Field Tests of Three Alternative Insecticides with Protein Bait for the Development of an Insecticide Rotation Program to Control Melon Flies, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Nu-Lure and Insecticide Application
2.2. Experimental Design
2.3. Statistical Analyses
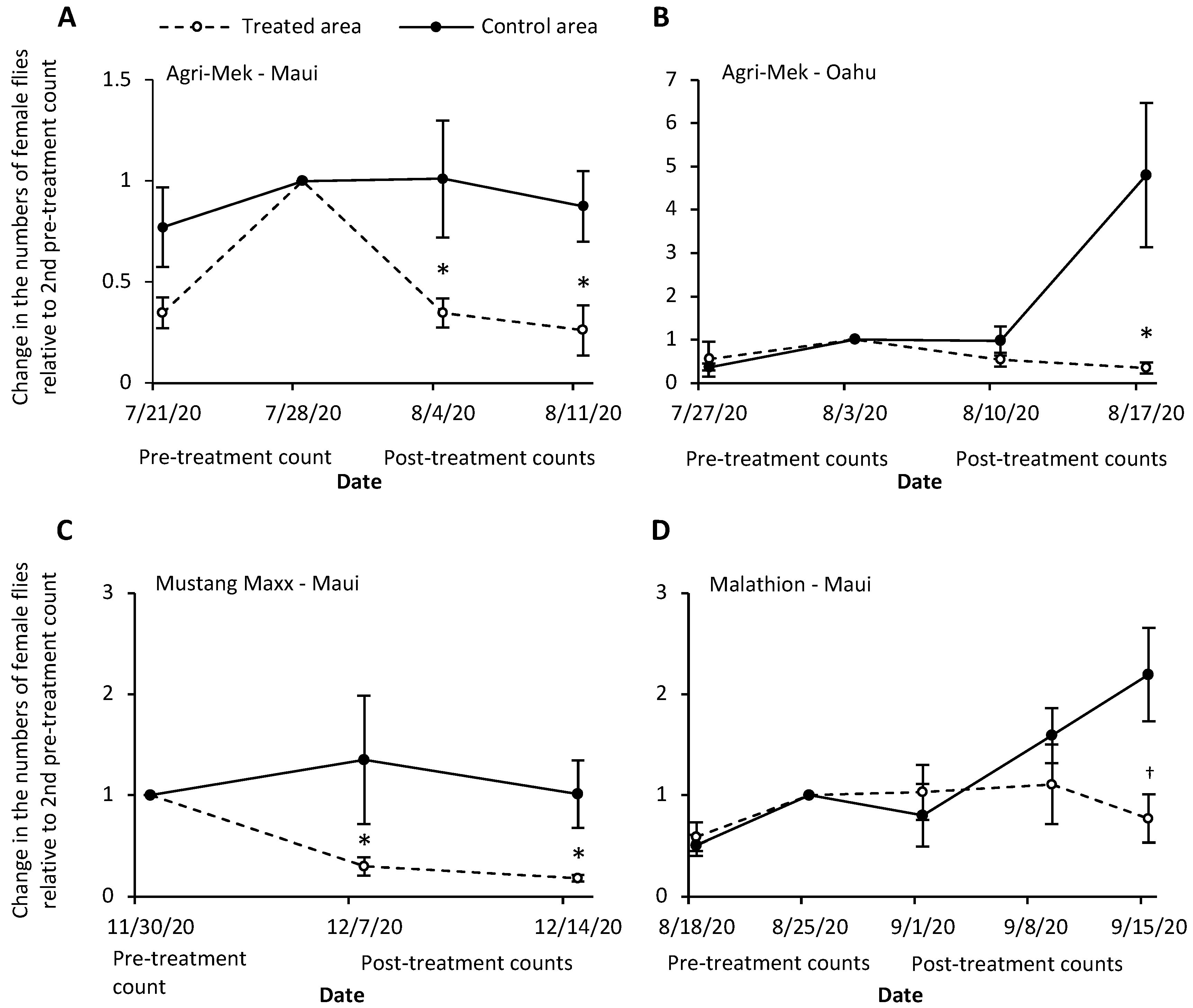
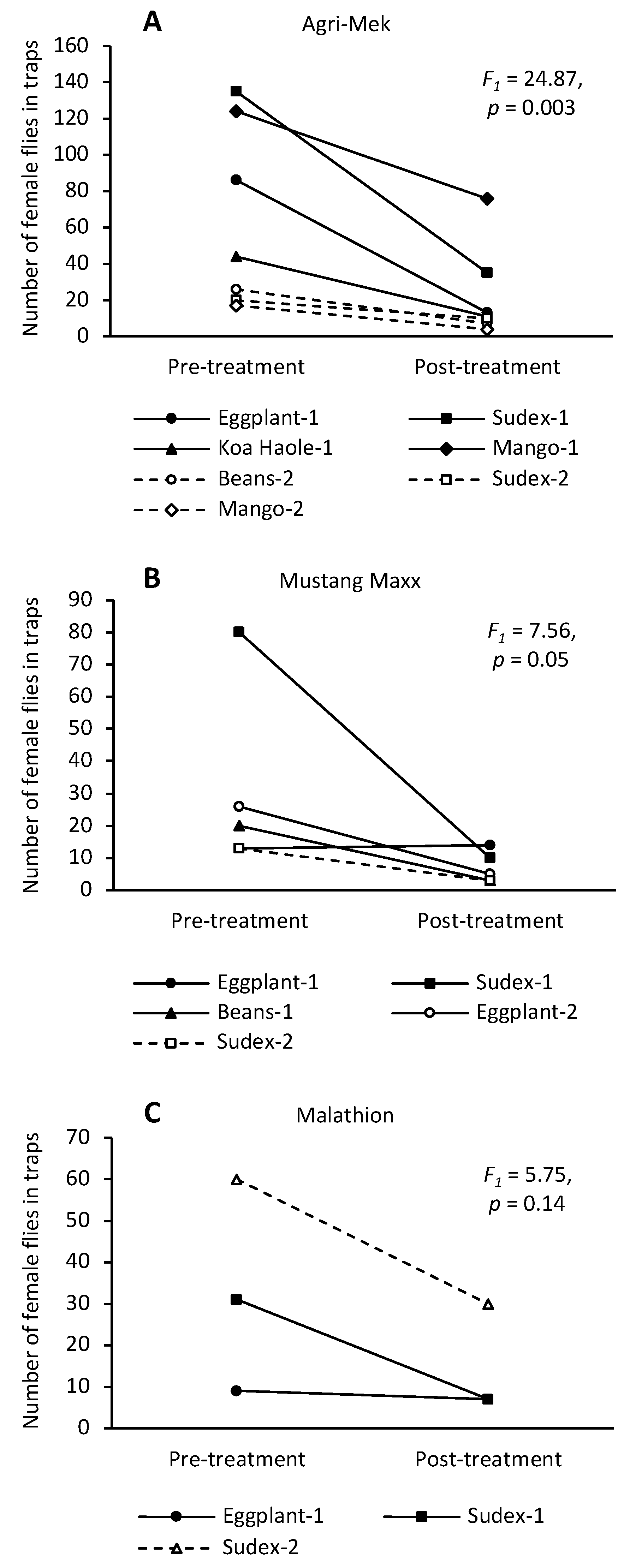
3. Results
3.1. Agri-Mek SC with Nu-Lure
3.2. Mustang Maxx with Nu-Lure
3.3. Malathion 5EC with Nu-Lure
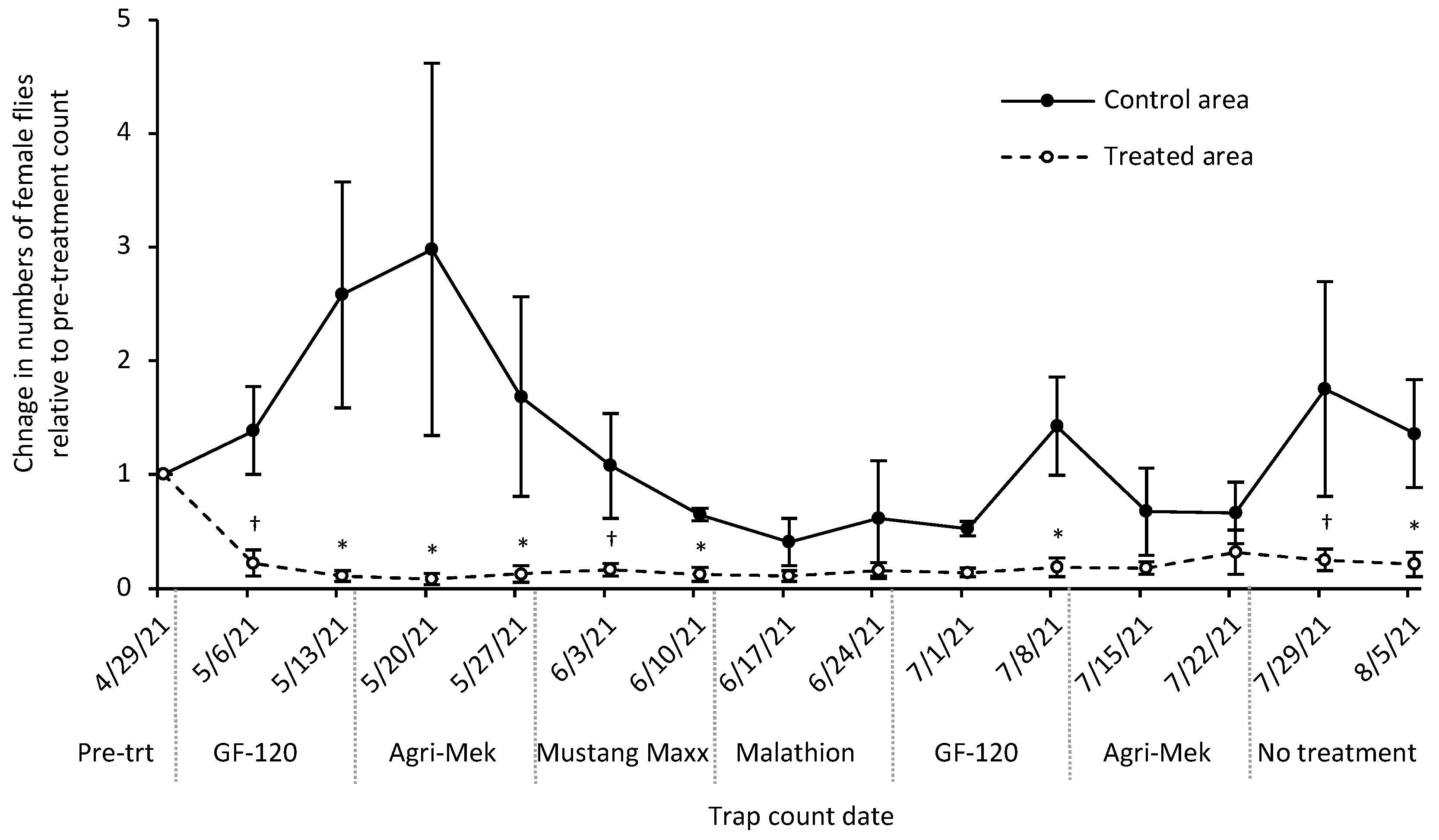
3.4. Insecticide Rotation Field Trial
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhillon, M.K.; Singh, R.; Naresh, J.S.; Sharma, H.C. The melon fruit fly, Bactrocera cucurbitae: A review of its biology and management. J. Insect Sci. 2005, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- McQuate, G.T.; Liquido, N.J.; Nakamichi, K.A.A. Annotated world bibliography of host plants of the melon fly, Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). Insecta Mundi 2017, 0527, 1–339. [Google Scholar]
- Nishida, T. Ecological Study of the Melon Fly, Dacus Cucurbitae, in the Hawaiian Islands; University of California: Berkley, CA, USA, 1953. [Google Scholar]
- McQuate, G.T.; Vargas, R.I. Assessment of attractiveness of plants as roosting sites for the melon fly, Bactrocera cucurbitae, and oriental fruit fly, Bactrocera dorsalis. J. Insect Sci. 2007, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Daane, K.M.; Canale, A.; Niu, C.Y.; Messing, R.H.; Vargas, R.I. Sexual communication and related behaviours in Tephritidae: Current knowledge and potential applications for Integrated Pest Management. J. Pest Sci. 2014, 87, 385–405. [Google Scholar] [CrossRef]
- Iwahashi, O.; Majima, T. Lek formation and male-male competition in the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae). Appl. Entomol. Zool. 1986, 21, 70–75. [Google Scholar] [CrossRef]
- McQuate, G.T. Assessment of attractiveness of cassava as a roosting plant for the melon fly, Bactrocera cucurbitae and the Oriental Fruit Fly, B. dorsalis. J. Insect Sci. 2011, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McQuate, G.T.; Jones, G.D.; Sylva, C.D. Assessment of corn pollen as a food source for two tephritid fruit fly species. Environ. Entomol. 2003, 32, 141–150. [Google Scholar] [CrossRef]
