A Bombyx mori Infection Model for Screening Antibiotics against Staphylococcus epidermidis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Bacterial Strain
2.3. Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs)
2.4. Injection of Larvae and Collection of Hemolymph
2.5. Lethal Dose 50 (LD50) for S. epidermidis
2.6. Administration of Antibiotics
2.7. Analysis of the Immunological Markers
2.7.1. Hemocyte Viability
2.7.2. Prophenoloxidase (proPO) System Activation
2.7.3. Lysozyme Activity
2.8. Statistical Analysis
3. Results
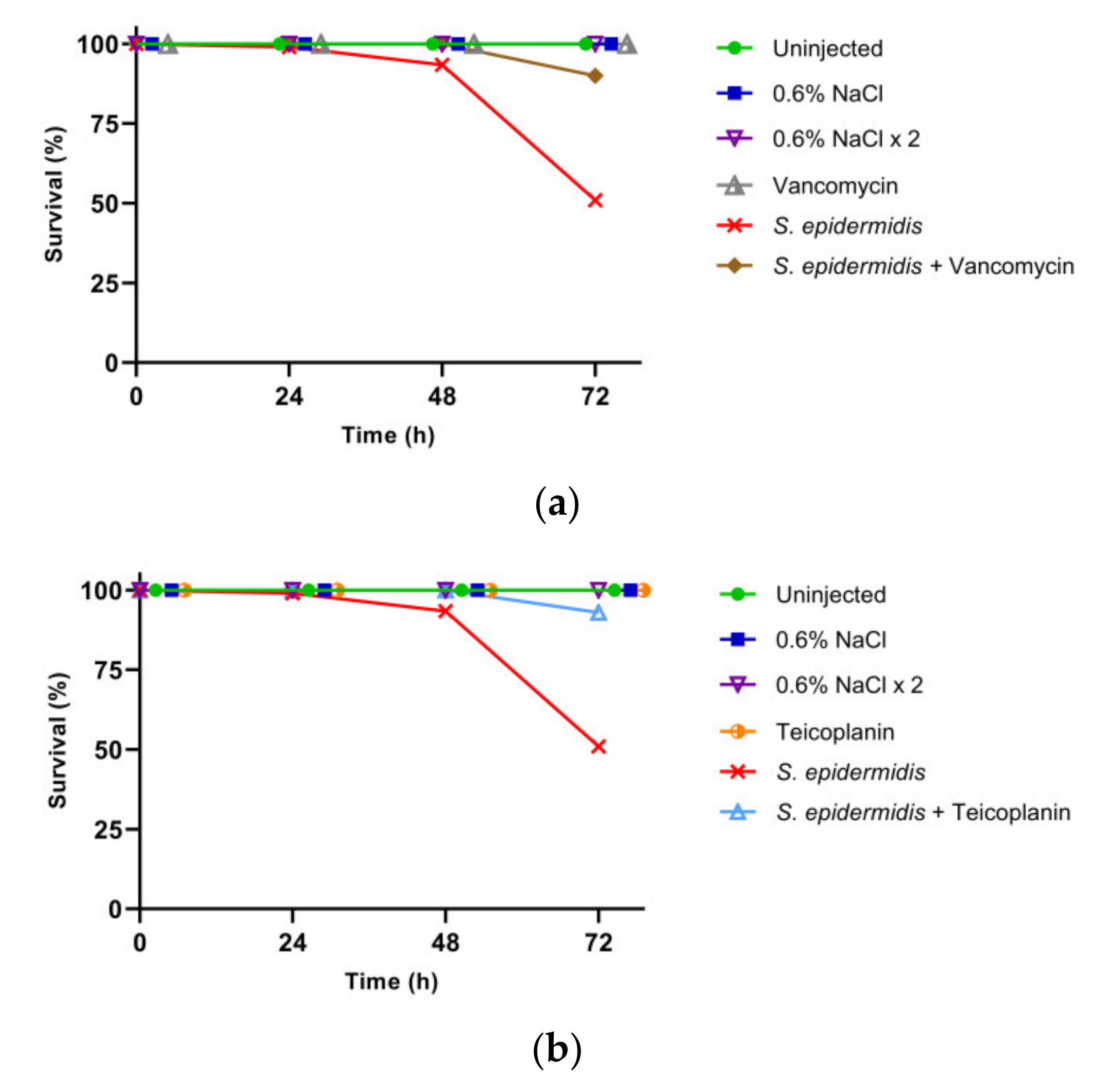
3.1. Larval Survival and Calculation of LD50
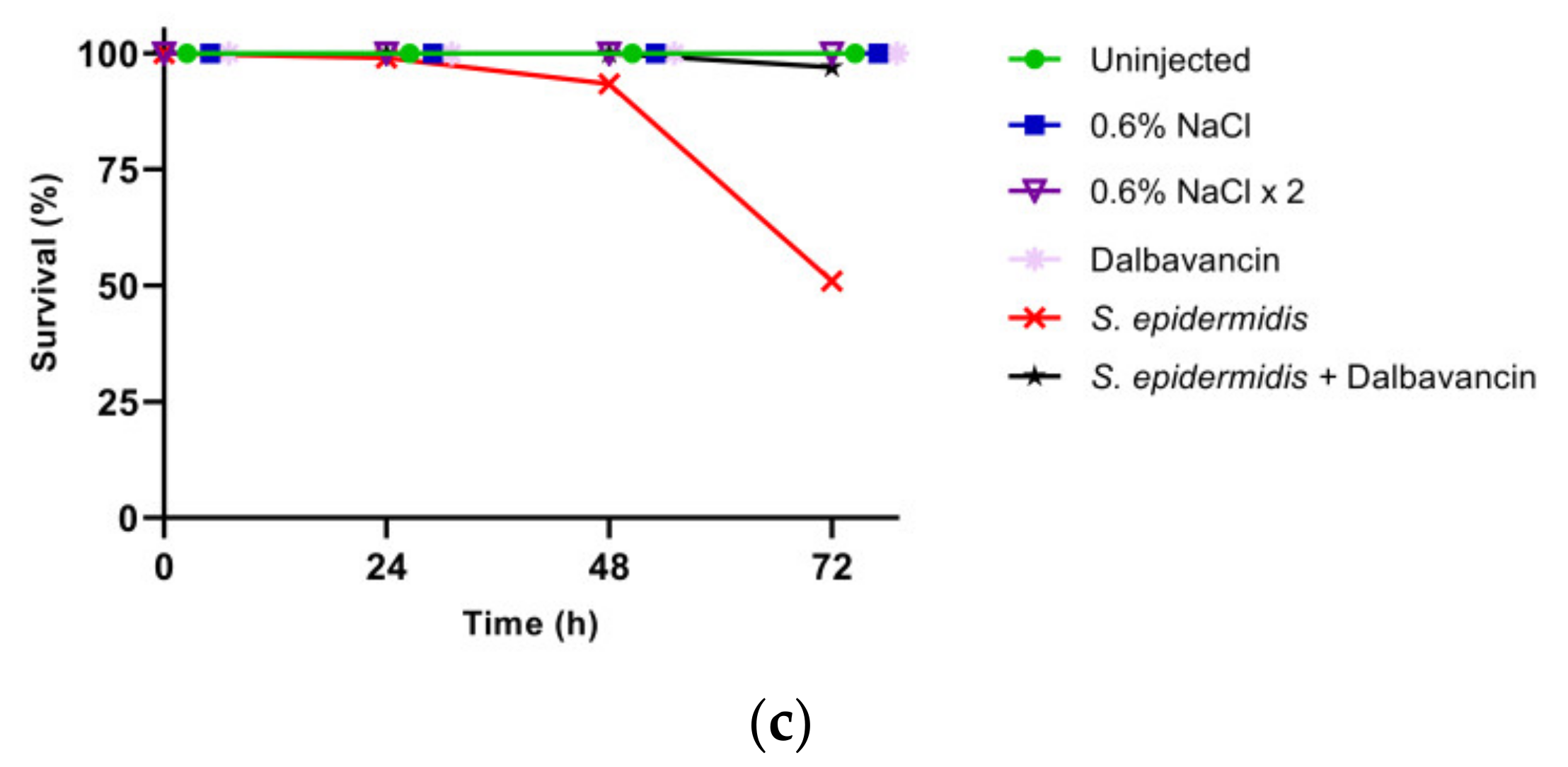
3.2. Effect of GPA Administration to the Larvae
3.3. Analysis of the Immunological Markers
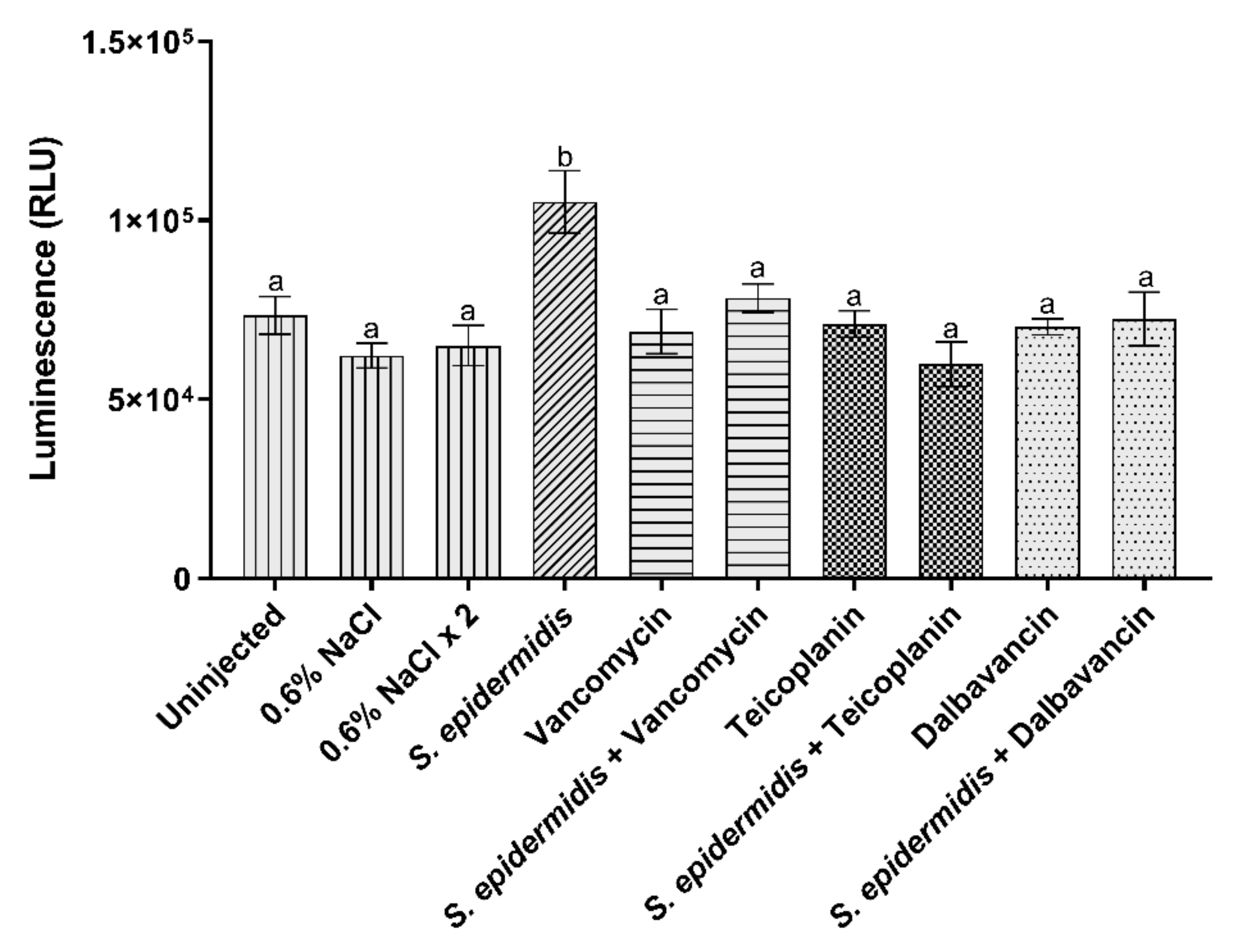
3.3.1. Hemocyte Viability
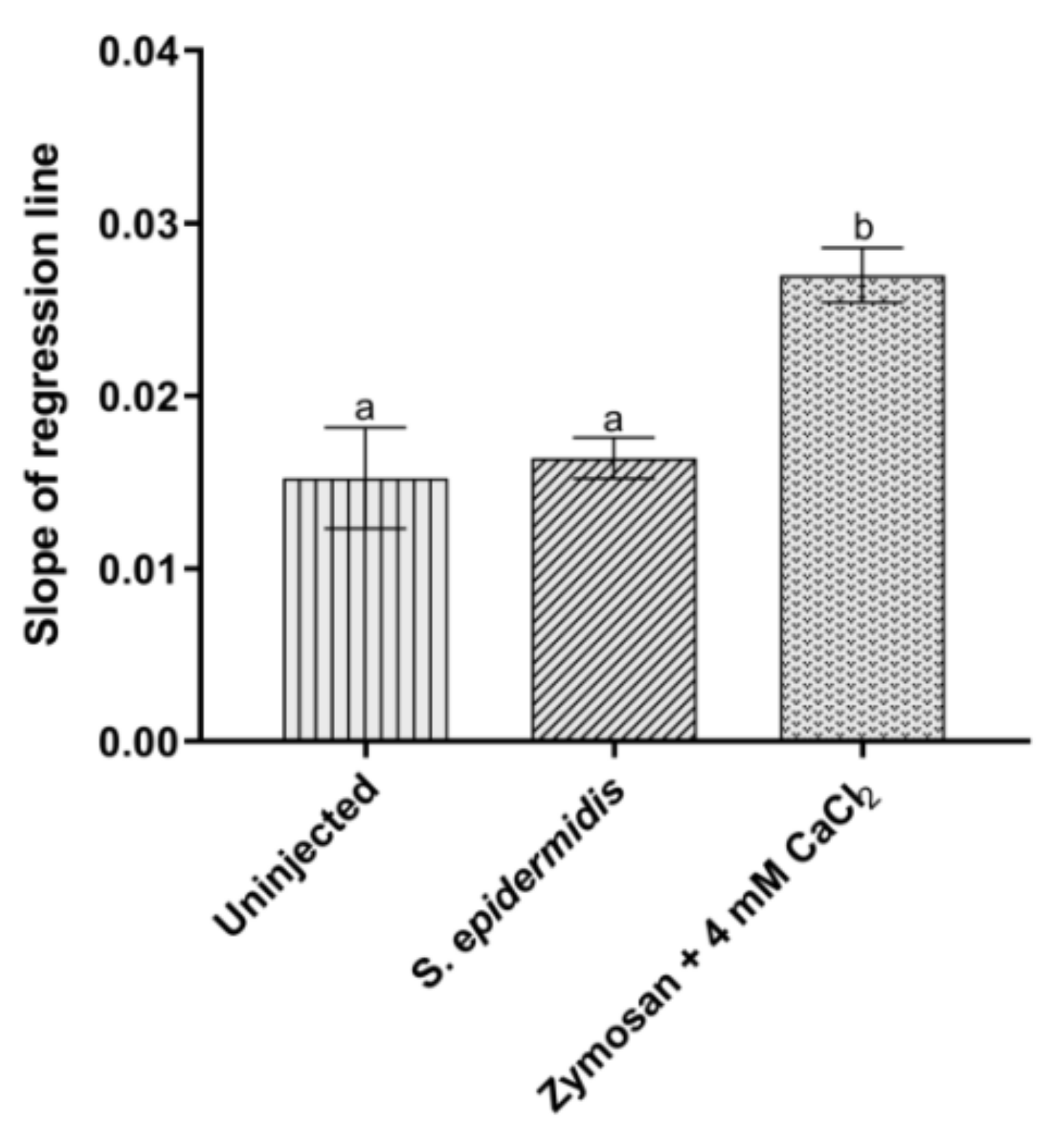
3.3.2. Activation of Prophenoloxidase System
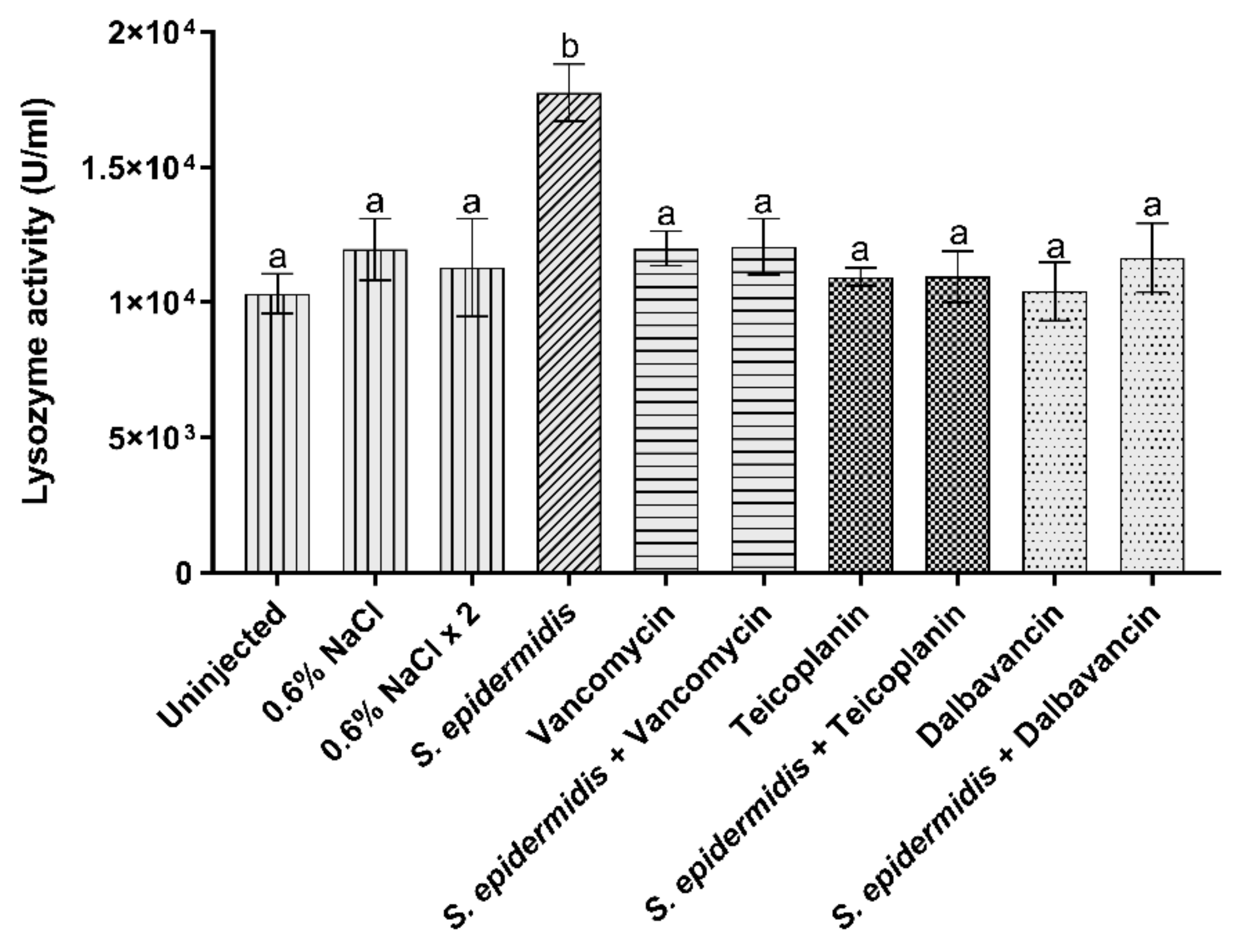
3.3.3. Lysozyme Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wencewicz, T.A. Crossroads of antibiotic resistance and biosynthesis. J. Mol. Biol. 2019, 431, 3370–3399. [Google Scholar] [CrossRef] [PubMed]
- Berini, F.; Orlandi, V.; Gornati, R.; Bernardini, G.; Marinelli, F. Nanoantibiotics to fight multidrug resistant infections by Gram-positive bacteria: Hope or reality? Biotechnol. Adv. 2022, 57, 107948. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Panthee, S.; Urai, M.; Hamamoto, H.; Ohwada, T.; Sekimizu, K. Pharmacokinetic parameters explain the therapeutic activity of antimicrobial agents in a silkworm infection model. Sci. Rep. 2018, 8, 1578. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J. The 3Rs–Past, present and future. Scand. J. Lab. Anim. Sci. 2000, 27, 84–92. [Google Scholar]
