Uridine Diphosphate Glycosyltransferases (UGTs) Involved in the Carotenoid-Based Body Color Difference between Tetranychus cinnabarinus (Red) and Tetranychus urticae (Green)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mites
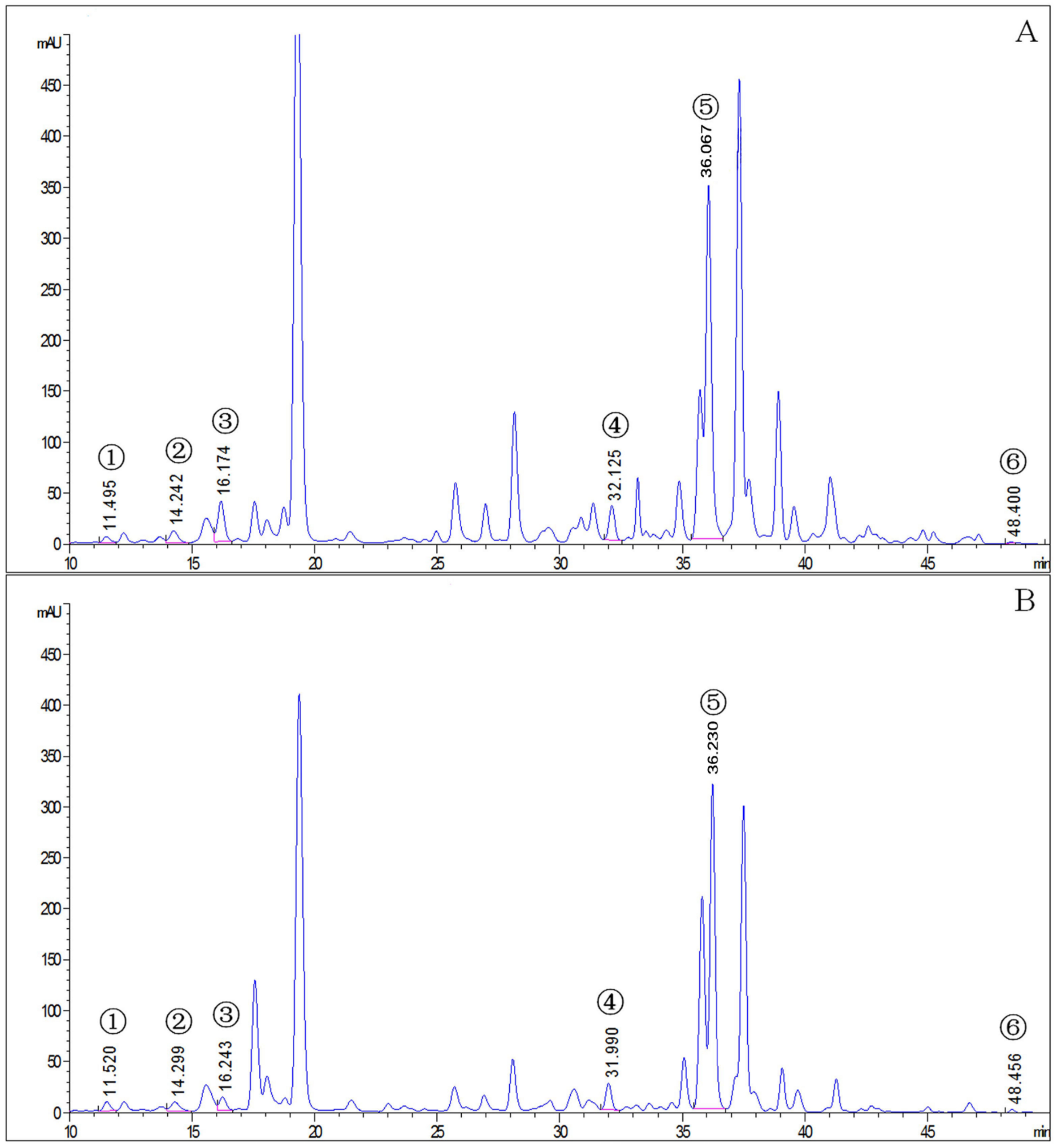
2.2. Determination of Carotenoid Content
2.3. Transcriptome Sequencing
2.4. Fluorescence Quantitative PCR (qPCR)
2.5. RNA Interference (RNAi)
2.6. Statistical Analysis
3. Results
3.1. Carotenoid Profiles
3.2. Transcriptome Sequencing and Carotenoid-Related DEGs Analysis
3.3. Analysis of Differential Gene Expression Patterns
3.4. Functional Validation of Carotenoid-Related DEGs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Auger, P.; Migeon, A.; Ueckermann, E.A.; Tiedt, L.; Navajas, M. Evidence for synonymy between Tetranychus urticae and Tetranychus cinnabarinus (Acari, Prostigmata, Tetranychidae): Review and new data. Acarologia 2013, 53, 383–415. [Google Scholar] [CrossRef]
- De Mendonca, R.S.; Navia, D.; Diniz, I.R.; Auger, P.; Navajas, M. A critical review on some closely related species of Tetranychus sensu stricto (Acari: Tetranychidae) in the public DNA sequences databases. Exp. Appl. Acarol. 2011, 55, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.G.; Qiao, Q.M.; Yuan, G.H. Progress of the research on body-color diversity in insects. Chin. Bull. Entomol. 2005, 42, 502–505. [Google Scholar]
- Ma, D.; Wu, C.; Yu, L.; Zhang, C.; Lin, W.; Yang, H. Comparative Transcriptome Analysis of Body-Color Segregated Progenies Produced by Crossing Albino Sea Cucumbers Apostichopus japonicus. Int. J. Agric. Biol. 2020, 24, 900–908. [Google Scholar]
- Sun, W.; Margam, V.M.; Sun, L.; Buczkowski, G.; Bennett, G.W.; Schemerhorn, B.; Muir, W.M.; Pittendrigh, B.R. Genome-wide analysis of phenobarbital-inducible genes in Drosophila melanogaster. Insect Mol. Biol. 2006, 15, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.B.; Li, Z.D.; Ye, Z.X.; Huang, H.J.; Chen, J.P.; Li, J.M.; Zhang, C.X. Long-wave opsin involved in body color plastic development in Nilaparvata lugens. BMC Genom. 2023, 24, 353. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Liu, Q.; Liu, Z.; Jiang, F.; Wang, H.; Guo, X.; Zhang, J.; Kang, L. A beta-carotene-binding protein carrying a red pigment regulates body-color transition between green am black in locusts. eLife 2019, 8, e41362. [Google Scholar] [CrossRef]
- Li, R.N.; Sun, Y.S.; Cui, R.; Zhang, X. Comprehensive Transcriptome Analysis of Different Skin Colors to Evaluate Genes Related to the Production of Pigment in Celestial Goldfish. Biology 2023, 12, 7. [Google Scholar] [CrossRef]