- Hendrichs, J.; Hendrichs, M.A. Mediterranean fruit fly (Diptera: Tephritidae) in nature: Location and diel pattern of feeding and other activities on fruiting and nonfruiting hosts and nonhosts. Ann. Entomol. Soc. Am. 1990, 83, 632–641. [Google Scholar] [CrossRef]
- Deguine, J.P.; Atiama-Nurbel, T.; Aubertot, J.N.; Augusseau, X.; Atiama, M.; Jacquot, M.; Reynaud, B. Agroecological management of cucurbit-infesting fruit fly: A review. Agron. Sustain. Dev. 2015, 35, 937–965. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Miller, N.W.; Piñero, J.C.; Oride, L.; Chaney, N.; Revis, H.; Vargas, R.I. How effective is GF-120 Fruit Fly Bait Spray applied to border area sorghum plants for control of melon flies (Diptera: Tephritidae)? Florida Entomol. 2004, 87, 354–360. [Google Scholar] [CrossRef]
- Vargas, R.I.; Mau, R.F.L.; Jang, E.B.; Faust, R.M.; Wong, L. The Hawaii fruit fly areawide pest management programme. In Areawide Pest Management: Theory and Implementation; Koul, O., Cuperus, G.W., Elliott, N.C., Eds.; CABI Publishing: Wallingford, UK, 2008; pp. 300–325. ISBN 9781845933722. [Google Scholar]
- Vargas, R.I.; Stark, J.D.; Nishida, T. Population dynamics, habitat preference, and seasonal distribution patterns of Oriental fruit fly and melon fly (Diptera: Tephritidae) in an agricultural area. Environ. Entomol. 1990, 19, 1820–1828. [Google Scholar] [CrossRef]
- Vargas, R.I.; Miyashita, D.; Nishida, T. Life history and demographic parameters of three laboratory-reared Tephritids (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1984, 77, 651–656. [Google Scholar] [CrossRef]
- Vargas, R.I.; Miller, N.W.; Stark, J.D. Field trials of spinosad as a replacement for Naled, DDVP, and Malathion in methyl eugenol and cue-lure bucket traps to attract and kill male Oriental fruit flies and melon flies (Diptera: Tephritidae) in Hawaii. J. Econ. Entomol. 2003, 96, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Mangan, R.L.; Moreno, D.S.; Thompson, G.D. Bait dilution, spinosad concentration, and efficacy of GF-120 based fruit fly sprays. Crop Prot. 2006, 25, 125–133. [Google Scholar] [CrossRef]
- Hsu, J.C.; Chou, M.Y.; Mau, R.F.L.; Maeda, C.; Shikano, I.; Manoukis, N.C.; Vargas, R.I. Spinosad resistance in field populations of melon fly, Zeugodacus cucurbitae (Coquillett), in Hawaii. Pest Manag. Sci. 2021, 77, 5439–5444. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Mau, R.F.L.; Gusukuma-Minuto, L. Diamondback moth, Plutella xylostella (L.), resistance management in Hawaii. In Proceedings of the 4th International Workshop, Melbourne, Australia, 26–29 November 2001; pp. 307–311. [Google Scholar]
