Urban Individuals of Three Rove Beetle Species Are Not More Exploratory or Risk-Taking Than Rural Conspecifics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area and Sampling Design
2.2. Test Organisms
2.3. Testing, Measuring, and Evaluating Behavioral Parameters
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Behavioral Measures and Personality
4.2. Sex-Specific Differences in Behavior
4.3. Urbanization and Behavioral Measures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.; Xu, S.; Qi, W.; Chen, C.; Deng, Y.; Pei, N.; König, H.J. Biodiversity constraint indicator establishment and its optimization for urban growth: Framework and application. Environ. Res. Lett. 2019, 14, 125006. [Google Scholar] [CrossRef]
- Song, X.; Chang, K.T.; Yang, L.; Scheffran, J. Change in environmental benefits of urban land use and its drivers in Chinese cities, 2000–2010. Int. J. Environ. Res. Public Health 2016, 13, 535. [Google Scholar] [CrossRef] [Green Version]
- Antrop, M. Changing patterns in the urbanized countryside of Western Europe. Landsc. Ecol. 2000, 15, 257–270. [Google Scholar] [CrossRef]
- United Nations. United Nations World Urbanization Prospects: The 2018 Revision; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019.
- Forman, R.T.T. Urban Regions: Ecology and Planning Beyond the City; Cambridge University Press: Cambridge, UK, 2008; ISBN 9780511754982. [Google Scholar]
- Niemelä, J. Ecology and urban planning. Biodivers. Conserv. 1999, 8, 119–131. [Google Scholar] [CrossRef]
- Simon, E.; Harangi, S.; Baranyai, E.; Braun, M.; Fábián, I.; Mizser, S.; Nagy, L.; Tóthmérész, B. Distribution of toxic elements between biotic and abiotic components of terrestrial ecosystem along an urbanization gradient: Soil, leaf litter and ground beetles. Ecol. Indic. 2016, 60, 258–264. [Google Scholar] [CrossRef]
- Kalnay, E.; Cai, M. Impact of urbanization and land-use change on climate. Nature 2003, 423, 528–531. [Google Scholar] [CrossRef]
- Xu, D.; Gao, J.; Lin, W.; Zhou, W. Differences in the ecological impact of climate change and urbanization. Urban Clim. 2021, 38, 100891. [Google Scholar] [CrossRef]
- Wang, H.; Marshall, C.W.; Cheng, M.; Xu, H.; Li, H.; Yang, X.; Zheng, T. Changes in land use driven by urbanization impact nitrogen cycling and the microbial community composition in soils. Sci. Rep. 2017, 7, 44049. [Google Scholar] [CrossRef]
- Enloe, H.A.; Lockaby, B.G.; Zipperer, W.C.; Somers, G.L. Urbanization effects on leaf litter decomposition, foliar nutrient dynamics and aboveground net primary productivity in the subtropics. Urban Ecosyst. 2015, 18, 1285–1303. [Google Scholar] [CrossRef]
- Pavao-Zuckerman, M.A. Urbanization, Soils, and Ecosystem Services. In Soil Ecology and Ecosystem Services; Wall, D., Bardgett, R.D., Behan-Pelletier, V., Herrick, J.E., Jones, T.H., Ritz, K., Six, J., Strong, D.R., van der Putten, V.H., Eds.; Oxford Scholarship Online: Oxford, UK, 2013. [Google Scholar]
- Miles, L.S.; Rivkin, L.R.; Johnson, M.T.J.; Munshi-South, J.; Verrelli, B.C. Gene flow and genetic drift in urban environments. Mol. Ecol. 2019, 28, 4138–4151. [Google Scholar] [CrossRef]
- Magura, T.; Lövei, G.L.; Tóthmérész, B. Conversion from environmental filtering to randomness as assembly rule of ground beetle assemblages along an urbanization gradient. Sci. Rep. 2018, 8, 16992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, M.N.; McKinney, M.L. Urbanization impacts on land snail community composition. Urban Ecosyst. 2018, 21, 721–735. [Google Scholar] [CrossRef]
- Sih, A.; Ferrari, M.C.O.; Harris, D.J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Lövei, G.L.; Magura, T. Body size and the urban heat island effect modulate the temperature-size relationship in ground beetles. J. Biogeogr. 2022, in press. [Google Scholar] [CrossRef]
- McIntyre, N.E. Urban ecology—Definitions and goals. In The Routledge Handbook of Urban Ecology; Douglas, I., Goode, D., Houck, M., Wang, R., Eds.; Routledge: London, UK, 2011; pp. 7–16. [Google Scholar]
- Magura, T.; Lövei, G.L. Consequences of urban living: Urbanization and ground beetles. Curr. Landsc. Ecol. Rep. 2021, 6, 9–21. [Google Scholar] [CrossRef]
- Eötvös, C.B.; Magura, T.; Lövei, G.L. A meta-analysis indicates reduced predation pressure with increasing urbanization. Landsc. Urban Plan. 2018, 180, 54–59. [Google Scholar] [CrossRef]