- Misawa, N.; Takemura, M.; Maoka, T. Carotenoid Biosynthesis in Animals: Case of Arthropods. In Carotenoids: Biosynthetic and Biofunctional Approaches; Misawa, N., Ed.; Advances in Experimental Medicine and Biology; Springer Nature: Berlin/Heidelberg, Germany, 2021; Volume 1261, pp. 217–220. [Google Scholar]
- Bryon, A.; Kurlovs, A.H.; Dermauw, W.; Greenhalgh, R.; Riga, M.; Grbic, M.; Tirry, L.; Osakabe, M.; Vontas, J.; Clark, R.M.; et al. Disruption of a horizontally transferred phytoene desaturase abolishes carotenoid accumulation and diapause in Tetranychus urticae. Proc. Natl. Acad. Sci. USA 2017, 114, 5871–5880. [Google Scholar] [CrossRef]
- Altincicek, B.; Kovacs, J.L.; Gerardo, N.M. Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae. Biol. Lett. 2012, 8, 253–257. [Google Scholar] [CrossRef]
- Hubbard, J.K.; Uy, J.A.C.; Hauber, M.E.; Hoekstra, H.E.; Safran, R.J. Vertebrate pigmentation: From underlying genes to adaptive function. Trends Genet. 2010, 26, 231–239. [Google Scholar] [CrossRef]
- Avalos, J.; Carmen Limon, M. Biological roles of fungal carotenoids. Curr. Genet. 2015, 61, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Toews, D.P.L.; Hofmeister, N.R.; Taylor, S.A. The Evolution and Genetics of Carotenoid Processing in Animals. Trends Genet. 2017, 33, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.J.; Cipollini, D.F.; Stireman, J.O., III. The role of carotenoids and their derivatives in mediating interactions between insects and their environment. Arthropod-Plant Interact. 2013, 7, 1–20. [Google Scholar] [CrossRef]
- Cooke, T.F.; Fischer, C.R.; Wu, P.; Jiang, T.-X.; Xie, K.T.; Kuo, J.; Doctorov, E.; Zehnder, A.; Khosla, C.; Chuong, C.-M.; et al. Genetic Mapping and Biochemical Basis of Yellow Feather Pigmentation in Budgerigars. Cell 2017, 171, 427–439. [Google Scholar] [CrossRef]
- Moran, N.A.; Jarvik, T. Lateral Transfer of Genes from Fungi Underlies Carotenoid Production in Aphids. Science 2010, 328, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Novakova, E.; Moran, N.A. Diversification of Genes for Carotenoid Biosynthesis in Aphids following an Ancient Transfer from a Fungus. Mol. Biol. Evol. 2012, 29, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.M.; Zhang, Y.Y.; Song, Z.R.; Xiong, X.H.; Hong, X.Y. The potential pigmentation-related genes in spider mites revealed by comparative transcriptomes of the red form of Tetranychus urticae. Insect Mol. Biol. 2021, 30, 580–593. [Google Scholar] [CrossRef]
