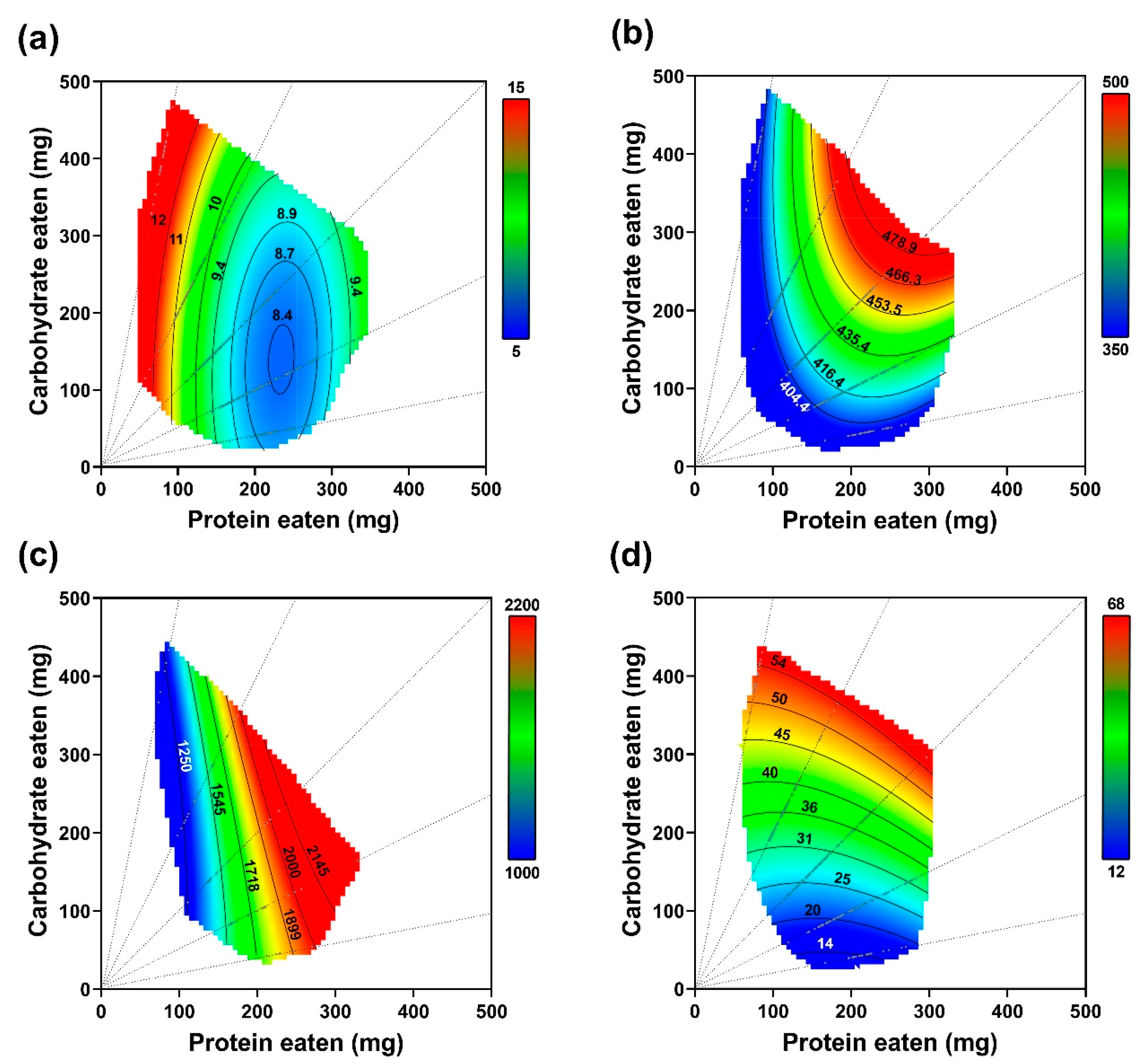
| Variable | P and C Consumed | Protein Consumed | Carbohydrate Consumed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | df | F | p | df | F | p | df | F | p |
| 5th instar | |||||||||
| Food pairing | 8,188 | 27.79 | 0.000 | 4,94 | 14.61 | 0.000 | 4,94 | 31.36 | 0.000 |
| Covariate | 2,93 | 0.27 | 0.767 | 1,94 | 0.21 | 0.648 | 1,94 | 0.004 | 0.951 |
| 6th instar | |||||||||
| Food pairing | 8,188 | 27.83 | 0.000 | 4,94 | 39.16 | 0.000 | 4,94 | 23.43 | 0.000 |
| Covariate | 2,93 | 2.59 | 0.080 | 1,94 | 4.74 | 0.032 | 1,94 | 0.23 | 0.630 |
| 5th Instar Larvae | 5th and 6th Instar Larvae | |||
|---|---|---|---|---|
| Model Terms | Food Consumption | Nutrient Intake | Food Consumption | Nutrient Intake |
| Full model | F6,321 = 151.57 p < 0.001 | F6,321 = 108.39 p < 0.001 | F6,321 = 387.88 p < 0.001 | F6,321 = 158.82 p < 0.001 |
| Initial mass | F1,321 = 73.20 p < 0.001 (51.22) | F1,321 = 78.90 p < 0.001 (18.46) | F1,321 = 3.59 p = 0.059 (43.26) | F1,321 = 7.50 p = 0.007 (24.34) |
| Protein (P) | F1,321 = 164.45 p < 0.001 (−44.85) | F1,321 = 7.82 p = 0.006 (3.39) | F1,321 = 1336.05 p < 0.001 (−470.37) | F1,321 = 2.03 p = 0.155 (7.14) |
| Carbohydrate (C) | F1,321 = 2.47 p = 0.117 (5.54) | F1,321 = 421.96 p < 0.001 (25.15) | F1,321 = 118.89 p < 0.001 (−140.93) | F1,321 = 769.62 p < 0.001 (139.59) |
| P2 | F1,321 = 139.43 p < 0.001 (62.61) | F1,321 = 51.93 p < 0.001 (13.27) | F1,321 = 305.83 p < 0.001 (506.73) | F1,321 = 83.21 p < 0.001 (102.89) |
| C2 | F1,321 = 2.51 p = 0.115 (8.45) | F1,321 = 0.57 p = 0.452 (1.40) | F1,321 = 46.04 p < 0.001 (197.86) | F1,321 = 7.12 p = 0.008 (30.28) |
| P × C | F1,321 = 93.72 p < 0.001 (53.51) | F1,321 = 17.44 p < 0.001 (8.01) | F1,321 = 138.79 p < 0.001 (355.82) | F1,321 = 0.39 p = 0.533 (−7.33) |
| Model Term | Developmental Time | Pupal Mass | Fecundity | Lipid Accumulation |
|---|---|---|---|---|
| Full model | F6,321 = 61.78 p < 0.001 | F6,316 = 41.84 p < 0.001 | F6,48 = 10.48 p < 0.001 | F6,206 = 256.81 p < 0.001 |