- Seto, K.C.; Parnell, S.; Elmqvist, T. A Global Outlook on Urbanization. In Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities: A Global Assessment; Elmqvist, T., Fragkias, M., Goodness, J., Güneralp, B., Marcotullio, P.J., McDonald, R.I., Parnell, S., Schewenius, M., Sendstad, M., Seto, K.C., et al., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 1–12. ISBN 978-94-007-7088-1. [Google Scholar]
- González-Oreja, J.A. Birds of different biogeographic origins respond in contrasting ways to urbanization. Biol. Conserv. 2011, 144, 234–242. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities are hotspots for threatened species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Kotze, D.J.; Lowe, E.C.; MacIvor, J.S.; Ossola, A.; Norton, B.A.; Hochuli, D.F.; Mata, L.; Moretti, M.; Gagné, S.A.; Handa, I.T.; et al. Urban forest invertebrates: How they shape and respond to the urban environment. Urban Ecosyst. 2022, in press. [Google Scholar] [CrossRef]
- Magura, T.; Kiss, E.; Lövei, G.L. No consistent diversity patterns in terrestrial mammal assemblages along rural-urban forest gradients. Basic Appl. Ecol. 2021, 52, 38–45. [Google Scholar] [CrossRef]
- Lin, P.; Yang, L.; Zhao, S. Urbanization effects on chinese mammal and amphibian richness: A multi-scale study using the urban-rural gradient approach. Environ. Res. Commun. 2020, 2, 125002. [Google Scholar] [CrossRef]
- Leveau, L.M.; Leveau, C.M.; Villegas, M.; Cursach, J.A.; Suazo, C.G. Bird communities along urbanization gradients: A comparative analysis among three neotropical cities. Ornitol. Neotrop. 2017, 28, 77–87. [Google Scholar]
- Batáry, P.; Kurucz, K.; Suarez-Rubio, M.; Chamberlain, D.E. Non-linearities in bird responses across urbanization gradients: A meta-analysis. Glob. Change Biol. 2018, 24, 1046–1054. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Hahs, A.K.; Vesk, P.A. Urbanisation, plant traits and the composition of urban floras. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 78–86. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Zhang, M.; Tong, F.; Xu, Z.; Ford, R.; Zhang, T.; Shi, X.; Wu, Z.; Luo, T. Plant Functional Groups Dominate Responses of Plant Adaptive Strategies to Urbanization. Front. Plant Sci. 2021, 12, 773676. [Google Scholar] [CrossRef]
- Fenoglio, M.S.; Rossetti, M.R.; Videla, M. Negative effects of urbanization on terrestrial arthropod communities: A meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1412–1429. [Google Scholar] [CrossRef]
- Lagucki, E.; Burdine, J.D.; McCluney, K.E. Urbanization alters communities of flying arthropods in parks and gardens of a medium-sized city. PeerJ 2017, 5, e3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef]
- Nagy, D.D.; Magura, T.; Horváth, R.; Debnár, Z.; Tóthmérész, B. Arthropod assemblages and functional responses along an urbanization gradient: A trait-based multi-taxa approach. Urban For. Urban Green. 2018, 30, 157–168. [Google Scholar] [CrossRef]
- Braschler, B.; Gilgado, J.D.; Rusterholz, H.P.; Buchholz, S.; Zwahlen, V.; Baur, B. Functional diversity and habitat preferences of native grassland plants and ground-dwelling invertebrates in private gardens along an urbanization gradient. Ecol. Evol. 2021, 11, 17043–17059. [Google Scholar] [CrossRef]
- Bátori, Z.; Gallé, R.; Gallé-Szpisjak, N.; Császár, P.; Nagy, D.D.; Lőrinczi, G.; Torma, A.; Tölgyesi, C.; Maák, I.E.; Frei, K.; et al. Topographic depressions provide potential microrefugia for ground-dwelling arthropods. Elem. Sci. Anthr. 2022, 10, 00084. [Google Scholar] [CrossRef]
- Magura, T.; Nagy, D.; Tóthmérész, B. Rove beetles respond heterogeneously to urbanization. J. Insect Conserv. 2013, 17, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Vergnes, A.; Pellissier, V.; Lemperiere, G.; Rollard, C.; Clergeau, P. Urban densification causes the decline of ground-dwelling arthropods. Biodivers. Conserv. 2014, 23, 1859–1877. [Google Scholar] [CrossRef]
- Gosling, S.D. From mice to men: What can we learn about personality from animal research? Psychol. Bull. 2001, 127, 45–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A.M.; Hankison, S.J.; Laskowski, K.L. The repeatability of behaviour: A meta-analysis. Anim. Behav. 2009, 77, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Lapiedra, O.; Chejanovski, Z.; Kolbe, J.J. Urbanization and biological invasion shape animal personalities. Glob. Change Biol. 2017, 23, 592–603. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Yasui, S.L.E.; Livingstone, S.; MacIvor, J.S. Are urban systems beneficial, detrimental, or indifferent for biological invasion? Biol. Invasions 2017, 19, 3489–3503. [Google Scholar] [CrossRef]