- Vargas, R.I.; Piñero, J.C.; Leblanc, L. An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects 2015, 6, 297–318. [Google Scholar] [CrossRef]
- Kiljanek, T.; Niewiadowska, A.; Semeniuk, S.; Gaweł, M.; Borzęcka, M.; Posyniak, A. Multi-residue method for the determination of pesticides and pesticide metabolites in honeybees by liquid and gas chromatography coupled with tandem mass spectrometry—Honeybee poisoning incidents. J. Chromatogr. A 2016, 1435, 100–114. [Google Scholar] [CrossRef]
- Albrecht, C.P.; Sherman, M. Lethal and sublethal effects of avermectin B1 on three fruit fly species (Diptera: Tephritidae). J. Econ. Entomol. 1987, 80, 344–347. [Google Scholar] [CrossRef]
- Hennessey, M.K.; King, J.R. Abamectin bait for Caribbean fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 1996, 89, 987–989. [Google Scholar] [CrossRef]
- Manrakhan, A.; Kotze, C.; Daneel, J.-H.; Stephen, P.R.; Beck, R.R. Investigating a replacement for malathion in bait sprays for fruit fly control in South African citrus orchards. Crop Prot. 2013, 43, 45–53. [Google Scholar] [CrossRef]
- Senior, L.J.; Missenden, B.P.; Wright, C. Comparative efficacy of insecticides on Bactrocera tryoni and Zeugodacus cucumis (Diptera: Tephritidae) in laboratory and semifield trials in fruiting vegetables. J. Econ. Entomol. 2017, 110, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, O.; Osborne, T.; Barchia, I. Efficacy of chemicals for the potential management of the Queensland fruit fly Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Insects 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Shamshad, A. The development of integrated pest management for the control of mushroom sciarid flies, Lycoriella ingenua (Dufour) and Bradysia ocellaris (Comstock), in cultivated mushrooms. Pest Manag. Sci. 2010, 66, 1063–1074. [Google Scholar] [CrossRef]
- Díaz-Fleischer, F.; Pé rez-Staples, D.; Valle-Mora, J.; García-Pé rez, J.A. Laboratory evaluation of two commercial abamectin-based insecticides against Anastrepha ludens (Diptera: Tephritidae): Lethal and sublethal effects. J. Econ. Entomol. 2016, 109, 2472–2478. [Google Scholar] [CrossRef]
