A Survey in Hawaii for Parasitoids of Citrus Whiteflies (Hemiptera: Aleyrodidae), for Introduction into Greece
Abstract
:Simple Summary
Abstract
1. Introduction
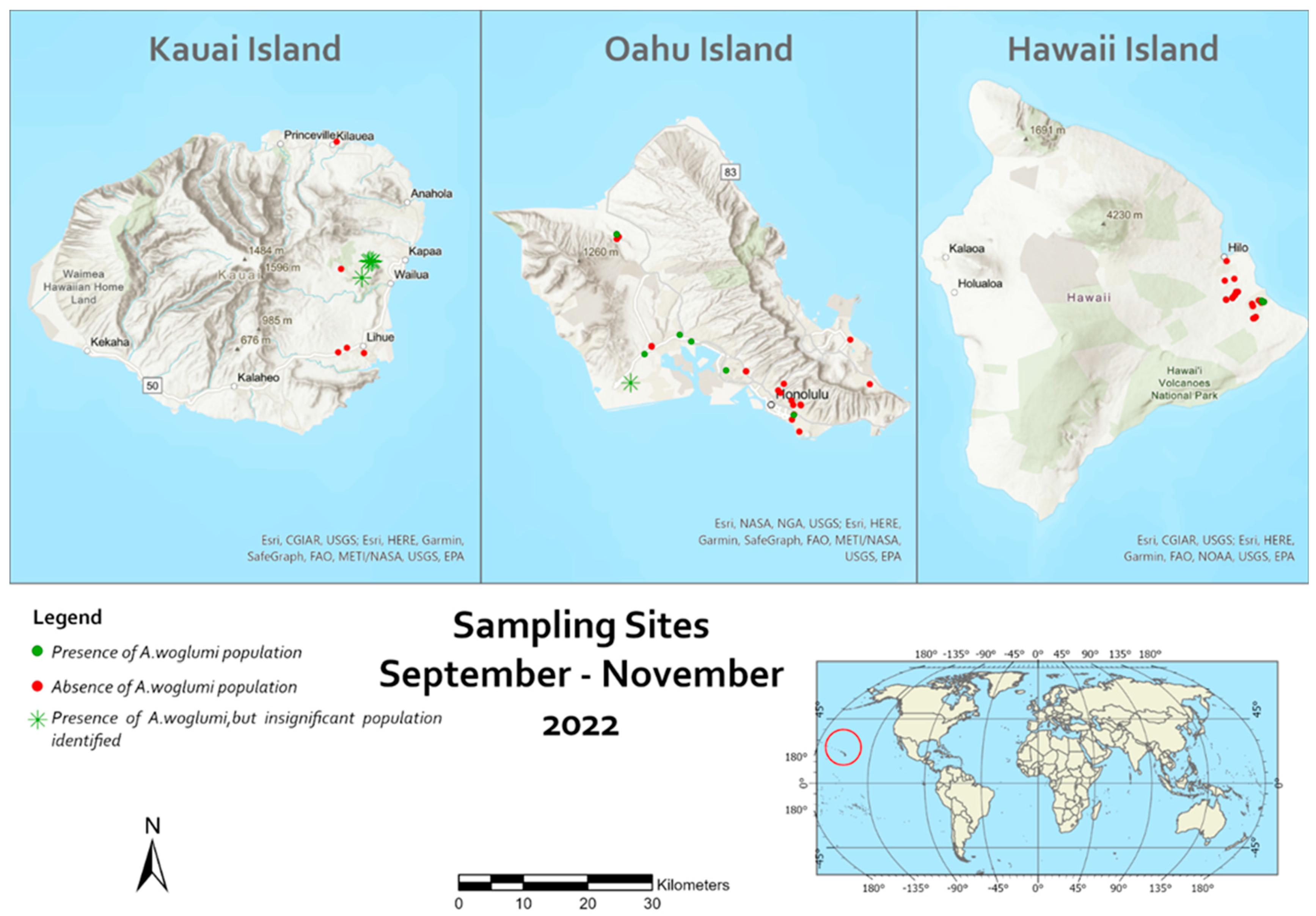
2. Materials and Methods
2.1. Collection and Emergence of Parasitoids
2.2. Identification of the Parasitoids
2.3. Infestation and Parasitism Rate
3. Results and Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapantaidaki, D.E.; Antonatos, S.; Kontodimas, D.; Milonas, P.; Papachristos, D.P. Presence of the invasive whitefly Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in Greece. Bull. OEPP/EPPO Bull. 2019, 49, 127–131. [Google Scholar] [CrossRef]
- Ramadan, M.M.; Kaufman, L.V.; Wright, M.G. Insect and weed biological control in Hawaii: Recent case studies and trends. Biol. Control 2023, 179, 105170. [Google Scholar] [CrossRef]
- EFSA Plant Health Panel (EFSA PLH Panel); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; et al. Scientific Opinion on the pest categorisation of Aleurocanthus spp. EFSA J. 2018, 16, e05436. [Google Scholar] [CrossRef] [PubMed]
- Akrivou, A.; Georgopoulou, I.; Papachristos, D.P.; Milonas, P.G.; Kriticos, D.J. Potential global distribution of Aleurocanthus woglumi considering climate change and irrigation. PLoS ONE 2021, 16, e0261626. [Google Scholar] [CrossRef]
- Porcelli, F. First record of Aleurocanthus spiniferus (Homoptera: Aleyrodidae) in Puglia, Southern Italy. EPPO Bull. 2008, 38, 516–518. [Google Scholar] [CrossRef]
- Nugnes, F.; Laudonia, S.; Jesu, G.; Jansen, M.G.M.; Bernardo, U.; Porcelli, F. Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in Some European Countries: Diffusion, Hosts, Molecular Characterization, and Natural Enemies. Insects 2020, 11, 42. [Google Scholar] [CrossRef]
- Šimala, M.; Masten Milek, T. First record of the orange spiny whitefly, Aleurocanthus spiniferus Quaintance, 1903 (Hemiptera: Aleyrodidae), in Croatia. In Plant Zbornik predavanj in Referatov 11. Slovenskega Posvetovanja o Varstvu Rastlin z Mednarodno Udeležbo Bled; Plant Protection Society of Slovenia: Ljubljana, Slovenia, 2013. [Google Scholar]
- Radonjic, S.; Hrncic, S.; Malumphy, C. First Record of Aleurocanthus spiniferus (Quaintance) (Hemiptera: Aleyrodidae) in Montenegro. CRA—Res. Cent. Agrobiol. Pedol. 2014, 97, 141–154. [Google Scholar]