- Mylonakis, E.; Casadevall, A.; Ausubel, F.M. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog. 2007, 3, e101. [Google Scholar] [CrossRef]
- Kong, C.; Eng, S.A.; Lim, M.P.; Nathan, S. Beyond traditional antimicrobials: A Caenorhabditis elegans model for discovery of novel anti-infectives. Front. Microbiol. 2016, 7, 1956. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Miyazaki, S.; Fukunaga, D.H.; Shimizu, K.; Kawamoto, S.; Sekimizu, K. Quantitative evaluation of cryptococcal pathogenesis and antifungal drugs using a silkworm infection model with Cryptococcus neoformans. J. Appl. Microbiol. 2012, 112, 138–146. [Google Scholar] [CrossRef]
- Panthee, S.; Paudel, A.; Hamamoto, H.; Sekimizu, K. Advantages of the silkworm as an animal model for developing novel antimicrobial agents. Front. Microbiol. 2017, 8, 373. [Google Scholar] [CrossRef]
- Buchmann, K. Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef]
- Ménard, G.; Rouillon, A.; Ghukasyan, G.; Emily, M.; Felden, B.; Donnio, P.Y. Galleria mellonella larvae as an infection model to investigate srna-mediated pathogenesis in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2021, 11, 631710. [Google Scholar] [CrossRef]
- The International Silkworm Genome Consortium. The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2008, 38, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; O’Brochta, D.A. Advanced technologies for genetically manipulating the silkworm Bombyx mori, a model Lepidopteran insect. Proc. Biol. Sci. 2015, 282, 20150487. [Google Scholar]