- Díaz-Fleischer, F.; Pérez-Staples, D.; Cabrera-Mireles, H.; Montoya, P.; Liedo, P. Novel insecticides and bait stations for the control of Anastrepha fruit flies in mango orchards. J. Pest Sci. 2017, 90, 865–872. [Google Scholar] [CrossRef]
- Yee, W.L.; Alston, D.G. Behavioral responses, rate of mortality, and oviposition of western cherry fruit fly exposed to malathion, zeta-cypermethrin, and spinetoram. J. Pest Sci. 2012, 85, 141–151. [Google Scholar] [CrossRef]
- Piñero, J.C.; Souder, S.K.; Vargas, R.I. Synergistic and additive interactions among components of food-based baits underlie female fruit fly attraction. Entomol. Exp. Appl. 2020, 168, 339–348. [Google Scholar] [CrossRef]
- Piñero, J.C.; Souder, S.K.; Smith, T.R.; Fox, A.J.; Vargas, R.I. Ammonium acetate enhances the attractiveness of a variety of protein-based baits to female Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2015, 108, 694–700. [Google Scholar] [CrossRef]
- Piñero, J.C.; Souder, S.K.; Smith, T.R.; Vargas, R.I. Attraction of Bactrocera cucurbitae and Bactrocera dorsalis (Diptera: Tephritidae) to beer waste and other protein sources laced with ammonium acetate. Florida Entomol. 2017, 100, 70–76. [Google Scholar] [CrossRef]
- Piñero, J.C.; Mau, R.F.L.; Vargas, R.I. A comparative assessment of the response of three fruit fly species (Diptera: Tephritidae) to a spinosad-based bait: Effect of ammonium acetate, female age, and protein hunger. Bull. Entomol. Res. 2011, 101, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Shelly, T.E.; Villalobos, E.M. Cue lure and the mating behavior of male melon flies (Diptera: Tephritidae). Florida Entomol. 1995, 78, 473–482. [Google Scholar] [CrossRef]
- Shelly, T.E.; Kurashima, R.S. Capture of melon flies and Oriental fruit flies (Diptera: Tephritidae) in traps baited with torula yeast-borax or CeraTrap in Hawaii. Fla. Entomol. 2018, 101, 144–146. [Google Scholar] [CrossRef]
- Nakamori, H.; Simizu, K. Comparison of flight ability between wild and mass-reared melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae), using a flight mill. Appl. Entomol. Zool. 1983, 18, 371–381. [Google Scholar] [CrossRef]
- Manoukis, N.C.; Carvalho, L.A.F.N. Flight burst duration as an indicator of flight ability and physical fitness in two species of tephritid fruit flies. J. Insect Sci. 2020, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Bess, H.A. Studies on the ecology and control of melon fly Dacus (Strumeta) cucurbitae. Coquillett (Diptera: Tephritidae). Tech. Bull. Hawaii Agric. Exp. Stn. 1957, 34, 1–47. [Google Scholar]
