Prospects for Biological Control of Macadamia Felted Coccid in Hawaii with Metaphycus macadamiae Polaszek & Noyes, a New Encyrtid Wasp Native to New South Wales, Australia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Insect Containment Facility Settings and Rearing Conditions
2.2. Host and Parasitoid Rearing
2.3. Life History, Longevity, and Fecundity of M. macadamiae
2.3.1. Life History
2.3.2. Longevity
2.3.3. Fecundity
2.4. Host Specificity Testing
2.5. Parasitoid Field Performance in Australia
2.6. Extant Natural Enemies in Hawaii
2.7. Statistical Analysis and Vouchers
3. Results
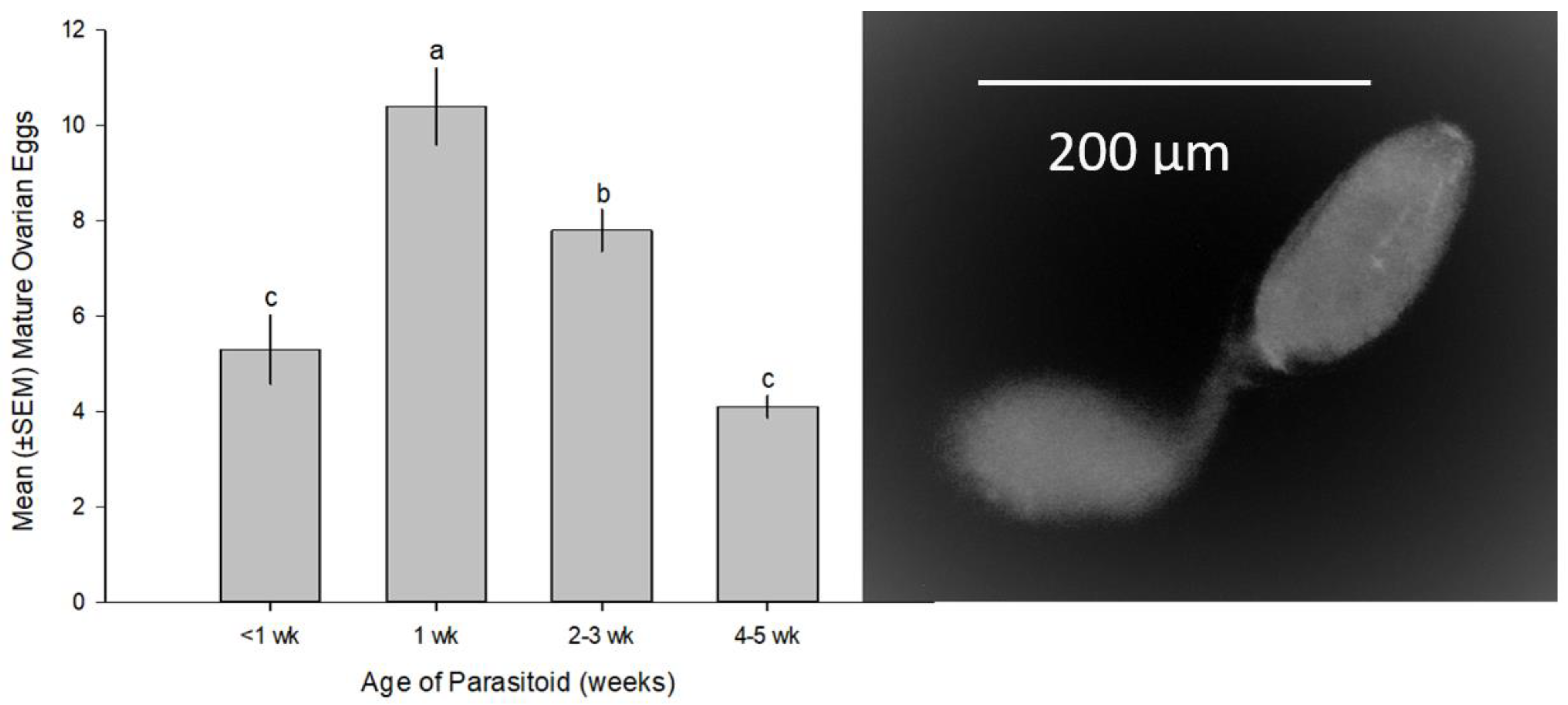
3.1. Life History, Longevity, and Fecundity of M. macadamiae
3.1.1. Life History and Longevity
3.1.2. Fecundity
3.2. Host Specificity Testing
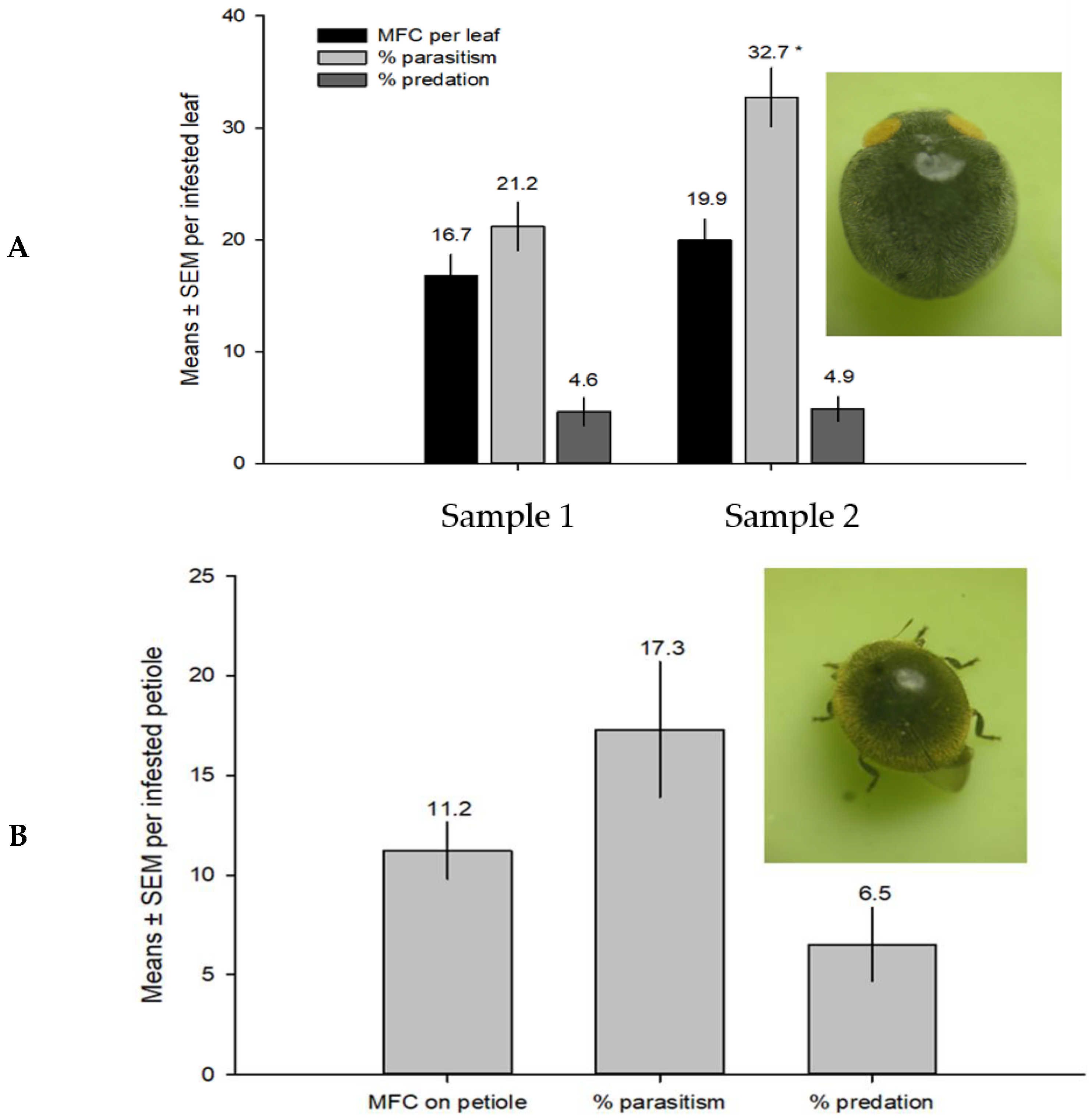