- Kaito, C.; Akimitsu, N.; Watanabe, H.; Sekimizu, K. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 2002, 32, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Hanaki, H.; Matsui, H.; Hamamoto, H.; Sekimizu, K.; Iwatsuki, M.; Kim, Y.P.; Tomoda, H. In vitro and in vivo anti-MRSA activities of nosokomycins. Drug Discov. Ther. 2014, 8, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Montali, A.; Berini, F.; Brivio, M.F.; Mastore, M.; Saviane, A.; Cappellozza, S.; Marinelli, F.; Tettamanti, G. A silkworm infection model for in vivo study of glycopeptide antibiotics. Antibiotics 2020, 9, 300. [Google Scholar] [CrossRef]
- Tuba, T.; Chowdhury, F.R.; Hossain, T.; Farzana, M.; Ahad, I.; Hossain, M.M.; Hossain, M.I.; Saleh, N.; Nawaar, N.; Uddin, M.A.; et al. Klebsiella pneumoniae pathogenicity in silk moth larvae infection model. FEMS Microbial. Lett. 2022, 368, 21–24. [Google Scholar] [CrossRef]
- Usui, K.; Miyazaki, S.; Kaito, C.; Sekimizu, K. Purification of a soil bacteria exotoxin using silkworm toxicity to measure specificactivity. Microb. Pathog. 2009, 46, 59–62. [Google Scholar] [CrossRef]
- Suzuki, J.; Uda, A.; Watanabe, K.; Shimizu, T.; Watarai, M. Sym-biosis with Francisella tularensisprovides resistance to patho-gens in the silkworm. Sci. Rep. 2016, 6, 31476. [Google Scholar] [CrossRef]
- Castillo, Y.; Suzuki, J.; Watanabe, K.; Shimizu, T.; Watarai, M. Effect of vitamin A on Listeria monocytogenes infection in a silkwormmodel. PLoS ONE 2016, 11, e0163747. [Google Scholar] [CrossRef]