- Ducatez, S.; Sayol, F.; Sol, D.; Lefebvre, L. Are urban vertebrates city specialists, artificial habitat exploiters, or environmental generalists? Integr. Comp. Biol. 2018, 58, 929–938. [Google Scholar] [CrossRef]
- Kuussaari, M.; Toivonen, M.; Heliölä, J.; Pöyry, J.; Mellado, J.; Ekroos, J.; Hyyryläinen, V.; Vähä-Piikkiö, I.; Tiainen, J. Butterfly species’ responses to urbanization: Differing effects of human population density and built-up area. Urban Ecosyst. 2021, 24, 515–527. [Google Scholar] [CrossRef]
- Mogi, M.; Armbruster, P.A.; Tuno, N. Differences in responses to urbanization between invasive mosquitoes, Aedes japonicus japonicus (Diptera: Culicidae) and Aedes albopictus, in their native range, Japan. J. Med. Entomol. 2019, 57, 104–112. [Google Scholar] [CrossRef]
- Schuett, W.; Delfs, B.; Haller, R.; Kruber, S.; Roolfs, S.; Timm, D.; Willmann, M.; Drees, C. Ground beetles in city forests: Does urbanization predict a personality trait? PeerJ 2018, 6, e4360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, A.; Merckx, T.; Van Dyck, H. An experimental test of changed personality in butterflies from anthropogenic landscapes. Behav. Ecol. Sociobiol. 2020, 74, 86. [Google Scholar] [CrossRef]
- Breck, S.W.; Poessel, S.A.; Mahoney, P.; Young, J.K. The intrepid urban coyote: A comparison of bold and exploratory behavior in coyotes from urban and rural environments. Sci. Rep. 2019, 9, 2104. [Google Scholar] [CrossRef]
- Dammhahn, M.; Mazza, V.; Schirmer, A.; Göttsche, C.; Eccard, J.A. Of city and village mice: Behavioural adjustments of striped field mice to urban environments. Sci. Rep. 2020, 10, 13056. [Google Scholar] [CrossRef] [PubMed]
- Magura, T.; Tóthmérész, B.; Molnár, T. Changes in carabid beetle assemblages along an urbanisation gradient in the city of Debrecen, Hungary. Landsc. Ecol. 2004, 19, 747–759. [Google Scholar] [CrossRef]
- Horváth, R.; Magura, T.; Tóthmérész, B. Ignoring ecological demands masks the real effect of urbanization: A case study of ground-dwelling spiders along a rural-urban gradient in a lowland forest in Hungary. Ecol. Res. 2012, 27, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Freude, H.; Harde, K.-W.; Lohse, G.A.; Klausnitzer, B. Die Käfer Mitteleuropas; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Koch, K. Die Käfer Mitteleuropas. Ökologie; Goecke & Evers Verlag: Krefeld, Germany, 1989. [Google Scholar]
- Tóth, L. Holyvák VI.—Staphylinidae VI; Akadémiai Kiadó: Budapest, Hyngary, 1989. [Google Scholar]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef] [Green Version]
- Kortet, R.; Hedrick, A.N.N. A behavioural syndrome in the field cricket Gryllus integer: Intrasexual aggression is correlated with activity in a novel environment. Biol. J. Linn. Soc. 2007, 91, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.A.; Godin, J.-G.J. Are fast explorers slow reactors? Linking personality type and anti-predator behaviour. Proc. R. Soc. B Biol. Sci. 2010, 277, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Labaude, S.; O’Donnell, N.; Griffin, C.T. Description of a personality syndrome in a common and invasive ground beetle (Coleoptera: Carabidae). Sci. Rep. 2018, 8, 17479. [Google Scholar] [CrossRef]
- Tremmel, M.; Müller, C. Insect personality depends on environmental conditions. Behav. Ecol. 2013, 24, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Gyuris, E.; Feró, O.; Tartally, A.; Barta, Z. Individual behaviour in firebugs (Pyrrhocoris apterus). Proc. R. Soc. B Biol. Sci. 2011, 278, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Wexler, Y.; Subach, A.; Pruitt, J.N.; Scharf, I. Behavioral repeatability of flour beetles before and after metamorphosis and throughout aging. Behav. Ecol. Sociobiol. 2016, 70, 745–753. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2019; ISBN 9781544336473. [Google Scholar]
- Venables, W.; Ripley, B. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for Descriptive Statistics. 2021. Available online: https://cran.r-project.org/web/packages/DescTools/DescTools.pdf (accessed on 1 March 2022).