3.3. Field Parasitism Evaluation of M. macadamiae in Australia
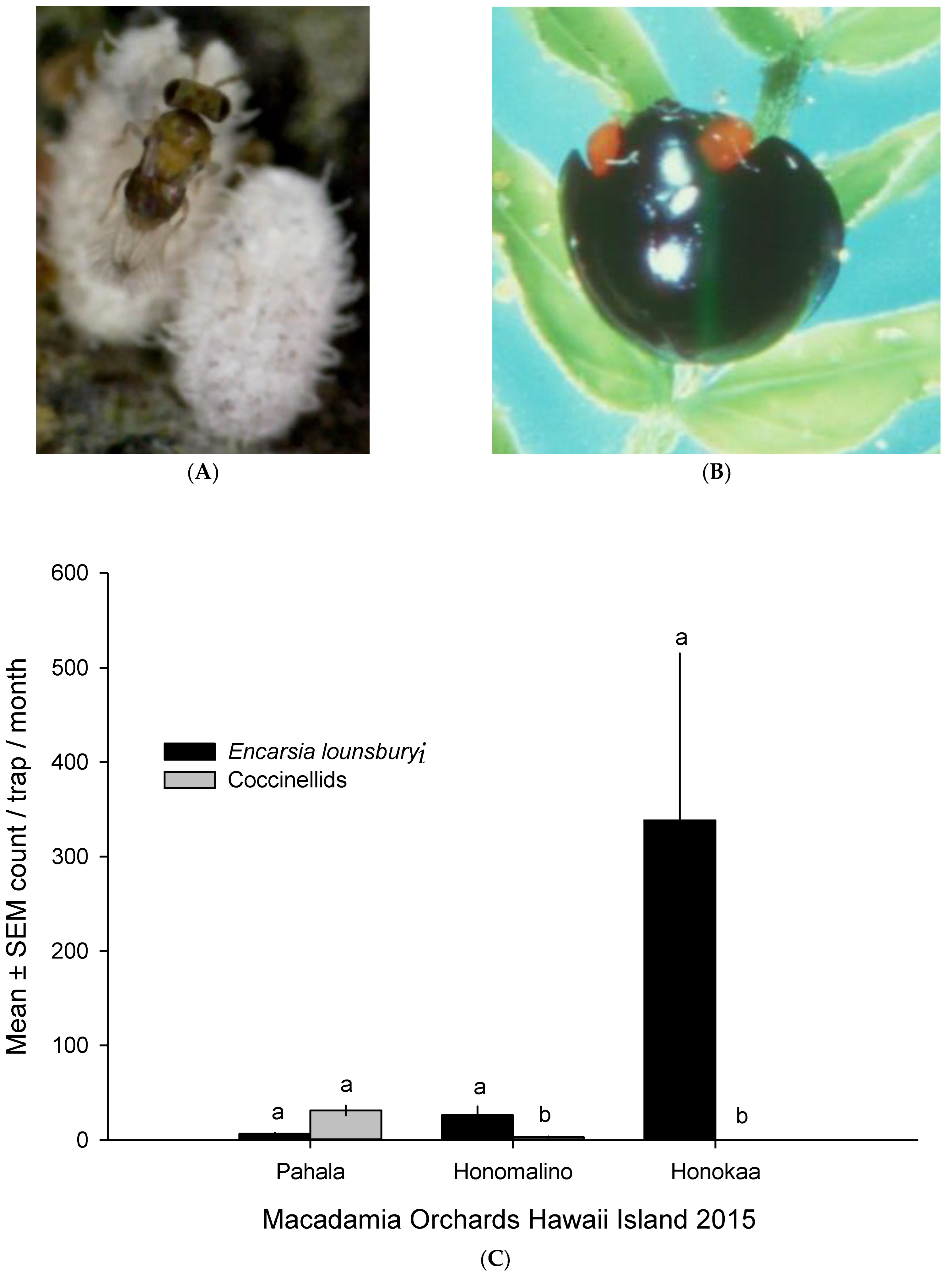
3.4. Extant Natural Enemies of MFC in Hawaii
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennell, M.R. Aspects of the Biology and Culture of Macadamia. Master’s Thesis, University of Sydney, Sydney, Australia, 1984; 143p. [Google Scholar]
- Jones, V.P. Macadamia felted coccid. In Macadamia Integrated Pest Management: IPM of Insects and Mites Attacking Macadamia Nuts in Hawaii; University of Hawaii: Honolulu, HI, USA, 2002; 98p. [Google Scholar]
- USDA National Agricultural Statics Service (NASS). Top 20 Commodities State of Hawaii. 2017. Available online: https://www.nass.usda.gov/Statistics_by_State/Hawaii/Publications/Miscellaneous/2017HawaiiTop20Commodities.pdf (accessed on 10 January 2022).