- Mo, Y.-D.; Yang, S.-X.; Zhao, J.-Y.; Jin, P.-Y.; Hong, X.-Y. Comparative transcriptomes and reciprocal best hit analysis revealed potential pigment genes in two color forms of Tetranychus urticae. Exp. Appl. Acarol. 2017, 73, 159–176. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, C.; Zhang, M.; Liu, P.; Zhang, P.; He, L. Transcription response of Tetranychus cinnabarinus to plant-mediated short-term and long -term selenium treatment. Chemosphere 2021, 263, 128007. [Google Scholar] [CrossRef]
- Sun, W.; Jin, Y.; He, L.; Lu, W.C.; Li, M. Suitable reference gene selection for different strains and developmental stages of the carmine spider mite, Tetranychus cinnabarinus, using quantitative real-time PCR. J. Insect Sci. 2010, 10, 208. [Google Scholar] [CrossRef]
- Dooms, M.; Chango, A.; Abdel-Nour, A. Quantitative PCR (qPCR) and the Guide to good practices MIQE: Adapting and relevance in the clinical biology context. Ann. De Biol. Clin. 2014, 72, 265–269. [Google Scholar] [CrossRef]
- Feng, K.; Liu, J.; Wei, P.; Ou, S.; Wen, X.; Shen, G.; Xu, Z.; Xu, Q.; He, L. lincRNA_Tc13743.2-miR-133-5p-TcGSTm02 regulation pathway mediates cyflumetofen resistance in Tetranychus cinnabarinus. Insect Biochem. Mol. Biol. 2020, 123, 103413. [Google Scholar] [CrossRef]
- Trissi, N.; Troczka, B.J.; Ozsanlav-Harris, L.; Singh, K.S.; Mallott, M.; Aishwarya, V.; O’Reilly, A.; Bass, C.; Wilding, C.S. Differential regulation of the Tor gene homolog drives the red/green pigmentation phenotype in the aphid Myzus persicae. Insect Biochem. Mol. Biol. 2023, 153, 103896. [Google Scholar] [CrossRef]
- Hinomoto, N.; Osakabe, M.; Gotoh, T.; Takafuji, A. Phylogenetic analysis of green and red forms of the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), in Japan, based on mitochondrial cytochrome oxidase subunit I sequences. Appl. Entomol. Zool. 2001, 36, 459–464. [Google Scholar] [CrossRef][Green Version]
- Veerman, A.; Helle, W. Evidence for functional involvement of carotenoids in photoperiodic reaction of spider mites. Nature 1978, 275, 234. [Google Scholar] [CrossRef]
- Sugasawa, J.; Kitashima, Y.; Gotoh, T. Hybrid affinities between the green and the red forms of the two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae) under laboratory and semi-natural conditions. Appl. Entomol. Zool. 2002, 37, 127–139. [Google Scholar] [CrossRef]
- Huo, S.-M.; Yan, Z.-C.; Zhang, F.; Chen, L.; Sun, J.-T.; Hoffmann, A.A.; Hong, X.-Y. Comparative genome and transcriptome analyses reveal innate differences in response to host plants by two color forms of the two-spotted spider mite Tetranychus urticae. BMC Genom. 2021, 22, 569. [Google Scholar] [CrossRef] [PubMed]