- Hosoda, K.; Koyama, N.; Hamamoto, H.; Yagi, A.; Uchida, R.; Kanamoto, A.; Tomoda, H. Evaluation of anti-mycobacterial compounds in a silkworm infection model with Mycobacteroides abscessus. Molecules 2020, 25, 4971. [Google Scholar] [CrossRef]
- Uchida, R.; Namiguchi, S.; Ishijima, H.; Tomoda, H. Therapeutic effects of three trichothecenes in the silkworm infection assay with Candida albicans. Drug Discov. Ther. 2016, 10, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Kanasaki, R.; Yoshikawa, K.; Furukawa, S.; Fujie, A.; Hamamoto, H.; Sekimizu, K. Discovery of a new antifungal agent ASP2397 using a silkworm model of Aspergillus fumigatus infection. J. Antibiot. 2017, 70, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Molecular basis of Staphylococcus epidermidis infections. Semin. Immunopathol. 2012, 34, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Marcone, G.L.; Binda, E.; Berini, F.; Marinelli, F. Old and new glycopeptide antibiotics: From product to gene and back in the post-genomic era. Biotechnol. Adv. 2018, 36, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Cappellozza, L.; Cappellozza, S.; Saviane, A.; Sbrenna, G. Artificial diet rearing system for the silkworm Bombyx mori (Lepidoptera: Bombycidae): Effect of vitamin C deprivation on larval growth and cocoon production. Appl. Entomol. Zool. 2005, 40, 405–412. [Google Scholar] [CrossRef]
- Franzetti, E.; Romanelli, D.; Caccia, S.; Cappellozza, S.; Congiu, T.; Rajagopalan, M.; Grimaldi, A.; de Eguileor, M.; Casartelli, M.; Tettamanti, G. The midgut of the silkmoth Bombyx mori is able to recycle molecules derived from degeneration of the larval midgut epithelium. Cell Tissue Res. 2015, 361, 509–528. [Google Scholar] [CrossRef]