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9-79. 2021. Available online: https://cran.r-project.org/web/packages/RVAideMemoire/RVAideMemoire.pdf (accessed on 1 March 2022).
- Stoffel, M.A.; Nakagawa, S.; Schielzeth, H. rptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 2017, 8, 1639–1644. [Google Scholar] [CrossRef] [Green Version]
- Legendre, P. Species associations: The Kendall coefficient of concordance revisited. J. Agric. Biol. Environ. Stat. 2005, 10, 226. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions. R Package Version 2.1.3. 2021. Available online: https://cran.r-project.org/web/packages/cluster/index.html (accessed on 1 April 2022).
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Carere, C.; Maestripieri, D. (Eds.) Introduction: Animal Personalities: Who Cares and Why? In Animal Personalities: Behavior, Physiology, and Evolution; The University of Chicago Press: Chicago, IL, USA; London, UK, 2013; pp. 1–11. [Google Scholar]
- Kralj-Fišer, S.; Schuett, W. Studying personality variation in invertebrates: Why bother? Anim. Behav. 2014, 91, 41–52. [Google Scholar] [CrossRef]
- Magura, T.; Mizser, S.; Horváth, R.; Nagy, D.D.; Tóth, M.; Csicsek, R.; Lövei, G.L. Are there personality differences between rural vs. urban-living individuals of a specialist ground beetle, Carabus convexus? Insects 2021, 12, 646. [Google Scholar] [CrossRef]
- Tremmel, M.; Müller, C. Diet dependent experience and physiological state shape the behavior of a generalist herbivore. Physiol. Behav. 2014, 129, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Chatzimanolis, S. Natural history and behavior of Nordus fungicola (Sharp) (Coleoptera: Staphylinidae). Ann. Entomol. Soc. Am. 2003, 96, 225–230. [Google Scholar] [CrossRef]
- Müller, T.; Müller, C. Behavioural phenotypes over the lifetime of a holometabolous insect. Front. Zool. 2015, 12, S8. [Google Scholar] [CrossRef] [Green Version]