- NASS. Pacific Region—Hawaii Macadamia Nuts Final Season Estimates. 2018. Available online: https://www.nass.usda.gov/Statistics_by_State/Hawaii/Publications/Fruits_and_Nuts/072020MacNutFinal.pdf (accessed on 20 January 2023).
- Williams, D.J. Scale insects (Homoptera: Coccoidea) on macadamia. J. Aust. Entomol. Soc. 1973, 12, 81–91. [Google Scholar] [CrossRef]
- Conant, P.; Tsuda, D.M.; Heu, R.A.; Teramoto, K.K. Macadamia Felted Coccid, Eriococcus ironsidei Williams; New Pest Advisory; No. 05-01; Hawaii Department of Agriculture: Honolulu, Hawaii, 2005. [Google Scholar]
- Ironside, D.A. The macadamia felted coccid. Qld. Agric. J. 1970, 96, 613–616. [Google Scholar]
- Ironside, D.A. Insect pests of macadamia. Qld. Agric. J. 1973, 99, 241–250. [Google Scholar]
- Ironside, D.A. The macadamia felted coccid. Qld. Agric. J. 1978, 104, 25–28. [Google Scholar]
- Miller, D.R.; Gimpel, M.E. A Systematic Catalogue of the Eriococcidae (Felt Scales) (Hemiptera: Coccoidea) of the World; Intercept Ltd.: Andover, UK, 2000; 589p. [Google Scholar]
- Wright, M.G.; Conant, P. Pest status and management of macadamia felted coccid (Hemiptera: Eriococcidae) in Hawaii. S. Afr. Macadamia Grow. Assoc. 2009, 17, 72–75. [Google Scholar]
- Pulakkatu-thodi, I.; Dzurisin, J.; Follett, P. Evaluation of macadamia felted coccid (Hemiptera: Eriococcidae) damage and cultivar susceptibility using imagery from a small unmanned aerial vehicle (sUAV), combined with ground truthing. Pest Manag. Sci. 2022, 78, 4533–4543. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. The Kaū Calendar News Briefs, Hawaii Island. 2014. Available online: http://kaunewsbriefs.blogspot.com/2014_01_05_archive.html?m=1 (accessed on 6 May 2023).