- Casati, B.; Terova, G.; Cattaneo, A.G.; Rimoldi, S.; Franzetti, E.; de Eguileor, M.; Tettamanti, G. Molecular cloning, characterization and expression analysis of ATG1 in the silkworm, Bombyx mori. Gene 2012, 511, 326–337. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Brady, D.; Saviane, A.; Romoli, O.; Tettamanti, G.; Sandrelli, F.; Cappellozza, S. Oral infection in a germ-free Bombyx mori model. In Immunity in Insects; Sandrelli, F., Tettamanti, G., Eds.; Humana: New York, NY, USA, 2020; pp. 217–231. [Google Scholar]
- Ashida, M.; Ishizaki, Y.; Iwahana, H. Activation of pro-phenoloxidase by bacterial cell walls or β-1,3-glucans in plasma of the silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 1983, 113, 562–568. [Google Scholar] [CrossRef]
- Bruno, D.; Montali, A.; Mastore, M.; Brivio, M.F.; Mohamed, A.; Tian, L.; Grimaldi, A.; Casartelli, M.; Tettamanti, G. Insights into the immune response of the black soldier fly larvae to bacteria. Front. Immunol. 2021, 12, 745160. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated immunity in insects: Cells, processes, and associated components in the fight against pathogens and parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef] [PubMed]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Hultmark, D. Insect lysozymes. EXS 1996, 75, 87–102. [Google Scholar] [PubMed]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef]
- Kaito, C.; Murakami, K.; Imai, L.; Furuta, K. Animal infection models using non-mammals. Microbiol. Immunol. 2020, 64, 585–592. [Google Scholar] [CrossRef]