- Monceau, K.; Moreau, J.; Richet, J.; Motreuil, S.; Moret, Y.; Dechaume-Moncharmont, F. Larval personality does not predict adult personality in a holometabolous insect. Biol. J. Linn. Soc. 2017, 120, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Lövei, G.L.; Sunderland, K.D. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.J.; Evans, J.C.; Parsons, S.; Morand-Ferron, J. Urbanization and individual differences in exploration and plasticity. Behav. Ecol. 2018, 29, 1415–1425. [Google Scholar] [CrossRef]
- Riyahi, S.; Björklund, M.; Mateos-Gonzalez, F.; Senar, J.C. Personality and urbanization: Behavioural traits and DRD4 SNP830 polymorphisms in great tits in Barcelona city. J. Ethol. 2017, 35, 101–108. [Google Scholar] [CrossRef]
- Biondi, L.M.; Fuentes, G.M.; Córdoba, R.S.; Bó, M.S.; Cavalli, M.; Paterlini, C.A.; Castano, M.V.; García, G.O. Variation in boldness and novelty response between rural and urban predatory birds: The Chimango Caracara, Milvago chimango as study case. Behav. Process. 2020, 173, 104064. [Google Scholar] [CrossRef]
- Mazza, V.; Dammhahn, M.; Lösche, E.; Eccard, J.A. Small mammals in the big city: Behavioural adjustments of non-commensal rodents to urban environments. Glob. Change Biol. 2020, 26, 6326–6337. [Google Scholar] [CrossRef]
- Oliveira, F.G.; da Luz Mathias, M.; Rychlik, L.; Tapisso, J.T.; von Merten, S. Metabolic and behavioral adaptations of greater white-toothed shrews to urban conditions. Behav. Ecol. 2020, 31, 1334–1343. [Google Scholar] [CrossRef]
- Lövei, G.L.; Elek, Z.; Howe, A.G.; Enggaard, M.K. The use of percentile-percentile plots to compare differences in seasonal dynamics, illustrated by the case of ground beetles (Coleoptera, Carabidae) reacting to urbanisation. Community Ecol. 2018, 19, 1–8. [Google Scholar] [CrossRef]
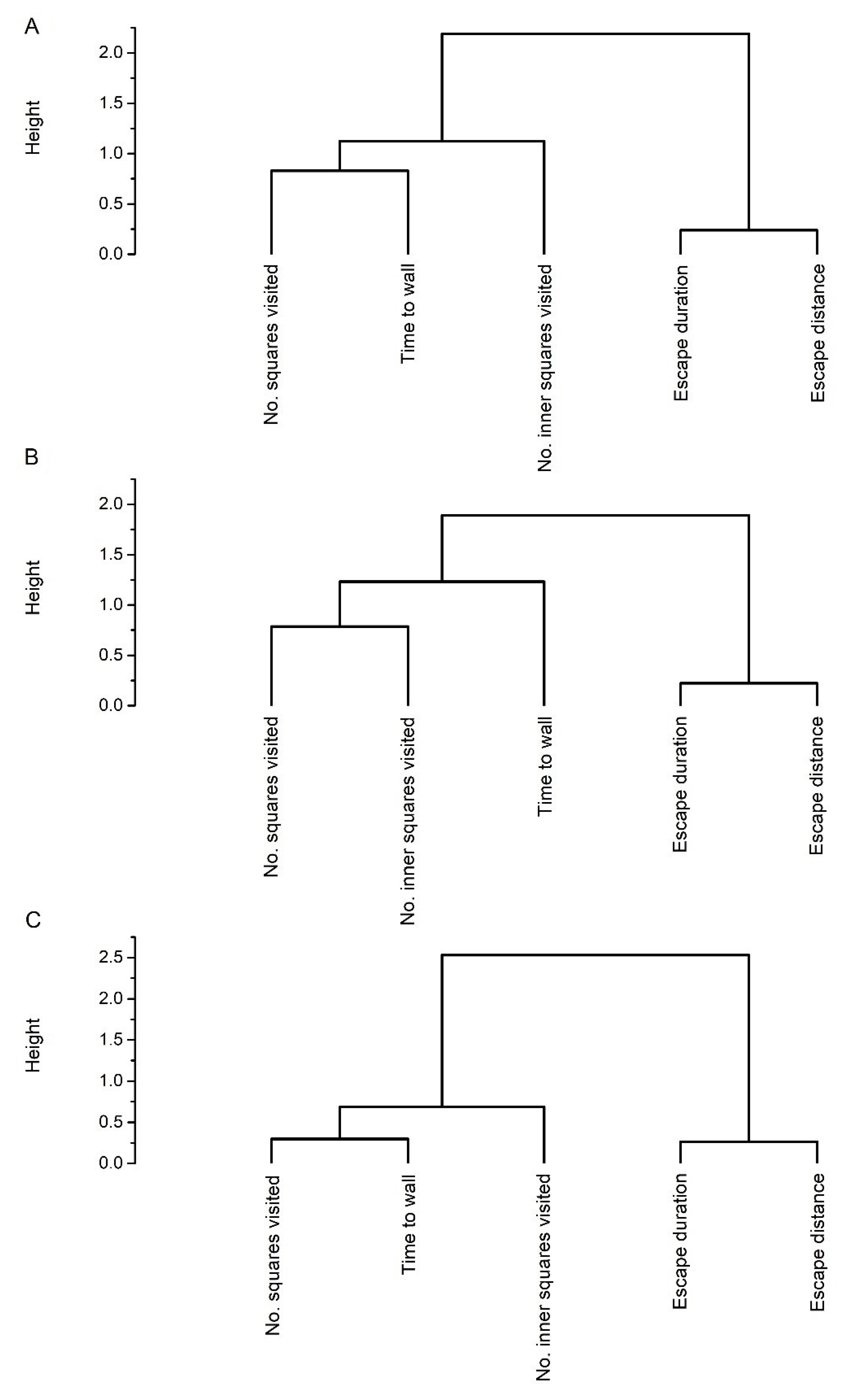
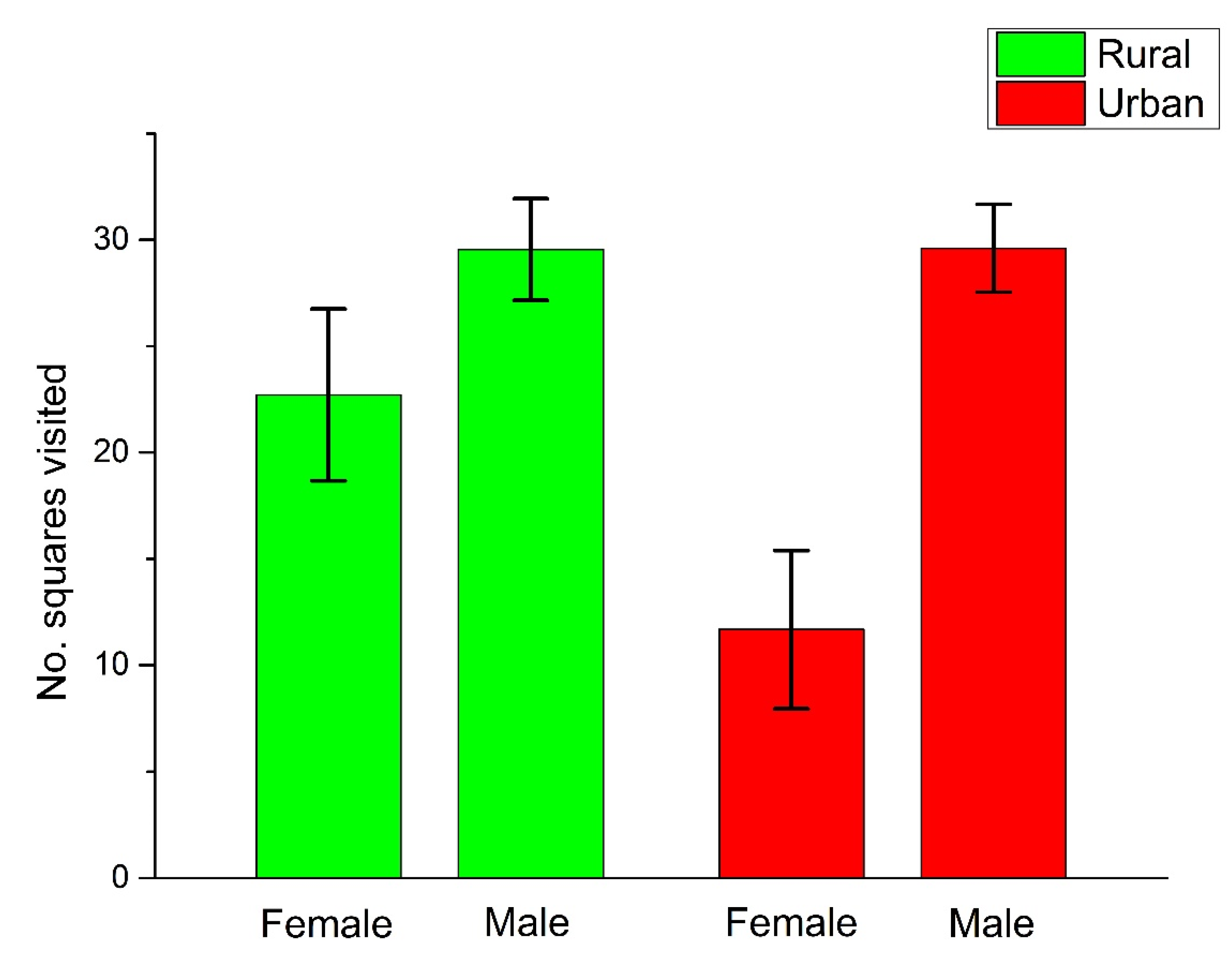
| Species | No. of Rural | No. of Urban | Total | ||
|---|---|---|---|---|---|
| Females | Males | Females | Males | ||
| Abemus chloropterus | 6 | 19 | 15 | 36 | 76 |