- EFSA (European Food Safety Authority); Schrader, G.; Camilleri, M.; Ciubotaru, R.M.; Diakaki, M.; Vos, S. Pest survey card on Aleurocanthus spiniferus and Aleurocanthus woglumi. EFSA Support. Publ. 2019, 16, 1565E. [Google Scholar] [CrossRef]
- Uesugi, R.; Yara, K.; Sato, Y. Changes in population density of Aleurocanthus camelliae (Hemiptera: Aleyrodidae) and parasitism rate of Encarsia smithi (Hymenoptera: Aphelinidae) during the early invasion stages. Appl. Entomol. Zool. 2016, 51, 581–588. [Google Scholar] [CrossRef]
- Nguyen, R.; Brasil, J.R.; Poucher, C. Population density of the citrus blackfly, Aleurocanthus woglumi Ashby (Homoptera: Aleyrodidae), and its parasites in urban Florida in 1979–1981. Environ. Entomol. 1983, 12, 878–884. [Google Scholar] [CrossRef]
- Dowell, R.V. Factors affecting the field effectiveness of Encarsia opulenta (Hymenoptera: Aphelinidae), a parasitoid of citrus blackfly, Aleurocanthus woglumi (Homoptera: Aleyrodidae). Trop. Agric. 1989, 66, 110–112. [Google Scholar]
- Lopez, V.F.; Kairo, M.T.; Pollard, G.V.; Pierre, C.; Commodore, N.; Dominique, D. Post-release survey to assess impact and potential host range expansion by Amitus hesperidum and Encarsia perplexa, two parasitoids introduced for the biological control of the citrus blackfly, Aleurocanthus woglumi in Dominica. BioControl 2009, 54, 497–503. [Google Scholar] [CrossRef]
- Yamashita, K.; Kasai, A.; Suzuki, Y.; Yoshiyasu, Y. Population dynamics of the camellia spiny whitefly, Aleurocanthus camelliae (Hemiptera: Aleyrodidae), in tea fields during the early phase of invasion into Kyoto, Japan. Appl. Entomol. Zool. 2016, 51, 117–124. [Google Scholar] [CrossRef]
- United States Department of Agriculture. New United States records—Orange spiny whitefly (Aleurocanthus spiniferus (Quaintance)—Hawaii. Coop. Econ. Insect Rep. 1974, 24, 585. [Google Scholar]
- Culliney, T.W.; Nagamine, W.T.; Teramoto, K.K. Introductions for Biological Control in Hawaii 1997–2001. Proc. Hawaii. Entomol. Soc. 2003, 36, 145–153. [Google Scholar]
- Nakao, H.K.; Funasaki, G.Y. Introductions for biological control in Hawaii: 1975 and 1976. Proc. Hawaii. Entomol. Soc. 1979, 23, 125–128. [Google Scholar]
- Heu, R.A.; Nagamine, W.T. Citrus Blackfly Aleurocanthus woglumi Ashby (Homoptera: Aleyrodidae). New Pest Advisory 2001, 99-03. Available online: https://hdoa.hawaii.gov/pi/files/2013/01/npa99-03_citrusbf.pdf (accessed on 10 July 2023).