- Hamamoto, H.; Kurokawa, K.; Kaito, C.; Kamura, K.; Manitra Razanajatovo, I.; Kasuhara, H.; Santa, T.; Sekimizu, K. Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob. Agents Chemother. 2004, 48, 774–779. [Google Scholar] [CrossRef]
- Kaito, C.; Kurokawa, K.; Matsumoto, Y.; Terao, Y.; Kawabata, S.; Hamada, S.; Sekimizu, K. Silkworm pathogenic bacteria infection model for identification of novel virulence genes. Mol. Microbiol. 2005, 56, 934–944. [Google Scholar] [CrossRef]
- Barman, T.K.; Arora, P.; Rao, M.; Bhadauriya, T.; Upadhyay, D.J. Utilization of Bombyx mori larvae as a surrogate animal model for evaluation of the anti-infective potential of oxazolidinones. J. Infect. Chemother. 2008, 14, 166–169. [Google Scholar] [CrossRef]
- Tabuchi, F.; Matsumoto, Y.; Ishii, M.; Tatsuno, K.; Okazaki, M.; Sato, T.; Moriya, K.; Sekimizu, K. Synergistic effects of vancomycin and β-lactams against vancomycin highly resistant Staphylococcus aureus. J. Antibiot. 2017, 70, 771–774. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef]
- Sabaté Brescó, M.; Harris, L.G.; Thompson, K.; Stanic, B.; Morgenstern, M.; O’Mahony, L.; Richards, R.G.; Moriarty, T.F. Pathogenic mechanisms and host interactions in Staphylococcus epidermidis device-related infection. Front. Microbiol. 2017, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Knafl, D.; Tobudic, S.; Cheng, S.C.; Bellamy, D.R.; Thalhammer, F. Dalbavancin reduces biofilms of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE). Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Candiani, G.; Abbondi, M.; Borgonovi, M.; Romanò, G.; Parenti, F. In-vitro and in-vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J. Antimicrob. Chemother. 