Diversity of Bacteria Associated with Guts and Gonads in Three Spider Species and Potential Transmission Pathways of Microbes within the Same Spider Host
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Dissection and DNA Extraction
2.3. PCR Amplification and Sequencing
2.4. Bioinformatics and Statistical Analysis
3. Results
3.1. Comparative Morphological Analysis of the Gut and Gonads in Three Spider Species
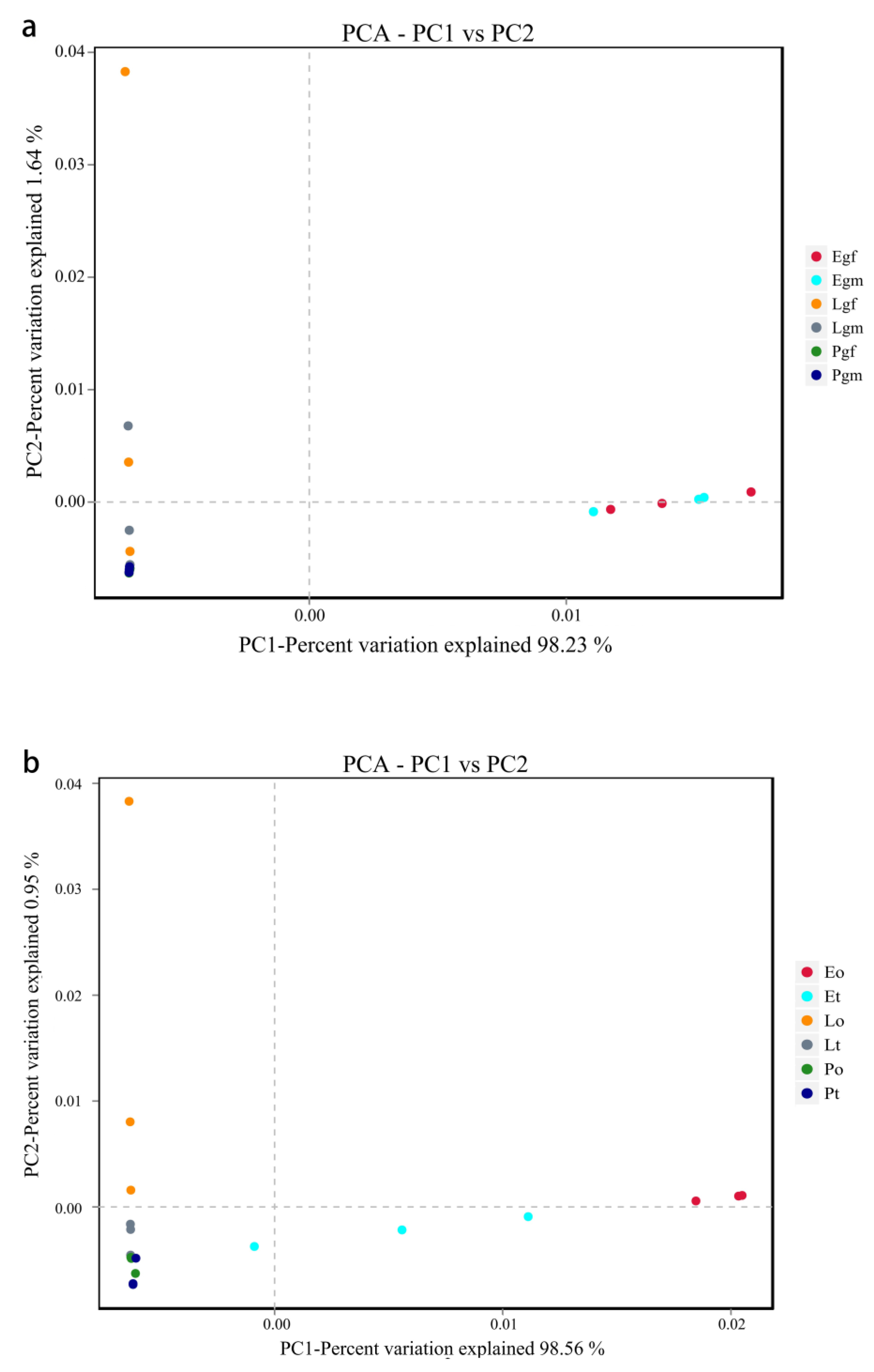
3.2. The Diversity Analysis of the Bacterial Community
3.3. Composition and Differential Analysis of Gut and Gonad Bacterial Communities
3.4. Differences in Relative Abundance of Bacteria between Spider Gut and Gonad in Males and Females
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Nair, S. Dynamics of insect–microbiome interaction influence host and microbial symbiont. Front. Microbiol. 2020, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Ravigné, V.; Becker, N.; Massol, F.; Guichoux, E.; Boury, C.; Mahé, F.; Facon, B. Fruit fly phylogeny imprints bacterial gut microbiota. Evol. Appl. 2022, 15, 1621–1638. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Segal, D.; Ringo, J.M.; Hefetz, A.; Zilber-Rosenberg, I.; Rosenberg, E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2010, 107, 20051–20056. [Google Scholar] [CrossRef]
- López-Madrigal, S.; Duarte, E.H. Titer regulation in arthropod-Wolbachia symbioses. FEMS Microbiol. Lett. 2019, 366, fnz232. [Google Scholar] [CrossRef]
- Hosokawa, T.; Koga, R.; Kikuchi, Y.; Meng, X.-Y.; Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA 2010, 107, 769–774. [Google Scholar] [CrossRef]
- Inagaki, T.; Yanagihara, S.; Fuchikawa, T.; Matsuura, K. Gut microbial pulse provides nutrition for parental provisioning in incipient termite colonies. Behav. Ecol. Sociobiol. 2020, 74, 64. [Google Scholar] [CrossRef]
- Hongoh, Y.; Deevong, P.; Inoue, T.; Moriya, S.; Trakulnaleamsai, S.; Ohkuma, M.; Vongkaluang, C.; Noparatnaraporn, N.; Kudo, T. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl. Environ. Microbiol. 2005, 71, 6590–6599. [Google Scholar] [CrossRef]
- Santana, R.H.; Catão, E.C.P.; Lopes, F.A.C.; Constantino, R.; Barreto, C.C.; Krüger, R.H. The gut microbiota of workers of the litter-feeding termite Syntermes wheeleri (Termitidae: Syntermitinae): Archaeal, bacterial, and fungal communities. Microb. Ecol. 2015, 70, 545–556. [Google Scholar] [CrossRef]
- He, B.; Chen, X.; Yang, H.; Cernava, T. Microbiome structure of the aphid Myzus persicae (Sulzer) is shaped by different solanaceae plant diets. Front. Microbiol. 2021, 12, 667257. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Luo, J.-Y.; Wang, C.-Y.; Lv, L.-M.; Cui, J.-J. Bacterial communities of the cotton aphid Aphis gossypii associated with Bt cotton in northern China. Sci. Rep. 2016, 6, 22958. [Google Scholar] [CrossRef] [PubMed]
- Ravenscraft, A.; Berry, M.; Hammer, T.; Peay, K.; Boggs, C. Structure and function of the bacterial and fungal gut microbiota of Neotropical butterflies. Ecol. Monogr. 2019, 89, e01346. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Rego, A.; Lucas, L.K.; Gompert, Z. Sources of variation in the gut microbial community of Lycaeides melissa caterpillars. Sci. Rep. 2017, 7, 11335. [Google Scholar] [CrossRef]