- Wang, L.L.; Huang, Y.; Lu, X.P.; Jiang, X.Z.; Smagghe, G.; Feng, Z.J.; Yuan, G.R.; Wei, D.; Wang, J.J. Overexpression of two α-esterase genes mediates metabolic resistance to malathion in the oriental fruit fly, Bactrocera dorsalis (Hendel). Insect Mol. Biol. 2015, 24, 467–479. [Google Scholar] [CrossRef]
- Couso-Ferrer, F.; Arouri, R.; Beroiz, B.; Perera, N.; Cervera, A.; Navarro-Llopis, V.; Castañera, P.; Hernández-Crespo, P.; Ortego, F. Cross-resistance to insecticides in a malathion-resistant strain of Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2011, 104, 1349–1356. [Google Scholar] [CrossRef]
- Magaña, C.; Hernández-Crespo, P.; Ortego, F.; Castañera, P. Resistance to malathion in field populations of Ceratitis capitata. J. Econ. Entomol. 2007, 100, 1836–1843. [Google Scholar] [CrossRef]
- Hsu, J.C.; Feng, H.T.; Wu, W.J. Resistance and synergistic effects of insecticides in Bactrocera dorsalis (Diptera: Tephritidae) in Taiwan. J. Econ. Entomol. 2004, 97, 1682–1688. [Google Scholar] [CrossRef]
- Vargas, R.; Prokopy, R. Attraction and feeding responses of melon flies and Oriental fruit flies (Diptera: Tephritidae) to various protein baits with and without toxicants. Proc. Hawaiian Entomol. Soc. 2006, 38, 49–60. [Google Scholar]
- Mahat, K.; Drew, R.A.I. Evaluation of protein bait laced with various insecticides on the Queensland fruit fly (Diptera: Tephritidae): Attraction, feeding, mortality and bait persistence. Acta Hortic. 2015, 1, 1041–1048. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Cooley, S.S.; Galarza, L.; Bergweiler, C.; Lauzon, C.R. Bird droppings compete with bait sprays for Rhagoletis pomonella (Walsh) flies (Diptera: Tephritidae). Can. Entomol. 1993, 125, 413–422. [Google Scholar] [CrossRef]
- Wang, W.; Mo, J.; Cheng, J.; Zhuang, P.; Tang, Z. Selection and characterization of spinosad resistance in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2006, 84, 180–187. [Google Scholar] [CrossRef]
- Jin, T.; Lin, Y.Y.; Jin, Q.A.; Wen, H.B.; Peng, Z.Q. Population susceptibility to insecticides and the development of resistance in bactrocera cucurbitae (Diptera: Tephritidae). J. Econ. Entomol. 2016, 109, 837–846. [Google Scholar] [CrossRef]
- Vargas, R.I.; Walsh, W.A.; Kanehisa, D.; Jang, E.B.; Armstrong, J.W. Demography of four Hawaiian fruit flies (Diptera: Tephritidae) reared at five constant temperatures. Ann. Entomol. Soc. Am. 1997, 90, 162–168. [Google Scholar] [CrossRef]