- Rossato, V. Ocorrência de Parasitóides de Aleurocanthus woglumi Ashby, 1903 (Hemiptera: Aleyrodidae) e seu Parasitismo por Cales noacki Howard, 1907 (Hymenoptera: Aphelinidae) nos Municípios de Belém, Capitão Poço e Irituia no Estado do Pará. Master’s Thesis, Universidade Federal Rural da Amazônia, Belém, Brazil, 2007; p. 40. [Google Scholar]
- MacGown, M.W.; Nebeker, T.E. Taxonomic review of Amitus (Hymenoptera: Proctotrupoidae, Platygastridae). Can. Ent. 1978, 110, 275–283. [Google Scholar] [CrossRef]
- Viggiani, G.; Mazzone, P. The Amitus Hald. (Hym. Platygastridae) of Italy, with description of three new species. Boll. Lab. Entomol. Agrar. “Filippo Silvestri” Portici 1978, 39, 59–69. [Google Scholar]
- Huang, J.; Polaszek, A. A revision of the Chinese species of Encarsia Forster (Hymenoptera: Aphelinidae): Parasitoids of whiteflies, scale insects and aphids (Hemiptera: Aleyrodidae, Diaspididae, Aphidoidea). J. Nat. Hist. 1998, 32, 1825–1966. [Google Scholar] [CrossRef]
- Nguyen, R. Amitus hesperidum (Hymenoptera: Platygasteridae), a parasite of the citrus blackfly (Aleurocanthus woglumi). Florida Department of Agricultural Consumer Services, Division Plant Industry. Entomol. Circular. 1988, 311, 1–2. [Google Scholar]
- Noyes, J.S. Universal Chalcidoidea Database. World Wide Web Electronic Publication. 2023. Available online: http://www.nhm.ac.uk/chalcidoids (accessed on 30 October 2023).
- Noyes, J.S. Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). J. Nat. Hist. 1982, 16, 315–334. [Google Scholar] [CrossRef]
- Noyes, J.S. Chapter 2.7.2.5. Chalcid parasitoids. In The Armored Scale Insects. Their Biology, Naural Enemies and Control; Rosen, D., Ed.; World Crop Pests; Elsevier: Amsterdam, The Netherlands; Oxford, UK; New York, NY, USA; Tokyo, Japan, 1990; Volume 4B, pp. 247–262. [Google Scholar]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Pentinsaari, M.; Salmela, H.; Mutanen, M.; Roslin, T. Molecular evolution of a widely-adopted taxonomic marker (COI) across the animal tree of life. Sci. Rep. 2016, 6, 35275. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Dowton, M.; Austin, A.D. Simultaneous analysis of 16S, 28S, COI and morphology in the Hymenoptera: Apocrita–evolutionary transitions among parasitic wasps. Biol. J. Linn. Soc. 2001, 74, 87–111. [Google Scholar]

| Target Whitefly (Hemiptera: Aleyrodidiae) | Introduced and Adventive Natural Enemies | ||||||
|---|---|---|---|---|---|---|---|
| Family | Species | Origin of Introduction | Year of Introduction and Release Period | Colonization Records (Total Numbers Released Per Island) | Establishment Records | Release Records | |
| Aleurocanthus spiniferus (Quaintance), citrus orange spiny whitefly | Platygastridae | Amitus hesperidum Silvestri | Mexico | 1974 | Recorded on Oahu | Only Oahu Island | |
| Platygastridae | Amitus spiniferus (Brethes) | California | 1979–1982 | Oahu (200) | Introduced for Aleurothrixus floccosus (Maskell). Established on Oahu Island. | Only on Oahu Island in 1982. | |