| Ocypus nitens | 12 | 20 | 3 | 39 | 74 |
| Platydracus fulvipes | 10 | 32 | 5 | 36 | 83 |
| Rove Beetle Species | Behavioral Variable | Spearman’s Rank-Correlation RS (95% CI) * | Repeatability r (95% CI) * |
|---|---|---|---|
| Abemus chloropterus | No. squares visited | 0.3106 (0.0894; 0.4977) | 0.183 (0; 0.382) |
| No. inner squares visited | 0.0968 (−0.1120; 0.3113) | 0 (0; 0.2) | |
| Time to wall (sec) | 0.3380 (0.0827; 0.5497) | 0.191 (0; 0.396) | |
| Escape duration (sec) | 0.1245 (−0.1161; 0.3519) | 0 (0; 0.208) | |
| Escape distance (no. segments) | 0.1765 (−0.0572; 0.3773) | 0.046 (0; 0.254) | |
| Ocypus nitens | No. squares visited | 0.4469 (0.2189; 0.6160) | 0.332 (0.114; 0.529) |
| No. inner squares visited | 0.1034 (−0.1202; 0.3274) | 0.067 (0; 0.243] | |
| Time to wall (sec) | 0.1194 (−0.1102; 0.3299) | 0 (0; 0.23) | |
| Escape duration (sec) | 0.2304 (0.0051; 0.4483) | 0.115 (0; 0.375) | |
| Escape distance (no. segments) | 0.3439 (0.1050; 0.5566) | 0.399 (0.113; 0.566) | |
| Platydracus fulvipes | No. squares visited | 0.4995 (0.2992; 0.6591) | 0.446 (0.221; 0.622) |
| No. inner squares visited | 0.1604 (−0.0620; 0.3718) | 0.143 (0; 0.317) | |
| Time to wall (sec) | 0.3918 (0.1651; 0.5879) | 0.247 (0.079; 0.444) | |
| Escape duration (sec) | 0.1606 (−0.0492; 0.3618) | 0 (0; 0.209) | |
| Escape distance (no. segments) | 0.0838 (−0.1313; 0.3166) | 0.051 (0; 0.224) |
| Response Variable | Fixed Effect | Estimate ± SE | χ2 | df | p |
|---|---|---|---|---|---|
| Abemus chloropterus | |||||
| No. squares visited | Urbanization level | −0.2114± 0.1895 | 1.2438 | 1 | 0.2647 |
| Sex | 0.0766 ± 0.1628 | 0.2213 | 1 | 0.6381 | |
| Urbanization level × Sex | −0.0689 ± 0.1938 | 0.1264 | 1 | 0.7222 | |
| No. inner squares visited | Urbanization level | 0.0477 ± 0.2059 | 0.0538 | 1 | 0.8166 |
| Sex | 0.3478 ± 0.1931 | 3.2445 | 1 | 0.0717 | |
| Urbanization level × Sex | −0.2927 ± 0.2287 | 1.6383 | 1 | 0.2006 | |