- Zorn, H.; Breithaupt, D.E.; Takenberg, M.; Schwack, W.; Berger, R.G. Enzymatic hydrolysis of carotenoid esters of marigold flowers (Tagetes erecta L.) and red paprika (Capsicum annuum L.) by commercial lipases and Pleurotus sapidus extracellular lipase. Enzym. Microb. Technol. 2003, 32, 623–628. [Google Scholar] [CrossRef]
- Carroll, S.B. Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell 2008, 134, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Henning, F.; Jones, J.C.; Franchini, P.; Meyer, A. Transcriptomics of morphological color change in polychromatic Midas cichlids. BMC Genom. 2013, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.T.; Roberto, N.M.; Lee, D.; Hahnel, S.R.; Andersen, E.C. The impact of species-wide gene expression variation on Caenorhabditis elegans complex traits. Nat. Commun. 2022, 13, 3462. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.B.; Keys, D.N.; Rocheleau, T.; Aronstein, K.; Blackburn, M.; Carroll, S.B.; Ffrench-Constant, R.H. Regulation of dopa decarboxylase expression during colour pattern formation in wild-type and melanic tiger swallowtail butterflies. Development 1998, 125, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, Q.; Xu, J.; Duan, C.; Zhou, M. Genetic regulation of body color in larvae of Galleria mellonella. Acta Entomol. Sin. 2002, 45, 717–723. [Google Scholar]
- Futahashi, R.; Fujiwara, H. Melanin-synthesis enzymes coregulate stage-specific larval cuticular markings in the swallowtail butterfly, Papilio xuthus. Dev. Genes Evol. 2005, 215, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Grbic, M.; Van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouze, P.; Grbic, V.; Osborne, E.J.; Dermauw, W.; Phuong Cao Thi, N.; Ortego, F.; et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Bryon, A.; Wybouw, N.; Dermauw, W.; Tirry, L.; Van Leeuwen, T. Genome wide gene-expression analysis of facultative reproductive diapause in the two-spotted spider mite Tetranychus urticae. BMC Genom. 2013, 14, 815. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Shen, Y.; Zheng, S.; Liu, H. Transcriptome Characterization of Pigment-Related Genes in Jujube (Ziziphus Jujuba Mill.) Peel at Different Growth Stages. Biochem. Genet. 2023, 1–18. [Google Scholar] [CrossRef]
- Zhao, X.T. The Function of Carotenoid Biosynthesis Genes in the Two-Spotted Spider Mite. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2015. [Google Scholar]
- Tsuchida, K.; Sakudoh, T. Recent progress in molecular genetic studies on the carotenoid transport system using cocoon-color mutants of the silkworm. Arch. Biochem. Biophys. 2015, 572, 151–157. [Google Scholar] [CrossRef]







| T. urticae ID | Abbreviation | Full Name |
|---|---|---|
| tetur17g01350 | KST | Ketoacyl-synthetase, C-terminal extension |