| Aphelinidae | Cales noacki Howard | California | February 1981–August 1982 | Lanai (275) Molokai (300) Oahu (12,149) | Introduced for Aleurothrixus floccosus (Maskell) Hawaii 1992 Kauai 1997 Oahu 1982 | Hawaii 1992 Lanai 1982 Molokai 1982 Oahu 1982 | |
| Aphelinidae | Encarsia clypealis (Silvestri) | Texas | 1975 | No recovery | Only on Oahu Island. | ||
| Aphelinidae | Encarsia perplexa Huang and Polaszek | Texas | 1975 | Oahu (165) | No recovery | Only on Oahu, released as Encarsia opulenta (Silvestri). | |
| Aphelinidae | Encarsia smithi (Silvestri) | Japan (Nagasaki) and Guam | September 1974 | Oahu (5330) | Established on Oahu September 1975, reported 1981, 1984, 1999 on Oahu. | Only on Oahu Island, some of the colonies from Guam in 1984 record (25 adults). | |
| Aleurocanthus woglumi Ashby, citrus blackfly | Platygastridae | Amitus hesperidum Silvestri | Guatemala | Introduced 1998, released May 1999 –August 2000 | Hawaii (20), Kauai (112), Maui (82), Oahu (8595) | Established | Hawaii, Kauai, Maui, Molokai, Oahu Islands |
| Aphelinidae | Cales noacki Howard | California | February 1981–August 1982 | Molokai (300) Oahu (12,149) | Introduced for Aleurothrixus floccosus (Maskell) | Only on Oahu Island in 1982. Recorded established on Oahu Island. | |
| Aphelinidae | Encarsia perplexa Huang and Polaszek | Guatemala | Introduced 1998, released April 1999–June 2002 | Hawaii (49,190) Kauai (17,090) Maui (47,025) Molokai (27,165) Oahu (5740) | Established | Hawaii, Kauai, Maui, Molokai, Oahu Islands. First identified as Encarsia opulenta (Silvestri) | |
| Aphelinidae | Encarsia nipponica Silvestri | Adventive, native to Japan and China | - | - | Hawaii 2000, Kauai 2001 Oahu 1997, 1999 | Fortuitous species | |
| Aphelinidae | Encarsia smithi (Silvestri) | Japan (Nagasaki) and Guam | September 1974 | Reported on Oahu 1989, 1999 on citrus | Established on Oahu September 1975 Reported in 1999 on Oahu | Only on Oahu Island. | |
| Survey Area and Habitat a | Citrus Plant | Coordinate and Elevation | Date | Total Number of Leaves Collected | Total Number of Nymphs | Level of Infestation | % Parasitism |
|---|---|---|---|---|---|---|---|
| University of Hawaii at Manoa | Sour orange | 21°18′17.98″ N 157°48′51.87″ W, 40 m | 26 September 2022 | 0 | 0 | 0 | - |
| University of Hawaii at Manoa | Sweet lime | 21°18′15.16″ N 157°48′48.99″ W, 38 m | “ | 0 | 0 | 0 | - |
| Ala Wai Community Garden | Lime | 21°17′02.50″ N 157°49′37.15″ W, 1.5 m | “ | 0 | 0 | 0 | - |
| Waimanalo Research Station | Citron, | 21°20′07.91″ N 157°42′55.01″ W, 23 m | “ | 0 | 0 | 0 | - |
| Kapaakea Lane, Honolulu | Tangerine | 21°17′27.82″ N 157°49′25.95″ W, 4 m | “ | 0 | 0 | 0 | - |
| Kapaakea Lane, Honolulu | Pummelo | 21°17′25.55″ N 157°49′26.61″ W, 3.6 m | “ | 9 | 118 | 1.44 ± 0.49 | 17.8% |