1999, 44, 179–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, A.Y.; Zervos, M.J.; Vazquez, J.A. Dalbavancin: A novel antimicrobial. Int. J. Clin. Pract. 2007, 61, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Ceccherini, F.; Sennati, S.; D’Agostino, F.; Arena, F.; D’Atanasio, N.; Di Giorgio, F.P.; Tongiani, S.; Pallecchi, L.; Rossolini, G.M. In vitro time-kill kinetics of dalbavancin against Staphylococcus spp. biofilms over prolonged exposure times. Diagn. Microbiol. Infect. Dis. 2020, 96, 114901. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.P.; Draghi, D.C.; Sheehan, D.J.; Hogan, P.; Sahm, D.F. Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob. Agents. Chemother. 2007, 51, 1150–1154. [Google Scholar] [CrossRef]
- Chen, K.; Lu, Z. Immune responses to bacterial and fungal infections in the silkworm, Bombyx mori. Dev. Comp. Immunol. 2018, 83, 3–11. [Google Scholar] [CrossRef]
- Li, T.; Yan, D.; Wang, X.; Zhang, L.; Chen, P. Hemocyte changes during immune melanization in Bombyx mori infected with Escherichia coli. Insects 2019, 10, 301. [Google Scholar] [CrossRef]
- Tsakas, S.; Marmaras, V.J. Insect immunity and its signalling: An overview. Invertebr. Surviv. J. 2010, 7, 228–238. [Google Scholar]
- Isao, M.; Tomoki, H.; Yoshiaki, Y. Lysozyme activity in immunized and non-immunized hemolymph during the development of the silkworm, Bombyx mori. Comp. Biochem. Physiol. 1994, 108, 311–314. [Google Scholar] [CrossRef]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Budd, A.; Kanost, M.R.; Michel, K. Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cell. Mol. Life Sci. 2011, 68, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, C.; Pan, G.; Li, Z.; Han, B.; Xu, J.; Lan, X.; Chen, J.; Yang, D.; Chen, Q.; et al. Genome-wide transcriptional response of silkworm (Bombyx mori) to infection by the microsporidian Nosema bombycis. PLoS ONE 2013, 8, e84137. [Google Scholar] [CrossRef] [PubMed]

| Antibiotic | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|
| Vancomycin | 2 | 16 |
| Teicoplanin | 1 | 32 |
| Dalbavancin | 0.25 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montali, A.; Berini, F.; Saviane, A.; Cappellozza, S.; Marinelli, F.; Tettamanti, G. A Bombyx mori Infection Model for Screening Antibiotics against Staphylococcus epidermidis. Insects 2022, 13, 748. https://doi.org/10.3390/insects13080748
Montali A, Berini F, Saviane A, Cappellozza S, Marinelli F, Tettamanti G. A Bombyx mori Infection Model for Screening Antibiotics against Staphylococcus epidermidis. Insects. 2022; 13(8):748. https://doi.org/10.3390/insects13080748
Chicago/Turabian StyleMontali, Aurora, Francesca Berini, Alessio Saviane, Silvia Cappellozza, Flavia Marinelli, and Gianluca Tettamanti. 2022. "A Bombyx mori Infection Model for Screening Antibiotics against Staphylococcus epidermidis" Insects 13, no. 8: 748. https://doi.org/10.3390/insects13080748
APA StyleMontali, A., Berini, F., Saviane, A., Cappellozza, S., Marinelli, F., & Tettamanti, G. (2022). A Bombyx mori Infection Model for Screening Antibiotics against Staphylococcus epidermidis. Insects, 13(8), 748. https://doi.org/10.3390/insects13080748