- Moran, N.A.; Hansen, A.K.; Powell, J.E.; Sabree, Z.L. Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS ONE 2012, 7, e36393. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Maes, P.; Anderson, K.E. The bacterial communities associated with honey bee (Apis mellifera) foragers. PLoS ONE 2014, 9, e95056. [Google Scholar] [CrossRef] [PubMed]
- Callegari, M.; Crotti, E.; Fusi, M.; Marasco, R.; Gonella, E.; De Noni, I.; Romano, D.; Borin, S.; Tsiamis, G.; Cherif, A.; et al. Compartmentalization of bacterial and fungal microbiomes in the gut of adult honeybees. npj Biofilms Microbiomes 2021, 7, 42. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Lv, Z.; Tian, J.; Peng, Y.; Peng, X.; Xu, X.; Song, Q.; Lv, B.; Chen, Z.; et al. Metatranscriptome analysis of the intestinal microorganisms in Pardosa pseudoannulata in response to cadmium stress. Ecotoxicol. Environ. Saf. 2018, 159, 1–9. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, L.; Yun, Y.; Peng, Y. Taking insight into the gut microbiota of three spider species: No characteristic symbiont was found corresponding to the special feeding style of spiders. Ecol. Evol. 2019, 9, 8146–8156. [Google Scholar] [CrossRef]
- Tyagi, K.; Tyagi, I.; Kumar, V. Interspecific variation and functional traits of the gut microbiome in spiders from the wild: The largest effort so far. PLoS ONE 2021, 16, e0251790. [Google Scholar] [CrossRef]
- Kennedy, S.R.; Tsau, S.; Gillespie, R.; Krehenwinkel, H. Are you what you eat? A highly transient and prey-influenced gut microbiome in the grey house spider Badumna longinqua. Mol. Ecol. 2020, 29, 1001–1015. [Google Scholar] [CrossRef]
- Marc, P.; Canard, A.; Ysne, F. Spiders (Araneae) useful for pest limitation and bioindication. Agric. Ecosyst. Environ. 1999, 74, 229–273. [Google Scholar] [CrossRef]
- Nyffeler, M.; Sunderland, K.D. Composition, abundance and pest control potential of spider communities in agroecosystems: A comparison of European and US studies. Agric. Ecosyst. Environ. 2003, 95, 579–612. [Google Scholar] [CrossRef]
- Michalko, R.; Pekár, S. The biocontrol potential of Philodromus (Araneae, Philodromidae) spiders for the suppression of pome fruit orchard pests. Biol. Control 2015, 82, 13–20. [Google Scholar] [CrossRef]
- Duron, O.; Bouchon, D.; Boutin, S.; Bellamy, L.; Zhou, L.; Engelstädter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 6, 27. [Google Scholar] [CrossRef]
- Goodacre, S.L.; Martin, O.Y.; Thomas, C.F.G.; Hewitt, G.M. Wolbachia and other endosymbiont infections in spiders. Mol. Ecol. 2006, 15, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Vilchez, I.; Mateos, M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS ONE 2010, 5, e12149. [Google Scholar] [CrossRef] [PubMed]
- Sanada-Morimura, S.; Matsumura, M.; Noda, H. Male killing caused by a Spiroplasma symbiont in the small brown planthopper, Laodelphax striatellus. J. Hered. 2013, 104, 821–829. [Google Scholar] [CrossRef]