| Pearl City Urban Botanical Garden | Sweet orange | 21°23′38.76″ N 157°58′38.08″ W, 8.5 m | 27 September 2022 | 71 | 1471 | 1.76 ± 0.78 | 56.6% |
| Diamond Head Community Garden | Tangelo | 21°16′02.09″ N 157°58′59.55″ W, 0.9 m | “ | 0 | 0 | 0 | - |
| Moanalua Gardens | Grapefruit | 21°20′52.68″ N 157°53′28.74″ W, 6.4 m | “ | 0 | 0 | 0 | - |
| North Shore. Poamoho Research Station | Sweet orange | 21°32′38.00″ N 158°05′16.28″ W, 189 m | 28 September 2022 | 345 | 11,981 | 2.17 ± 0.95 | 19.7% |
| Salt Lake Blvd | Sweet lime | 21°21′09.13″ N 157°55′30.36″ W, 29 m | “ | 75 | 158 | 1.37 ± 0.48 | 53.8% |
| North Shore, Hawaii Queen bees, Hinshaw Farms | Sour orange | 21°32′11.10″ N 158°05′16.82″ W, 226 m | “ | 0 | 0 | 0 | - |
| North Shore, Hawaii Queen bees, Hinshaw Farms | Lemon | 21°32′17.54″ N 158°05′14.90″ W, 225 m | “ | 0 | 0 | 0 | - |
| Hawaii Agriculture Research Center, Kunia | Tangelo | 21°23′09.02″ N 158°02′14.67″ W, 82.6 m | 2 October 2022 | 0 | 0 | 0 | - |
| Hawaii Agriculture Research Center 2 | Lemon | 21°23′07.28″ N 158°02′17.16″ W, 84.4 m | “ | 0 | 0 | 0 | - |
| Manoa valley community gardens | Citron | 21°18′53.77″ N 157°48′25.49″ W, 57 m | “ | 0 | 0 | 0 | - |
| Nuuanu Mauna ala Royal mausoleum | Pummelo | 21°19′30.33″ N 157°50′49.81″ W, 64 m | 26 October 2022 | 0 | 0 | 0 | - |
| Nuuanu old Pali drive | Lemon | 21°21′14.24″ N 157°48′37.68″ W, 308 m | “ | 0 | 0 | 0 | - |
| Tantalus lookout | Tangerine | 21°19′06.92″ N 157°49′48.23″ W, 277 m | “ | 0 | 0 | 0 | - |
| Kailua | Lemon | 21°23′47.09″ N 157°44′44.49″ W, 0.6 m | “ | 0 | 0 | 0 | - |
| Mahiole street, Moanaloa | Sour orange | 21°20′49.79″ N 157°53′21.81″ W, 11 m | 31 October 2022 | 0 | 0 | 0 | - |
| North Shore Poamoho Research Station | Sweet orange and sweet lime | 21°32′38.00″ N 158°05′16.28″ W, 189 m | “ | 573 | 23,168 | 2.34 ± 0.81 | 24.9% |
| Waikele | Tangerine | 21°24′03.18″ N 158°00′13.75″ W, 60.6 m | 1 November 2022 | 13 | 389 | 2.00 ± 1.03 | 34.4% |
| Aloun farm | Pummelo | 21°22′28.47″ N 158°02′42.94″ W, 56.7 m | “ | 14 | 212 | 1.78 ± 0.67 | 11.5% |
| Royal Mausoleum of Hawaii | Lime | 21°19′30.33″ N 157°50′49.81″ W, 64 m | “ | 0 | 0 | 0 | - |
| Survey Area and Habitat a | Citrus Plant | GPS and Elevation | Date | Total Number of Leaves Collected | Total Number of Nymphs | Level of Infestation | % Parasitism |
|---|---|---|---|---|---|---|---|
| Waiakea | Sweet orange | 19°38′30.98″ N 155°04′51.67″ W, 194 m | 27 October 2022 | 0 | 0 | 0 | - |
| Waiakea Research station | Lime | 19°38′41.46″ N 155°04′37.82″ W, 173 m | “ | 0 | 0 | 0 | - |
| Hawaii Department of Agriculture, Hilo | Lemon | 19°42′23.03″ N 155°04′26.25″ W, 11 m | “ | 0 | 0 | 0 | - |
| Kurtistown 1 | Pummelo | 19°35′35.98″ N 155°03′30.41″ W, 206 m | “ | 0 | 0 | 0 | - |
| Kurtistown 2 | Pummelo | 19°35′30.36″ N 155°03′29.91″ W, 199 m | “ | 0 | 0 | 0 | - |
| Kurtistown 3 | Sweet orange | 19°35′00.29″ N 155°03′36.53″ W, 242 m | “ | 0 | 0 | 0 | - |