- Harwood, J.F.; Chen, K.; Müller, H.-G.; Wang, J.-L.; Vargas, R.I.; Carey, J.R. Effects of diet and host access on fecundity and lifespan in two fruit fly species with different life-history patterns. Physiol. Entomol. 2013, 38, 81–88. [Google Scholar] [CrossRef]
- Forrester, N.W.; Cahill, M. Management of insecticide resistance in Heliothis armigera (Hubner) in Australia. In Combating Resistance to Xenobiotics: Biological and Chemical Approaches; Ford, M.G., Holloman, D.W., Khambay, B.P.S., Sawicki, R.M., Eds.; Ellis Horwood, Ltd.: Chichester, UK, 1987; pp. 127–137. [Google Scholar]
- Chen, X.D.; Neupane, S.; Gossett, H.; Pelz-Stelinski, K.S.; Stelinski, L.L. Insecticide rotation scheme restores insecticide susceptibility in thiamethoxam-resistant field populations of Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), in Florida. Pest Manag. Sci. 2021, 77, 464–473. [Google Scholar] [CrossRef]
- Lasota, J.A.; Dybas, R.A. Abamectin as a pesticide for agricultural use. Acta Leiden. 1990, 59, 217–225. [Google Scholar]
- Wang, X.; Lu, H. Acute toxic effect of abamectin on fresh-water aquatic animals. J. Environ. Health 2009, 26, 593–597. [Google Scholar]
- Newhart, K. Environmental fate of malathion. Calif. Environ. Prot. Agency 2006, 11, 1–20. [Google Scholar]
- Cabrera-Marín, N.V.; Liedo, P.; Sánchez, D. The effect of application rate of GF-120 (spinosad) and Malathion on the mortality of Apis mellifera (Hymenoptera: Apidae) foragers. J. Econ. Entomol. 2016, 109, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J.P.; Grant, A.K. IPM-compatibility of foliar insecticides for citrus: Indices derived from toxicity to beneficial insects from four orders. J. Insect Sci. 2003, 3, 18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Dyke, M.; Mullen, E.; Wixted, D.; McArt, S. A Pesticide Decision-Making Guide to Protect Pollinators in Tree Fruit Orchards; Cornell University: New York, NY, USA, 2018. [Google Scholar]
- Sattar, M. Impact of proteins in adult artificial diet of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) on biological parameters. Pak. J. Zool. 2017, 49, 1491–1497. [Google Scholar] [CrossRef]