- White, J.A.; Styer, A.; Rosenwald, L.C.; Curry, M.M.; Welch, K.D.; Athey, K.J.; Chapman, E.G. Endosymbiotic bacteria are prevalent and diverse in agricultural spiders. Microb. Ecol. 2020, 79, 472–481. [Google Scholar] [CrossRef]
- Stefanini, A.; Duron, O. Exploring the effect of the Cardinium endosymbiont on spiders. J. Evol. Biol. 2012, 25, 1521–1530. [Google Scholar] [CrossRef]
- Duron, O.; Hurst, G.D.D.; Hornett, E.A.; Josling, J.A.; Engelstädter, J. High incidence of the maternally inherited bacterium Cardinium in spiders. Mol. Ecol. 2008, 17, 1427–1437. [Google Scholar] [CrossRef]
- Laura, C.R.; Michael, I.S.; Jennifer, A.W. Endosymbiotic Rickettsiella causes cytoplasmic incompatibility in a spider host. bioRxiv 2020, 287, 20201107. [Google Scholar]
- Stouthamer, R.; Breeuwer, J.A.J.; Hurst, G.D.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, B.; Goodacre, S.L.; Hewitt, G.M. Sex ratio, mating behaviour and Wolbachia infections in a sheetweb spider. Biol. J. Linn. Soc. 2009, 98, 181–186. [Google Scholar] [CrossRef]
- Vanthournout, B.; Swaegers, J.; Hendrickx, F. Spiders do not escape reproductive manipulations by Wolbachia. BMC Evol. Biol. 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Vanthournout, B.; Vandomme, V.; Hendrickx, F. Sex ratio bias caused by endosymbiont infection in the dwarf spider Oedothorax retusus. J. Arachnol. 2014, 42, 24–33. [Google Scholar] [CrossRef]
- Vanthournout, B.; Hendrickx, F. Endosymbiont dominated bacterial communities in a dwarf spider. PLoS ONE 2015, 10, e0117297. [Google Scholar] [CrossRef]
- Dedeine, F.; Vavre, F.; Fleury, F.; Loppin, B.; Hochberg, M.E.; Boulétreau, M. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. USA 2001, 98, 6247–6252. [Google Scholar] [CrossRef]
- Mao, Q.; Wu, W.; Huang, L.; Yi, G.; Jia, D.; Chen, Q.; Chen, H.; Wei, T. Insect bacterial symbiont-mediated vitellogenin uptake into oocytes to support egg development. mBio 2020, 11, e01142–e01220. [Google Scholar] [CrossRef]
- Perlmutter, J.I.; Bordenstein, S.R. Microorganisms in the reproductive tissues of arthropods. Nat. Rev. Microbiol. 2020, 18, 97–111. [Google Scholar] [CrossRef]
- Zhang, L.; Yun, Y.; Hu, G.; Peng, Y. Insights into the bacterial symbiont diversity in spiders. Ecol. Evol. 2018, 8, 4899–4906. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Yun, Y.; Peng, Y. Bacterial community of a spider, Marpiss magister (Salticidae). 3 Biotech 2017, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Tyagi, K.; Tyagi, I.; Kumar, V. Insights into the gut bacterial communities of spider from wild with no evidence of phylosymbiosis. Saudi J. Biol. Sci. 2021, 28, 5913–5924. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Xie, J.; Zhang, Z. Diversity and function of wolf spider gut microbiota revealed by shotgun metagenomics. Front. Microbiol. 2021, 12, 758794. [Google Scholar] [CrossRef]
- Chen, B.; Teh, B.-S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis. Sci. Rep. 2016, 6, 29505. [Google Scholar] [CrossRef]
- Xia, X.; Gurr, G.M.; Vasseur, L.; Zheng, D.; Zhong, H.; Qin, B.; Lin, J.; Wang, Y.; Song, F.; Li, Y.; et al. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef]