| tetur05g02630 | SDR | Short-chain dehydrogenase/reductase |
| tetur21g01400 | UGT | UDP-glycosyltransferase |
| tetur02g13630 | PPs | Protein prenyltransferase |
| tetur01g11260 | PSLC | lycopene cyclase/phytoene synthase |
| tetur11g04810 | PDs | phytoene desaturase |
| tetur08g00550 | RDH2 | Putative retinol dehydrogenase |
| tetur05g05060 | β-UGT | Sterol 3-beta-glucosyltransferase |
| tetur11g05720 | PLAT10 | Lipase/lipooxygenase; PLAT/LH2 |
| tetur11g05520 | CYP385C4 | Cytochrome P450 |
| tetur11g05730 | PLAT11 | Lipase/lipooxygenase; PLAT/LH2 |
| tetur13g01900 | CaaX PPR | CaaX prenyl protease Rce1 |
| Gene | (TU/TC) Fold Change | Mite | RNAi Efficiency | Change of Carotenoids | ||
|---|---|---|---|---|---|---|
| α-Carotene | β-Carotene | γ-Carotene | ||||
| SDR | 0.55 | TC | 40.4% | - | - | - |
| PPs | 101.72 | TU | 51.5% | - | - | - |
| CaaX PPR | 6115.17 | TU | 94.4% | - | - | - |
| PSLC | 0.28 | TC | 40.9% | - | - | −20% |
| CYP385C4 | 30,323.34 | TU | 57.3% | - | +20% | +29% |
| PDs | 31.70 | TU | 47.0% | - | +41% | +142% |
| PLAT10 | 1114.28 | TU | 30.2% | - | +64% | +98% |
| PLAT11 | 164.60 | TU | 36.7% | - | +64% | +98% |
| β-UGT | 93.71 | TU | 59.5% | +41% | +48% | +39% |
| UGT | 3.62 | TU | 83.9% | +132% | +59% | +91% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Lin, T.; Wang, T.; Hu, Y.; Shen, G.; Feng, K.; Zhang, P.; He, L. Uridine Diphosphate Glycosyltransferases (UGTs) Involved in the Carotenoid-Based Body Color Difference between Tetranychus cinnabarinus (Red) and Tetranychus urticae (Green). Insects 2023, 14, 823. https://doi.org/10.3390/insects14100823
Xu Z, Lin T, Wang T, Hu Y, Shen G, Feng K, Zhang P, He L. Uridine Diphosphate Glycosyltransferases (UGTs) Involved in the Carotenoid-Based Body Color Difference between Tetranychus cinnabarinus (Red) and Tetranychus urticae (Green). Insects. 2023; 14(10):823. https://doi.org/10.3390/insects14100823
Chicago/Turabian StyleXu, Zhifeng, Ting Lin, Tongyang Wang, Yuan Hu, Guangmao Shen, Kaiyang Feng, Ping Zhang, and Lin He. 2023. "Uridine Diphosphate Glycosyltransferases (UGTs) Involved in the Carotenoid-Based Body Color Difference between Tetranychus cinnabarinus (Red) and Tetranychus urticae (Green)" Insects 14, no. 10: 823. https://doi.org/10.3390/insects14100823
APA StyleXu, Z., Lin, T., Wang, T., Hu, Y., Shen, G., Feng, K., Zhang, P., & He, L. (2023). Uridine Diphosphate Glycosyltransferases (UGTs) Involved in the Carotenoid-Based Body Color Difference between Tetranychus cinnabarinus (Red) and Tetranychus urticae (Green). Insects, 14(10), 823. https://doi.org/10.3390/insects14100823