| Initial mass | F1,321 = 16.18 p < 0.001 (−0.96) | F1,316 = 32.70 p < 0.001 (26.81) | F1,48 = 0.48 p = 0.493 (96.05) | F1, 206 = 4.52 p = 0.035 (1.97) |
| Protein (P) | F1,321 = 97.06 p < 0.001 (−2.71) | F1,316 = 113.58 p < 0.001 (60.52) | F1,48 = 43.07 p < 0.001 (1054.37) | F1,206 = 21.76 p < 0.001 (5.20) |
| Carbohydrate (C) | F1,321 = 3.18 p = 0.076 (0.34) | F1,316 = 98.33 p < 0.001 (38.09) | F1,48 = 2.59 p = 0.114 (175.02) | F1,206 = 828.32 p < 0.001 (21.85) |
| P2 | F1,321 = 22.64 p < 0.001 (3.86) | F1,316 = 8.96 p = 0.003 (−53.69) | F1,48 = 0.62 p = 0.434 (−323.21) | F1,206 = 5.72 p = 0.018 (8.67) |
| C2 | F1,321 = 3.07 p = 0.081 (0.52) | F1,316 = 3.66 p = 0.057 (−11.68) | F1,48 = 0.12 p = 0.734 (64.99) | F1,206 = 0.84 p = 0.361 (−1.08) |
| P × C | F1,321 = 0.201 p = 0.654 (−0.33) | F1,316 = 7.00 p = 0.009 (40.95) | F1,48 = 0.21 p = 0.650 (216.24) | F1,206 = 4.62 p = 0.033 (6.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Yang, Z.; Ren, J.; Liu, T.; Jing, X. Fitness of Nutrition Regulation in a Caterpillar Pest Mythimna separata (Walker): Insights from the Geometric Framework. Insects 2023, 14, 937. https://doi.org/10.3390/insects14120937
Sun S, Yang Z, Ren J, Liu T, Jing X. Fitness of Nutrition Regulation in a Caterpillar Pest Mythimna separata (Walker): Insights from the Geometric Framework. Insects. 2023; 14(12):937. https://doi.org/10.3390/insects14120937
Chicago/Turabian StyleSun, Shaolei, Zhen Yang, Jinchan Ren, Tongxian Liu, and Xiangfeng Jing. 2023. "Fitness of Nutrition Regulation in a Caterpillar Pest Mythimna separata (Walker): Insights from the Geometric Framework" Insects 14, no. 12: 937. https://doi.org/10.3390/insects14120937
APA StyleSun, S., Yang, Z., Ren, J., Liu, T., & Jing, X. (2023). Fitness of Nutrition Regulation in a Caterpillar Pest Mythimna separata (Walker): Insights from the Geometric Framework. Insects, 14(12), 937. https://doi.org/10.3390/insects14120937