- Wang, A.; Yao, Z.; Zheng, W.; Zhang, H. Bacterial communities in the gut and reproductive organs of Bactrocera minax (Diptera: Tephritidae) based on 454 pyrosequencing. PLoS ONE 2014, 9, e106988. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Ramirez, J.L.; Rooney, A.P.; Kim, C.-H. Comparative analysis of gut microbiota of mosquito communities in central Illinois. PLoS Neglected Trop. Dis. 2017, 11, e0005377. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, P.; Cui, S.; Ali, A.; Zheng, G. Divergence in gut bacterial community between females and males in the wolf spider Pardosa astrigera. Ecol. Evol. 2022, 12, e8823. [Google Scholar] [CrossRef]
- Busck, M.M.; Settepani, V.; Bechsgaard, J.; Lund, M.B.; Bilde, T.; Schramm, A. Microbiomes and specific symbionts of social spiders: Compositional patterns in host species, populations, and nests. Front. Microbiol. 2020, 11, 1845. [Google Scholar] [CrossRef] [PubMed]
- Serbus, L.R.; Casper-Lindley, C.; Landmann, F.; Sullivan, W. The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 2008, 42, 683–707. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef]
- Talarico, G.; Lipke, E.; Alberti, G. Gross morphology, histology, and ultrastructure of the alimentary system of Ricinulei (Arachnida) with emphasis on functional and phylogenetic implications. J. Morphol. 2011, 272, 89–117. [Google Scholar] [CrossRef]
- Foelix, R. Biology of Spiders; OUP USA: New York, NY, USA, 2011. [Google Scholar]
- Freitak, D.; Schmidtberg, H.; Dickel, F.; Lochnit, G.; Vogel, H.; Vilcinskas, A. The maternal transfer of bacteria can mediate trans-generational immune priming in insects. Virulence 2014, 5, 547–554. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, L.; Zhang, H. Low diversity bacterial community and the trapping activity of metabolites from cultivable bacteria species in the female reproductive system of the oriental fruit fly, Bactrocera dorsalis Hendel (Diptera: Tephritidae). Int. J. Mol. Sci. 2012, 13, 6266–6278. [Google Scholar] [CrossRef]
- Elgart, M.; Stern, S.; Salton, O.; Gnainsky, Y.; Heifetz, Y.; Soen, Y. Impact of gut microbiota on the fly’s germ line. Nat. Commun. 2016, 7, 11280. [Google Scholar] [CrossRef] [PubMed]
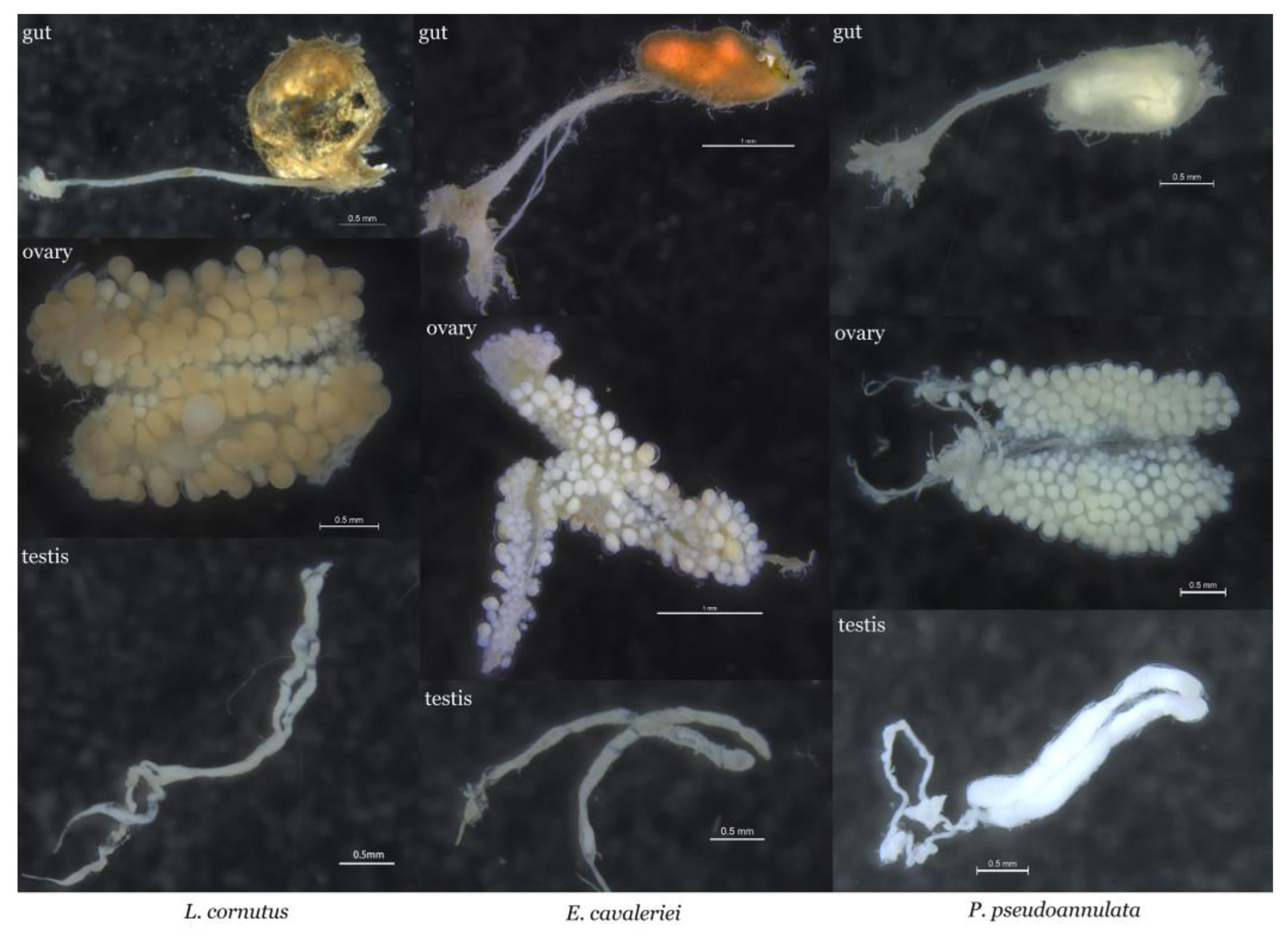

| Spider Species | Tissue | Sample ID |
|---|---|---|
| E. cavaleriei | Female gut | Egf |
| Male gut | Egm | |
| Ovary | Eo | |
| Testis | Et | |
| L. cornutus | Female gut | Lgf |
| Male gut | Lgm | |
| Ovary | Lo | |
| Testis | Lt | |
| P. pseudoannulata | Female gut | Pgf |
| Male gut | Pgm | |
| Ovary | Po | |
| Testis | Pt |
| Sample ID | Number of OTUs | Community Diversity | Species Richness | ||
|---|---|---|---|---|---|
| Shannon | Simpson | Ace | Chao1 | ||
| Egf | 958 | 5.35 ± 0.16 | 0.01 ± 0.00 | 806.45 ± 62.59 | 709.32 ± 98.94 |
| Egm | 902 | 5.34 ± 0.03 | 0.01 ± 0.00 | 803.78 ± 48.88 | 743.17 ± 21.24 |
| Eo | 853 | 0.85 ± 0.03 | 0.79 ± 0.01 | 1006.97 ± 111.60 | 798.71 ± 29.12 |
| Et | 1229 | 3.17 ± 0.52 | 0.31 ± 0.10 | 920.92 ± 37.22 | 910.88 ± 45.84 |