| Time to wall, s | Urbanization level | −0.0192 ± 0.2065 | 0.0087 | 1 | 0.9258 |
| Sex | −0.1047 ± 0.2002 | 0.2735 | 1 | 0.6010 | |
| Urbanization level × Sex | −0.1138 ± 0.2394 | 0.2261 | 1 | 0.6345 | |
| Escape duration, s | Urbanization level | −0.0697 ± 0.1137 | 0.3761 | 1 | 0.5397 |
| Sex | 0.0213 ± 0.0993 | 0.0459 | 1 | 0.8304 | |
| Urbanization level × Sex | −0.0359 ± 0.1177 | 0.0930 | 1 | 0.7604 | |
| Escape distance, no. segments | Urbanization level | −0.3215 ± 0.2792 | 1.3254 | 1 | 0.2496 |
| Sex | −0.0775 ± 0.2213 | 0.1226 | 1 | 0.7263 | |
| Urbanization level × Sex | 0.0116 ± 0.2721 | 0.0018 | 1 | 0.9659 | |
| Ocypus nitens | |||||
| No. squares visited | Urbanization level | −0.7211 ± 0.4642 | 2.4129 | 1 | 0.1203 |
| Sex | 0.5603 ± 0.1934 | 8.3955 | 1 | 0.0038 | |
| Urbanization level × Sex | 0.3110 ± 0.3911 | 0.6323 | 1 | 0.4265 | |
| No. inner squares visited | Urbanization level | −0.3355 ± 0.3120 | 1.1562 | 1 | 0.2822 |
| Sex | 0.1008 ± 0.1628 | 0.3830 | 1 | 0.5360 | |
| Urbanization level × Sex | 0.3740 ± 0.3344 | 1.2504 | 1 | 0.2635 | |
| Time to wall, s | Urbanization level | 0.4853 ± 0.3343 | 2.2398 | 2 | 0.3263 |
| Sex | −0.3049 ± 0.1891 | 2.5991 | 1 | 0.1069 | |
| Urbanization level × Sex | −0.6724 ± 0.3636 | 3.4190 | 1 | 0.0644 | |
| Escape duration, s | Urbanization level | −0.0721 ± 0.1425 | 0.2561 | 1 | 0.6128 |
| Sex | 0.0236 ± 0.0849 | 0.0773 | 1 | 0.7810 | |
| Urbanization level × Sex | 0.1457 ± 0.1552 | 0.8824 | 1 | 0.3475 | |
| Escape distance, no. segments | Urbanization level | −0.1644 ± 0.4079 | 0.1624 | 1 | 0.6870 |
| Sex | 0.0817 ± 0.2340 | 0.1218 | 1 | 0.7271 | |
| Urbanization level × Sex | 0.2663 ± 0.4376 | 0.3704 | 1 | 0.5428 | |
| Platydracus fulvipes | |||||
| No. squares visited | Urbanization level | −0.6457 ± 0.5083 | 1.6137 | 1 | 0.2040 |
| Sex | 0.1687 ± 0.2800 | 0.3628 | 1 | 0.5469 | |
| Urbanization level × Sex | 0.7405 ± 0.4737 | 2.4436 | 1 | 0.1180 | |
| No. inner squares visited | Urbanization level | −0.2783 ± 0.3037 | 0.8398 | 1 | 0.3595 |