- Zarders, D.R.; Wright, M.G. Macadamaia felted coccid, Eriococcus ironsidei: Biology and Life Cycle in Hawaii. Proc. Hawaii Entomol. Soc. 2016, 48, 51–55. [Google Scholar]
- Gutierrez-Coarite, R.; Pulakkatu-Thodi, I.; Zarders, D.; Mollinedo, J.; Yalemar, J.; Wright, M.G.; Cho, A. Macadamia Felted Coccid Eriococcus ironsidei (Hemiptera: Eriococidae) Description, Monitoring, and Control. Coll. Trop. Agric. Hum. Resour. Univ. Hawaii Manoa Insect Pests 2017, IP-43, 1–5. [Google Scholar]
- Swaine, G.; Ironside, D.A.; Yarrow, W.H.T. Macadamia felted coccid. In Insect Pests of Fruits and Vegetables; Information Series; Queensland Department of Primary Industries: Brisbane, Australia, 1985; Q183021. [Google Scholar]
- Pfeiffer Douglas, G. 3.3.8 Deciduous Fruit Trees. In World Crop Pests, 1997, Soft Scale Insects—Their Biology, Natural Enemies and Control; Ben-Dov, Y., Hodgson, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 7B, pp. 3–442. [Google Scholar]
- Lotfalizadeh, H. The genus Metaphycus Mercet (Hymenoptera: Encyrtidae) of the Iranian fauna with description of a new species. North-West. J. Zool. 2010, 6, 255–261. [Google Scholar]
- Polaszek, A.; Noyes, J.S.; Russell, S.; Ramadan, M.M. Metaphycus macadamiae (Hymenoptera: Encyrtidae)—A biological control agent of macadamia felted coccid Acanthococcus ironsidei (Hemiptera: Eriococcidae) in Hawaii. PLoS ONE 2020, 15, e0230944. [Google Scholar] [CrossRef] [PubMed]
- Zilahi-Balogh, G.M.G.; Shipp, J.L.; Cloutier, C.; Brodeur, J. Influence of Light Intensity, Photoperiod, and Temperature on the Efficacy of Two Aphelinid Parasitoids of the Greenhouse Whitefly. Environ. Entomol. 2006, 35, 581–589. [Google Scholar] [CrossRef]
- Prinsloo Gerhard, L. Chapter 2.3 Parasitoids 2.3.1 Encyrtidae. In Soft Scale Insects—Their Biology, Natural Enemies and Control; Ben-Dov, Y., Hodgson, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 7B. [Google Scholar]
- Nishida, G.M. Hawaiian Terrestrial Arthropod Checklist, 4th ed.; Hawaiian Biological Survey, Bishop Museum Technical Report 22; Bishop Museum Press: Honolulu, HI, USA, 2002; 313p. [Google Scholar]
- Noyes, J.S. Universal Chalcidoidea Database. World Wide Web Electronic Publication. 2019. Available online: http://www.nhm.ac.uk/chalcidoids (accessed on 10 May 2023).
- Triapitsyn, S.V.; Andreason, S.A.; Power, N.; Ganjisaffar, F.; Fusu, L.; Dominguez, C.; Perring, T.M. Two new species of Ooencyrtus (Hymenoptera, Encyrtidae), egg parasitoids of the bagrada bug Bagrada hilaris (Hemiptera, Pentatomidae), with taxonomic notes on Ooencyrtus telenomicida. J. Hymenopt. Res. 2020, 76, 57–98. [Google Scholar] [CrossRef]
- Slipinski, A. Australian Ladybird Beetles (Coleoptera: Coccinellidae): Their Biology and Classification; Australian Biological Resources Study: Canberra, Australia, 2007; p. 286. [Google Scholar]
- Java Memory Profiler (JMP); Version II; SAS Institute Inc.: Cary, NC, USA, 2023; Available online: https://www.jmp.com/en_us/home.html (accessed on 10 February 2022).