| Lgf | 1242 | 5.44 ± 0.20 | 0.02 ± 0.00 | 873.03 ± 64.70 | 869.33 ± 64.47 |
| Lgm | 1208 | 5.47 ± 0.06 | 0.01 ± 0.00 | 846.85 ± 41.92 | 849.58 ± 37.85 |
| Lo | 1311 | 5.05 ± 0.43 | 0.05 ± 0.03 | 888.44 ± 64.50 | 880.04 ± 71.39 |
| Lt | 1293 | 5.53 ± 0.01 | 0.01 ± 0.00 | 868.51 ± 9.29 | 868.24 ± 11.28 |
| Pgf | 1469 | 5.67 ± 0.07 | 0.01 ± 0.00 | 1024.92 ± 64.37 | 1028.60 ± 65.46 |
| Pgm | 1353 | 5.57 ± 0.10 | 0.01 ± 0.00 | 890.14 ± 69.14 | 894.97 ± 67.27 |
| Po | 1469 | 5.63 ± 0.16 | 0.01 ± 0.00 | 982.96 ± 154.58 | 989.46 ± 154.28 |
| Pt | 1323 | 5.46 ± 0.12 | 0.01 ± 0.00 | 1033.31 ± 77.99 | 1004.71 ± 37.93 |
| Phylum/Genus | Abundance (%) | |||||
|---|---|---|---|---|---|---|
| Egf | Egm | Lgf | Lgm | Pgf | Pgm | |
| Proteobacteria | 88.70 ± 3.37 a | 88.20 ± 3.08 a | 15.38 ± 2.69 b | 14.30 ± 1.37 b | 17.20 ± 1.18 b | 12.30 ± 0.56 b |
| Wolbachia | 86.20 ± 4.45 a | 86.10 ± 3.56 a | 0.10 ± 0.01 b | 0.08 ± 0.01 b | 0.12 ± 0.03 b | 0.01 ± 0.01 b |
| Massilia | 0.45 ± 0.13 a | 0.46 ± 0.12 a | 0.34 ± 0.09 a | 0.56 ± 0.11 a | 3.30 ± 0.33 b | 3.85 ± 0.22 b |
| Desulfovibrio | 0.22 ± 0.05 a | 0.25 ± 0.10 a | 0.36 ± 0.09 a | 0.28 ± 0.06 a | 1.16 ± 0.10 b | 1.58 ± 0.11 b |
| Escherichia-Shigella | 0.12 ± 0.04 a | 0.15 ± 0.05 a | 0.31 ± 0.12 a | 0.29 ± 0.05 a | 0.75 ± 0.08 b | 0.93 ± 0.04 b |
| Halomonas | 0.06 ± 0.02 a | 0.10 ± 0.03 a | 0.23 ± 0.00 b | 0.43 ± 0.01 b | 0.75 ± 0.05 c | 0.68 ± 0.07 c |
| Firmicutes | 4.18 ± 1.25 a | 4.22 ± 0.96 a | 22.42 ± 2.53 b | 29.90 ± 1.09 b | 29.00 ± 2.49 b | 28.62 ± 1.29 b |
| Virgibacillus | 0.70 ± 0.21 a | 0.69 ± 0.15 a | 4.35 ± 0.10 b | 5.28 ± 0.06 b | 4.66 ± 0.38 b | 5.22 ± 0.25 b |
| Lachnospiraceae_NK4A136_group | 0.54 ± 0.13 a | 0.62 ± 0.17 a | 2.90 ± 0.63 b | 3.98 ± 0.05 c | 3.96 ± 0.36 c | 4.42 ± 0.37 c |
| uncultured_bacterium_f_Lachnospiraceae | 0.18 ± 0.04 a | 0.22 ± 0.06 a | 1.16 ± 0.29 b | 1.33 ± 0.30 b | 1.33 ± 0.25 b | 1.42 ± 0.19 b |
| Lactobacillus | 0.16 ± 0.05 a | 0.12 ± 0.05 a | 0.67 ± 0.19 ab | 1.11 ± 0.10 ab | 1.49 ± 0.41 b | 1.10 ± 0.10 ab |
| Ruminiclostridium_9 | 0.13 ± 0.05 a | 0.15 ± 0.04 a | 0.97 ± 0.12 b | 0.98 ± 0.08 b | 1.01 ± 0.09 b | 1.04 ± 0.04 b |
| uncultured_bacterium_f_Ruminococcaceae | 0.13 ± 0.04 a | 0.14 ± 0.01 a | 0.70 ± 0.10 c | 1.25 ± 0.09 b | 0.89 ± 0.03 c | 1.05 ± 0.12 c |
| Bacillus | 0.34 ± 0.20 ab | 0.13 ± 0.01 a | 0.71 ± 0.12 ab | 0.88 ± 0.18 b | 0.87 ± 0.16 b | 0.87 ± 0.01 b |
| Ruminococcaceae_UCG-005 | 0.12 ± 0.05 a | 0.13 ± 0.03 a | 0.76 ± 0.19 b | 0.90 ± 0.13 b | 0.84 ± 0.07 b | 0.94 ± 0.15 b |
| Ruminococcaceae_UCG-014 | 0.13 ± 0.04 a | 0.18 ± 0.06 a | 0.74 ± 0.14 b | 0.89 ± 0.08 b | 0.86 ± 0.11 b | 0.79 ± 0.06 b |
| Quinella | 0.13 ± 0.03 a | 0.14 ± 0.04 a | 0.79 ± 0.17 b | 0.86 ± 0.07 b | 0.74 ± 0.16 b | 0.67 ± 0.07 b |
| Ruminococcus_1 | 0.10 ± 0.03 a | 0.13 ± 0.03 a | 0.58 ± 0.09 b | 0.83 ± 0.09 b | 0.63 ± 0.10 b | 0.72 ± 0.05 b |
| Turicibacter | 0.09 ± 0.03 a | 0.09 ± 0.01 a | 0.57 ± 0.19 b | 0.67 ± 0.01 b | 0.69 ± 0.04 b | 0.65 ± 0.05 b |
| Bacteroidetes | 3.16 ± 0.91 a | 3.44 ± 1.04 a | 18.75 ± 3.22 b | 22.50 ± 0.99 b | 24.40 ± 1.00 b | 2.10 ± 0.50 a |