| Kurtistown 4 | Sour orange | 19°34′38.83″ N 155°03′56.19″ W, 271 m | “ | 0 | 0 | 0 | - |
| Hawaiian Paradise Park 1 | Grapefruit | 19°36′12.88″ N 154°56′51.46″ W, 13 m | 28 October 2022 | 173 | 17,848 | 3.14 ± 0.76 | 2.7% |
| Hawaiian Paradise Park 2 | Pummelo | 19°36′12.88″ N 154°56′51.46″ W, 13 m | “ | 7 | 423 | 3.00 ± 0.50 | 0.0% |
| Hawaiian Paradise Park 3 | Pummelo | 19°34′17.70″ N 154°57′21.50″ W, 50 m | “ | 6 | 212 | 2.50 ± 0.80 | 28.3% |
| Hawaiian Paradise Park 4 | Sweet orange | 19°34′35.47″ N 154°57′18.39″ W, 9 m | “ | 0 | 0 | 0 | - |
| Hawaiian Paradise Park 5 | Citron | 19°34′52.95″ N 154°57′39.69″ W, 39 m | 29 October 2022 | 0 | 0 | 0 | - |
| Hawaiian Paradise Park 6 | Lime | 19°34′14.46″ N 154°57′45.16″ W, 40 m | 30 October 2022 | 0 | 0 | 0 | - |
| Hawaiian Paradise Park 7 | Lemon | 19°35′39.96″ N 154°57′21.77″ W, 28 m | 31 October 2022 | 0 | 0 | 0 | - |
| Keeau 1 | Sour orange | 19°37′06.79″ N 155°02′37.40″ W, 121 m | 29 October 2022 | 0 | 0 | 0 | - |
| Keeau 2 | Lemon | 19°36′52.38″ N 155°02′48.90″ W, 149 m | “ | 0 | 0 | 0 | - |
| Keeau 3 | Tangerine | 19°36′23.02″ N 155°02′59.69″ W, 177 m | “ | 0 | 0 | 0 | - |
| Keeau 4 | Sweet orange | 19°37′11.96″ N 155°02′32.56″ W, 117 m | “ | 0 | 0 | 0 | - |
| Keeau 5 | Pummelo | 19°37′42.04″ N 155°02′19.12″ W, 123 m | “ | 0 | 0 | 0 | - |
| Keeau 6 | Tangelo | 19°38′42.04″ N 155°02′32.56″ W, 79 m | “ | 0 | 0 | 0 | - |
| Ainaloa 1 | Tangerine | 19°31′40.76″ N 154°59′35.89″ W, 217 m | “ | 0 | 0 | 0 | - |
| Ainaloa 2 | Lime | 19°31′20.12″ N 155°00′05.43″ W, 226 m | “ | 0 | 0 | 0 | - |
| Ainaloa 3 | Lemon | 19°31′47.83″ N 154°59′49.05″ W, 201 m | “ | 0 | 0 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giakoumaki, M.-V.; Milonas, P.; Antonatos, S.; Evangelοu, V.; Partsinevelos, G.; Papachristos, D.; Ramadan, M.M. A Survey in Hawaii for Parasitoids of Citrus Whiteflies (Hemiptera: Aleyrodidae), for Introduction into Greece. Insects 2023, 14, 858. https://doi.org/10.3390/insects14110858
Giakoumaki M-V, Milonas P, Antonatos S, Evangelοu V, Partsinevelos G, Papachristos D, Ramadan MM. A Survey in Hawaii for Parasitoids of Citrus Whiteflies (Hemiptera: Aleyrodidae), for Introduction into Greece. Insects. 2023; 14(11):858. https://doi.org/10.3390/insects14110858
Chicago/Turabian StyleGiakoumaki, Maria-Vasiliki, Panagiotis Milonas, Spyridon Antonatos, Vasiliki Evangelοu, George Partsinevelos, Dimitrios Papachristos, and Mohsen M. Ramadan. 2023. "A Survey in Hawaii for Parasitoids of Citrus Whiteflies (Hemiptera: Aleyrodidae), for Introduction into Greece" Insects 14, no. 11: 858. https://doi.org/10.3390/insects14110858
APA StyleGiakoumaki, M.-V., Milonas, P., Antonatos, S., Evangelοu, V., Partsinevelos, G., Papachristos, D., & Ramadan, M. M. (2023). A Survey in Hawaii for Parasitoids of Citrus Whiteflies (Hemiptera: Aleyrodidae), for Introduction into Greece. Insects, 14(11), 858. https://doi.org/10.3390/insects14110858