- Conant, P.; Hirayama, C. Natural enemies of macadamia felted coccid (Eriococcus ironsidei Williams, (Homoptera: Eriococcidae) in Hawaii. In 45th Annual Conference Proceedings; Hawaii Macadamia Nut Association: Hilo, HI, USA, 2005; pp. 30–34. [Google Scholar]
- Viggiani, G. La Specie Italiane del Genre Encarsia Foerster (Hymenoptera: Aphelinidae); Bollettino del Laboratorio di Entomologia Agraria ‘Filippo Silvestri’: Portici, Italy, 1987; Volume 44, pp. 121–179. (In Italian) [Google Scholar]
- Gutierrez-Coarite, R.; Cho, A.H.; Mollenido, J.; Pulakkatu-Thodi, I.; Wright, M.G. Macadamia felted coccid impact on macadamia nut yield in the absence of a specialized natural enemy, and economic injury levels. Crop Prot. 2021, 139, 105378. [Google Scholar] [CrossRef]
- Houping, L.; Mottern, J. An Old Remedy for a New Problem? Identification of Ooencyrtus kuvanae (Hymenoptera: Encyrtidae), an Egg Parasitoid of Lycorma delicatula (Hemiptera: Fulgoridae) in North America. J. Insect Sci. 2017, 17, 18. [Google Scholar] [CrossRef]
- Beardsley, J.W. Synopsis of the Encyrtidae of the Hawaiian Islands with Keys to General and Species (Hymenoptera: Chalcidoidae). Proc. Hawaii Entomol. Soc. 1976, 12, 181–195. [Google Scholar]
- Schoeman, P.S.; Millar, I.M. First report of Eriococcus ironsidei Williams (Hemiptera: Coccomorpha: Eriococcidae) on macadamia (Macadamia integrifolia Maiden & Betche and Macadamia tetraphylla Johnson: Proteaceae) in South Africa. Afr. Entomol. 2018, 26, 247–249. [Google Scholar] [CrossRef]
- Gutierrez-Coarite, R.; Pulakkatu-Thodi, I.; Wright, M.G. Binomial sequential sampling plans for macadamia felted coccid (Hemiptera: Eriococcidae) infesting Hawaii macadamia orchards. Environ. Entomol. 2019, 48, 219–226. [Google Scholar] [CrossRef]
- Anonymous. The Macadamia Magazine, Beware the Felted Coccid. 2019. Available online: https://themacadamia.co.za/2019/02/19/beware-the-felted-coccid/ (accessed on 9 June 2022).
- Yerton, S. Hawaii Farmers Struggle as Worldwide Macadamia Market Goes Nuts. Honolulu Civil Beat. 2022. Available online: https://www.civilbeat.org/2022/11/hawaii-farmers-struggle-as-worldwide-macadamia-market-goes-nuts/ (accessed on 2 December 2022).
- Workman, D. Top Macadamia Nuts Exports & Imports by Country Plus Average Prices. 2023. Available online: https://www.worldstopexports.com/top-macadamia-nuts-exports-imports-by-country-plus-average (accessed on 10 August 2023).
- Gullan, P.J.; Giliomee, J.H.; Hodgson, C.J.; Cook, L.G. The systematics and biology of the South African gall-inducing scale insect, Calycicoccus merwei Brain (Hemiptera: Coccoidea: Eriococcidae). Afr. Entomol. 2006, 14, 13–33. [Google Scholar]
- Kapranas, A.; Luck, R.F. Egg maturation, host feeding, and longevity in two Metaphycus parasitoids of soft scale insects. Biol. Control 2008, 47, 147–153. [Google Scholar] [CrossRef]
- Chirinos, D.T.; Kondo, T. Description and Biological Studies of a New Species of Metaphycus Mercet, 1917 (Hymenoptera: Encyrtidae), A Parasitoid of Capulinia linarosae Kondo & Gullan. Int. J. Insect Sci. 2020, 11, 1179543319857962. [Google Scholar] [CrossRef]



| Non-Target Insect Hosts and Host Plants | Mean% Parasitism Based on Metaphycus emergence and Non-Target Dissections (n = 3) | ||||||
|---|---|---|---|---|---|---|---|