| Prevotellaceae_UCG-003 | 0.66 ± 0.18 a | 0.82 ± 0.24 a | 4.86 ± 0.28 b | 5.27 ± 0.36 b | 5.13 ± 0.56 b | 6.35 ± 0.44 b |
| Bacteroidales_S24-7_group | 0.65 ± 0.17 a | 0.67 ± 0.26 a | 3.78 ± 0.14 b | 5.02 ± 0.02 c | 4.55 ± 0.13 bc | 4.49 ± 0.18 bc |
| Prevotella_9 | 0.49 ± 0.14 a | 0.55 ± 0.21 a | 2.88 ± 0.19 b | 3.19 ± 0.02 b | 3.22 ± 0.33 b | 3.24 ± 0.21 b |
| Bacteroides | 0.30 ± 0.08 a | 0.30 ± 0.09 a | 1.37 ± 0.08 b | 1.86 ± 0.01 b | 1.84 ± 0.18 b | 1.89 ± 0.33 b |
| Prevotellaceae_NK3B31_group | 0.21 ± 0.05 a | 0.26 ± 0.04 a | 1.31 ± 0.30 b | 1.71 ± 0.25 b | 1.44 ± 0.09 b | 1.65 ± 0.26 b |
| Prevotella_1 | 0.10 ± 0.03 a | 0.10 ± 0.01 a | 0.55 ± 0.02 ab | 0.95 ± 0.19 b | 0.78 ± 0.17 b | 0.99 ± 0.06 b |
| Actinobacteria | 1.69 ± 0.49 a | 1.80 ± 0.50 a | 10.37 ± 1.82 b | 12.40 ± 0.64 b | 11.40 ± 0.14 b | 5.27 ± 0.08 a |
| uncultured_bacterium_c_MB-A2-108 | 0.22 ± 0.05 a | 0.26 ± 0.09 a | 1.25 ± 0.06 b | 1.83 ± 0.35 b | 1.41 ± 0.18 b | 1.81 ± 0.19 b |
| Rubrobacter | 0.12 ± 0.02 a | 0.13 ± 0.02 a | 0.62 ± 0.03 b | 0.85 ± 0.12 b | 0.74 ± 0.09 b | 0.86 ± 0.10 b |
| Geodermatophilus | 0.08 ± 0.04 a | 0.11 ± 0.06 a | 0.55 ± 0.16 ab | 0.74 ± 0.07 b | 0.55 ± 0.09 ab | 0.84 ± 0.14 b |
| Streptomyces | 0.09 ± 0.02 a | 0.11 ± 0.11 a | 0.58 ± 0.22 a | 0.46 ± 0.07 a | 0.56 ± 0.14 a | 0.66 ± 0.06 a |
| Tenericutes | 0.10 ± 0.04 a | 0.09 ± 0.03 a | 4.46 ± 0.65 a | 6.04 ± 3.86 a | 0.47 ± 0.09 a | 1.01 ± 0.77 a |
| Spiroplasma | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 18.73 ± 16.25 a | 5.55 ± 5.55 a | 0.01 ± 0.01 a | 0.00 ± 0.00 a |
| Spirochaetae | 0.78 ± 0.18 a | 0.91 ± 0.22 a | 4.46 ± 0.28 b | 5.87 ± 0.12 c | 5.19 ± 0.14 bc | 5.27 ± 0.12 bc |
| Treponema_2 | 0.78 ± 0.18 a | 0.91 ± 0.22 a | 4.44 ± 0.10 b | 5.86 ± 0.01 c | 5.01 ± 0.55 bc | 5.59 ± 0.04 bc |
| Cyanobacteria | 0.43 ± 0.13 a | 0.52 ± 0.12 a | 2.83 ± 0.49 b | 3.71 ± 0.19 b | 3.31 ± 0.10 b | 2.70 ± 0.87 b |
| Microcoleus | 0.28 ± 0.09 a | 0.32 ± 0.07 a | 1.74 ± 0.16 b | 2.36 ± 0.02 bc | 2.16 ± 0.15 bc | 2.71 ± 0.16 c |
| Phylum/Genus | Abundance (%) | |||||
|---|---|---|---|---|---|---|
| Eo | Et | Lo | Lt | Po | Pt | |
| Proteobacteria | 93.08 ± 0.33 a | 65.19 ± 7.88 b | 13.60 ± 2.15 c | 14.40 ± 0.48 c | 14.60 ± 1.07 c | 8.82 ± 4.36 c |
| Wolbachia | 91.94 ± 0.27 a | 57.31 ± 9.30 b | 0.01 ± 0.00 c | 0.00 ± 0.00 c | 0.46 ± 0.43 c | 0.79 ± 0.34 c |
| Massilia | 0.29 ± 0.03 a | 1.45 ± 0.36 ab | 2.97 ± 0.09 ab | 3.18 ± 0.71 b | 2.66 ± 0.78 ab | 1.23 ± 0.79 ab |
| Desulfovibrio | 0.14 ± 0.03 a | 0.73 ± 0.25 ab | 1.39 ± 0.11 ab | 1.68 ± 0.41 b | 1.14 ± 0.22 ab | 0.76 ± 0.32 ab |
| Escherichia-Shigella | 0.07 ± 0.02 a | 0.43 ± 0.03 ab | 0.80 ± 0.05 ab | 1.20 ± 0.13 b | 0.73 ± 0.18 ab | 0.52 ± 0.40 ab |
| Firmicutes | 2.47 ± 0.13 a | 13.50 ± 2.83 b | 24.70 ± 2.50 c | 32.60 ± 0.50 c | 30.60 ± 2.59 c | 26.60 ± 1.43 c |
| Virgibacillus | 0.47 ± 0.02 a | 2.56 ± 0.67 ab | 4.36 ± 0.24 ab | 4.99 ± 0.38 b | 4.57 ± 1.13 ab | 2.04 ± 1.51 ab |
| Lachnospiraceae_NK4A136_group | 0.35 ± 0.01 a | 1.82 ± 0.33 ab | 3.69 ± 0.41 b | 4.73 ± 0.50 b | 3.78 ± 0.87 b | 1.75 ± 0.93 ab |