- Vargas, R.I.; Miller, N.W.; Prokopy, R.J. Attraction and feeding responses of Mediterranean fruit fly and a natural enemy to protein baits laced with two novel toxins, phloxine B and spinosad. Entomol. Exp. Appl. 2002, 102, 273–282. [Google Scholar] [CrossRef]
| Brand Name (Common Name) | Active Ingredient | IRAC Group/Subgroup | Primary Site of Action/Mode of Action (Based on IRAC MoA Classification) |
|---|---|---|---|
| Nu-Lure | - | - | - |
| Agri-Mek SC | 8% abamectin | 6 (Avermectins and milbemycins) | Chloride channel activator |
| Mustang Maxx | 9.15% zeta-cypermethrin | 3B (DDT and analogs) | Voltage gated sodium channel modulator |
| Malathion 5EC | 57% malathion | 1B (organophosphates) | Acetylcholinesterase inhibitor |
| Ground Sprayer | |||||
|---|---|---|---|---|---|
| Brand Name (Common Name) | Amount (fl oz/acre) | Amount of Water (gal Water/acre) | mL of Product/L Water | g/L of Active Ingredient | g of Active Ingredient/acre |
| Nu-Lure | 48 | 10 | 37.5 | - | - |
| Agri-Mek SC | 3.5 | 20 | 1.37 | 0.115 | 8.68 |
| Mustang Maxx | 4 | 10 | 3.125 | 0.30 | 11.34 |
| Malathion 5EC | 44.8 | 10 | 35.0 | 20.97 | 793.79 |
| Aerial sprayer | |||||
| Nu-Lure | 48 | 1 | 375 | - | - |
| Agri-Mek SC | 3.5 | 5 | 5.48 | 0.46 | 8.68 |
| Mustang Maxx | 4 | 2 | 15.63 | 1.50 | 11.34 |
| Malathion 5EC | 44.8 | 2 | 175 | 104.85 | 793.79 |
| Insecticide Product | Trial Location (Year) | Total Volume of Spray | Water | Nu-Lure | Amount of Insecticide Product | Ammonium Acetate (% of Final Mixture) | Vol. per Spot Spray | No. of Spot Sprays | Approx. Area Covered by Treated Spots | g of Active Ingredient/Acre |
|---|---|---|---|---|---|---|---|---|---|---|
| Agri-Mek SC | Maui commercial farm (2020) | 0.95 gal (3.6 L) | 0.75 gal (2.83 L) | 24 fl oz (0.71 L) | 1.758 fl oz (52 mL) | 36 g (1%) | 3 fl oz (90 mL) | 40 | 5 acres (2 hectares) | 0.872 |
| Oahu commercial farm (2020) | 8 gal (30.28 L) | 6.31 gal (23.88 L) | 201.7 fl oz (5.96 L) | 14.77 fl oz (437 mL) | 151.4 g (0.5%) | 3 fl oz (90 mL) | 336 | 96 acres (39 hectares) | 0.382 | |
| Maui research station (2021) | 1.43 gal (5.4 L) | 1.32 gal (4.98 L) | 14.2 fl oz (0.415 L) | 0.25 fl oz (7.4 mL) | 54 g (1%) | 3 fl oz (90 mL) | 60 | 6.3 acres (2.5 hectares) | 0.099 | |
| Mustang Maxx | Maui commercial farm (2020) | 1.43 gal (5.4 L) | 1.12 gal (4.25 L) | 36 fl oz (1.065 L) | 3 fl oz (89 mL) | 54 g (1%) | 3 fl oz (90 mL) | 60 | 5 acres (2 hectares) | 1.706 |