| Scientific Name, Order, and Family | Stage Tested | Status | Source | Host Plant and Infested Plant Part Used | MFC (Control) % M. macadamiae Emergence | Total Non-Target Insects Dissected | Non-Target Insects % Parasitism |
| Tectococcus ovatus (Hempel) Hemiptera: Eriococcidae | Adult and Nymph | Biocontrol agent | Lab-reared HDOA, Oahu | Psidium cattleianum Whole plants and seedlings | 6.7 | 200 | 0 |
| Acanthococcus araucariae (Maskell) Hemiptera: Eriococcidae | Adult and Nymph | Immigrant | Field collected, Molokai | Araucaria sp. Cuttings | 17.5 | 420 | 0 |
| Thysanococcus pandani (Stickney) Hemiptera: Halimococcidae | Adult and Nymph | Immigrant | Field collected, Maui | Pandanus tectorius Whole plants | 21.7 | 300 | 0 |
| Colobopyga pritchardiae (Stickney) Hemiptera: Halimococcidae | Adult and Nymph | Endemic | Field collected, Hawaii | Pritchardia sp. Cuttings | 15.2 | 500 | 0 |
| Dactylopius opuntiae (Cockerell) Hemiptera: Dactylopiidae | Adult and Nymph | Biocontrol agent | Field collected, Oahu | Opuntia ficus-indica Cuttings | 11.7 | 1100 | 0 |
| Saissetia oleae (Oliver) Hemiptera: Coccidae | Adult and Nymph | Immigrant | Lab-reared HDOA, Oahu | Erythrina variegata Whole plants and seedlings | 30.6 | 1642 | 0 |
| Pseudococcid montanus (Erhorn) Hemiptera: Pseudococcidae | Nymphs and pupae | Endemic | Field collected, Oahu | Freycetia arborea Cuttings | 62.5 | 354 | 0 |
| Pariaconus ohiacola (Crawford) Hemiptera: Triozidae | Nymphs and pupae | Endemic | Field collected, Oahu | Metrosideros sp. Cuttings | 54.3 | 300 | 0 |
| Tetraleurodes acaciae (Quaintance) Hemiptera: Aleyrodidae | Egg | Immigrant | Field collected, Oahu | Erythrina variegata, Seedling | 38.3 | 300 | 0 |
| Vanessa tameamea (Eschscholtz) Lepidoptera: Nymphalidae | Egg | Endemic | Lab-reared PEPS, UHM | Pipturus albidus Eggs placed on filter paper | 57.3 | 55 | 0 |
| Danaus plexippus (L.) Lepidoptera: Nymphalidae | Egg | Naturalized | Field collected, Oahu | Colotropis gigantea Foliage | 19.4 | 30 | 0 |
| Secusio extensa (Butler) Lepidoptera: Erebidae | Egg | Biocontrol agent | Lab-reared HDOA, Oahu | Senecio madgascariensis Foliage | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalemar, J.A.; Tateno-Bisel, A.P.; Chun, S.G.; Ramadan, M.M. Prospects for Biological Control of Macadamia Felted Coccid in Hawaii with Metaphycus macadamiae Polaszek & Noyes, a New Encyrtid Wasp Native to New South Wales, Australia. Insects 2023, 14, 793. https://doi.org/10.3390/insects14100793
Yalemar JA, Tateno-Bisel AP, Chun SG, Ramadan MM. Prospects for Biological Control of Macadamia Felted Coccid in Hawaii with Metaphycus macadamiae Polaszek & Noyes, a New Encyrtid Wasp Native to New South Wales, Australia. Insects. 2023; 14(10):793. https://doi.org/10.3390/insects14100793
Chicago/Turabian StyleYalemar, Juliana A., Amber P. Tateno-Bisel, Stacey G. Chun, and Mohsen M. Ramadan. 2023. "Prospects for Biological Control of Macadamia Felted Coccid in Hawaii with Metaphycus macadamiae Polaszek & Noyes, a New Encyrtid Wasp Native to New South Wales, Australia" Insects 14, no. 10: 793. https://doi.org/10.3390/insects14100793
APA StyleYalemar, J. A., Tateno-Bisel, A. P., Chun, S. G., & Ramadan, M. M. (2023). Prospects for Biological Control of Macadamia Felted Coccid in Hawaii with Metaphycus macadamiae Polaszek & Noyes, a New Encyrtid Wasp Native to New South Wales, Australia. Insects, 14(10), 793. https://doi.org/10.3390/insects14100793