| uncultured_bacterium_f_Lachnospiraceae | 0.08 ± 0.01 a | 0.71 ± 0.19 ab | 1.17 ± 0.09 bc | 1.80 ± 0.15 c | 1.34 ± 0.29 bc | 0.73 ± 0.23 ab |
| uncultured_bacterium_f_Ruminococcaceae | 0.06 ± 0.00 a | 0.51 ± 0.14 ab | 0.81 ± 0.09 ab | 1.15 ± 0.27 b | 0.96 ± 0.19 ab | 0.70 ± 0.27 ab |
| Ruminococcaceae_NK4A214_group | 0.04 ± 0.01 a | 0.29 ± 0.08 a | 0.49 ± 0.09 ab | 0.69 ± 0.17 ab | 1.05 ± 0.30 ab | 1.62 ± 0.45 b |
| Succiniclasticum | 0.02 ± 0.01 a | 0.04 ± 0.03 a | 0.12 ± 0.11 a | 0.06 ± 0.03 a | 0.96 ± 0.86 a | 3.03 ± 1.50 a |
| Bacillus | 0.09 ± 0.02 a | 0.50 ± 0.10 ab | 0.81 ± 0.10 ab | 1.31 ± 0.12 b | 1.07 ± 0.31 b | 0.39 ± 0.21 b |
| Ruminococcaceae_UCG-014 | 0.04 ± 0.00 a | 0.47 ± 0.07 ab | 0.88 ± 0.21 ab | 1.16 ± 0.24 b | 0.69 ± 0.01 b | 0.70 ± 0.08 ab |
| Ruminiclostridium_9 | 0.11 ± 0.01 a | 0.48 ± 0.19 ab | 0.86 ± 0.06 ab | 1.24 ± 0.11 b | 0.93 ± 0.29 ab | 0.34 ± 0.25 ab |
| Lactobacillus | 0.10 ± 0.06 a | 0.35 ± 0.05 ab | 0.69 ± 0.12 ab | 1.04 ± 0.23 b | 0.75 ± 0.19 ab | 0.41 ± 0.27 ab |
| Ruminococcaceae_UCG-005 | 0.07 ± 0.01 a | 0.36 ± 0.12 a | 0.64 ± 0.11 ab | 1.13 ± 0.11 b | 0.71 ± 0.12 ab | 0.36 ± 0.23 a |
| Bacteroidetes | 2.04 ± 0.11 a | 11.10 ± 2.62 a | 20.40 ± 2.77 ab | 24.60 ± 0.95 ab | 29.80 ± 5.28 ab | 47.70 ± 12.30 b |
| Prevotellaceae_UCG-003 | 0.49 ± 0.01 a | 2.46 ± 0.52 ab | 5.25 ± 0.54 b | 6.11 ± 1.02 b | 5.07 ± 1.12 b | 3.13 ± 0.73 ab |
| Bacteroidales_S24-7_group | 0.42 ± 0.05 a | 2.60 ± 0.62 ab | 3.92 ± 0.54 ab | 5.66 ± 0.93 b | 4.30 ± 1.23 ab | 2.06 ± 1.33 ab |
| Prevotella_1 | 0.10 ± 0.03 a | 0.43 ± 0.10 a | 1.05 ± 0.32 a | 0.81 ± 0.22 a | 3.69 ± 2.92 a | 11.3 ± 5.35 a |
| Rikenellaceae_RC9_gut_group | 0.06 ± 0.03 a | 0.24 ± 0.09 a | 0.29 ± 0.14 a | 0.37 ± 0.13 a | 2.76 ± 2.45 a | 10.10 ± 4.91 a |
| uncultured_rumen_bacterium | 0.05 ± 0.01 a | 0.16 ± 0.08 a | 0.23 ± 0.11 a | 0.26 ± 0.03 a | 2.96 ± 2.67 a | 9.62 ± 4.69 a |
| Prevotella_9 | 0.27 ± 0.03 a | 1.58 ± 0.49 ab | 2.85 ± 0.32 ab | 3.63 ± 0.16 b | 3.06 ± 0.79 ab | 1.22 ± 0.90 ab |
| Bacteroides | 0.00 ± 0.00 a | 0.19 ± 0.07 a | 1.66 ± 0.30 a | 2.48 ± 0.59 a | 1.58 ± 0.38 a | 1.48 ± 1.21 a |
| Prevotellaceae_NK3B31_group | 0.16 ± 0.01 a | 0.82 ± 0.22 ab | 1.48 ± 0.29 b | 1.54 ± 0.27 b | 1.76 ± 0.12 b | 1.38 ± 0.05 b |
| Bacteroidales_BS11_gut_group | 0.02 ± 0.01 a | 0.04 ± 0.01 a | 0.05 ± 0.03 a | 0.07 ± 0.04 a | 0.86 ± 0.76 a | 3.02 ± 1.46 a |
| Actinobacteria | 1.11 ± 0.08 a | 5.53 ± 1.13 ab | 11.00 ± 1.01 b | 12.60 ± 0.98 b | 11.00 ± 2.09 b | 4.93 ± 3.20 ab |
| uncultured_bacterium_c_MB-A2-108 | 0.15 ± 0.03 a | 0.71 ± 0.22 a | 1.61 ± 0.20 a | 1.56 ± 0.15 a | 1.51 ± 0.40 a | 0.67 ± 0.47 a |
| Spirochaetae | 0.43 ± 0.03 a | 2.60 ± 0.45 ab | 4.93 ± 0.43 b | 5.42 ± 0.48 b | 4.19 ± 0.96 b | 2.55 ± 1.00 ab |
| Treponema_2 | 0.43 ± 0.03 a | 2.60 ± 0.45 ab | 4.93 ± 0.43 b | 5.42 ± 0.84 b | 4.16 ± 0.99 ab | 2.47 ± 1.04 ab |
| Tenericutes | 0.05 ± 0.01 a | 0.26 ± 0.06 a | 15.90 ± 8.86 a | 1.85 ± 0.59 a | 0.55 ± 0.09 a | 0.39 ± 0.02 a |
| Spiroplasma | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 15.30 ± 9.00 a | 1.07 ± 0.91 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Cyanobacteria | 0.31 ± 0.01 a | 1.52 ± 0.19 a | 3.20 ± 0.3 bc | 3.50 ± 0.08 b | 2.76 ± 0.63 bc | 1.35 ± 0.65 a |