| Sex | −0.1170 ± 0.1879 | 0.3877 | 1 | 0.5335 | |
| Urbanization level × Sex | 0.3035 ± 0.3224 | 0.8860 | 1 | 0.3466 | |
| Time to wall, s | Urbanization level | 0.0525 ± 0.2071 | 0.0643 | 1 | 0.7998 |
| Sex | −0.0281 ± 0.1064 | 0.0696 | 1 | 0.7919 | |
| Urbanization level × Sex | −0.1364 ± 0.1680 | 0.6592 | 1 | 0.4168 | |
| Escape duration, s | Urbanization level | −0.0250 ± 0.0946 | 0.0697 | 1 | 0.7917 |
| Sex | −0.0019 ± 0.0638 | 0.0009 | 1 | 0.9759 | |
| Urbanization level × Sex | 0.0456 ± 0.1028 | 0.1964 | 1 | 0.6576 | |
| Escape distance, no. segments | Urbanization level | 0.2747 ± 0.2750 | 0.9975 | 1 | 0.3179 |
| Sex | 0.2554 ± 0.1951 | 1.7147 | 1 | 0.1904 | |
| Urbanization level × Sex | −0.2015 ± 0.2977 | 0.4582 | 1 | 0.4984 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magura, T.; Horváth, R.; Mizser, S.; Tóth, M.; Nagy, D.D.; Csicsek, R.; Balla, E.; Lövei, G.L. Urban Individuals of Three Rove Beetle Species Are Not More Exploratory or Risk-Taking Than Rural Conspecifics. Insects 2022, 13, 757. https://doi.org/10.3390/insects13080757
Magura T, Horváth R, Mizser S, Tóth M, Nagy DD, Csicsek R, Balla E, Lövei GL. Urban Individuals of Three Rove Beetle Species Are Not More Exploratory or Risk-Taking Than Rural Conspecifics. Insects. 2022; 13(8):757. https://doi.org/10.3390/insects13080757
Chicago/Turabian StyleMagura, Tibor, Roland Horváth, Szabolcs Mizser, Mária Tóth, Dávid D. Nagy, Réka Csicsek, Emőke Balla, and Gábor L. Lövei. 2022. "Urban Individuals of Three Rove Beetle Species Are Not More Exploratory or Risk-Taking Than Rural Conspecifics" Insects 13, no. 8: 757. https://doi.org/10.3390/insects13080757
APA StyleMagura, T., Horváth, R., Mizser, S., Tóth, M., Nagy, D. D., Csicsek, R., Balla, E., & Lövei, G. L. (2022). Urban Individuals of Three Rove Beetle Species Are Not More Exploratory or Risk-Taking Than Rural Conspecifics. Insects, 13(8), 757. https://doi.org/10.3390/insects13080757