| Microcoleus | 0.21 ± 0.02 a | 1.07 ± 0.14 ab | 2.11 ± 0.21 b | 2.22 ± 0.16 b | 1.80 ± 0.46 b | 0.78 ± 0.44 ab |
| Gemmatimonadetes | 0.12 ± 0.01 a | 0.73 ± 0.13 ab | 1.83 ± 0.28 b | 1.90 ± 0.07 b | 1.62 ± 0.36 b | 0.71 ± 0.47 ab |
| uncultured_bacterium_f_Longimicrobiaceae | 0.05 ± 0.01 a | 0.35 ± 0.07 ab | 0.81 ± 0.08 ab | 1.03 ± 0.27 b | 0.73 ± 0.16 ab | 0.33 ± 0.26 ab |
| Phylum/Genus | Comparison of Differences in Relative Abundance (p-Value) | |||||
|---|---|---|---|---|---|---|
| Egf/Eo | Egm/Et | Lgf/Lo | Lgm/Lt | Pgf/Po | Pgm/Pt | |
| Proteobacteria | - | * | - | - | - | - |
| Wolbachia | - | * | - | - | - | - |
| Massilia | - | * | - | - | - | * |
| Desulfovibrio | - | - | - | - | - | - |
| Escherichia-Shigella | - | ** | - | * | - | - |
| Firmicutes | - | * | - | - | - | - |
| Virgibacillus | - | * | - | - | - | - |
| Lachnospiraceae_NK4A136_group | - | * | - | * | - | * |
| uncultured_bacterium_f_Lachnospiraceae | * | - | - | - | - | - |
| Lactobacillus | - | * | - | - | - | - |
| Ruminiclostridium_9 | - | - | - | - | - | * |
| uncultured_bacterium_f_Ruminococcaceae | - | * | - | - | - | - |
| Bacillus | - | * | - | - | - | * |
| Ruminococcaceae_UCG-005 | - | - | - | - | - | - |
| Ruminococcaceae_UCG-014 | * | * | - | - | - | - |
| Bacteroidetes | - | - | - | - | - | - |
| Prevotellaceae_UCG-003 | - | * | - | - | - | * |
| Bacteroidales_S24-7_group | - | * | - | - | - | - |
| Prevotella_9 | - | - | - | * | - | - |
| Bacteroides | - | ** | - | - | - | - |
| Prevotellaceae_NK3B31_group | - | * | - | - | - | - |
| Prevotella_1 | - | * | - | - | - | - |
| Actinobacteria | - | * | - | - | - | * |
| uncultured_bacterium_c_MB-A2-108 | - | - | - | - | - | - |
| Tenericutes | - | - | - | - | - | - |
| Spiroplasma | - | - | - | - | - | - |
| Spirochaetae | - | * | - | - | - | * |
| Treponema_2 | - | * | - | - | - | * |
| Cyanobacteria | - | ** | - | - | - | * |
| Microcoleus | - | ** | - | - | - | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, J.; Zhang, X.; Yun, Y. Diversity of Bacteria Associated with Guts and Gonads in Three Spider Species and Potential Transmission Pathways of Microbes within the Same Spider Host. Insects 2023, 14, 792. https://doi.org/10.3390/insects14100792
Liu Y, Liu J, Zhang X, Yun Y. Diversity of Bacteria Associated with Guts and Gonads in Three Spider Species and Potential Transmission Pathways of Microbes within the Same Spider Host. Insects. 2023; 14(10):792. https://doi.org/10.3390/insects14100792
Chicago/Turabian StyleLiu, Yue, Jia Liu, Xiaopan Zhang, and Yueli Yun. 2023. "Diversity of Bacteria Associated with Guts and Gonads in Three Spider Species and Potential Transmission Pathways of Microbes within the Same Spider Host" Insects 14, no. 10: 792. https://doi.org/10.3390/insects14100792
APA StyleLiu, Y., Liu, J., Zhang, X., & Yun, Y. (2023). Diversity of Bacteria Associated with Guts and Gonads in Three Spider Species and Potential Transmission Pathways of Microbes within the Same Spider Host. Insects, 14(10), 792. https://doi.org/10.3390/insects14100792