| Maui research station (2021) | 1.43 gal (5.4 L) | 1.31 gal (4.97 L) | 13.8 fl oz (0.408 L) | 0.52 fl oz (15.5 mL) | 54 g (1%) | 3 fl oz (90 mL) | 60 | 6.3 acres (2.5 hectares) | 0.236 | |
| Malathion 5EC | Maui commercial farm (2020) | 1.43 gal (5.4 L) | 1.00 gal (3.77 L) | 36 fl oz (1.065 L) | 19.2 fl oz (568 mL) | 54 g (1%) | 3 fl oz (90 mL) | 60 | 5 acres (2 hectares) | 68.061 |
| Maui research station (2021) | 1.43 gal (5.4 L) | 1.29 gal (4.90 L) | 14.0 fl oz (0.414 L) | 3.11 fl oz (92 mL) | 54 g (1%) | 3 fl oz (90 mL) | 60 | 6.3 acres (2.5 hectares) | 8.749 |
| Insecticide Product | Application Dates | Total Volume of Spray | Water | Nu-Lure | Amount of Insecticide Product | Ammonium Acetate (% of Final Mixture) | Vol. Per Spot Spray | No. of Spot Sprays | Approx. Area Covered by Treated Spots | g of Active Ingredient/Acre |
|---|---|---|---|---|---|---|---|---|---|---|
| GF-120 | 29 April 21, 6 May 21, 1 July 21, 8 July 21 | 1.43 gal (5.4 L) | 0.86 gal (3.24 L) | - | 0.57 gal (2.16 L) | - | 3 fl oz (90 mL) | 40 | 5 acres (2 hectares) | 0.104 |
| Agri-Mek SC | 13 May 21, 20 May 21, 15 July 21, 22 July 21 | 1.43 gal (5.4 L) | 1.32 gal (4.98 L) | 14.2 fl oz (0.415 L) | 0.25 fl oz (7.4 mL) | 54 g (1%) | 3 fl oz (90 mL) | 60 | 5 acres (2 hectares) | 0.124 |
| Mustang Maxx | 3 June 21, 10 June 21 | 1.43 gal (5.4 L) | 1.31 gal (4.97 L) | 13.8 fl oz (0.408 L) | 0.52 fl oz (15.5 mL) | 54 g (1%) | 3 fl oz (90 mL) | 60 | 5 acres (2 hectares) | 0.297 |
| Malathion 5EC | 17 June 21, 24 June 21 | 1.43 gal (5.4 L) | 1.29 gal (4.90 L) | 14.0 fl oz (0.414 L) | 3.11 fl oz (92 mL) | 54 g (1%) | 3 fl oz (90 mL) | 60 | 5 acres (2 hectares) | 11.024 |
| Treated Area | Control Area | ||||||
|---|---|---|---|---|---|---|---|
| Zucchini Harvest Date | Insecticide Treatment the Week Prior to Harvest | No. Buckets of Marketable Fruits | No. Buckets of Infested Fruits | Marketable Harvest (%) | No. Buckets of Marketable Fruits | No. Buckets of Infested Fruits | Marketable Harvest (%) |
| 29 April 2021 | No treatment | - | - | - | - | - | - |
| 6 May 2021 | GF-120 | 24 | 23 | 51% | 28 | 25 | 53% |
| 13 May 2021 | GF-120 | 35 | 28 | 55% | 26 | 21 | 55% |
| 20 May 2021 | Agri-Mek SC | 48 | 20 | 71% | 32 | 24 | 57% |
| 27 May 2021 | Agri-Mek SC | 47 | 8 | 85% | 27 | 21 | 56% |
| 3 June 2021 | Mustang Maxx | 45 | 3 | 94% | 17 | 12 | 59% |
| 10 June 2021 | Mustang Maxx | 46 | 3 | 94% | No harvest | No harvest | - |
| 17 June 2021 | Malathion 5EC | 42 | 1 | 98% | 23 | 14 | 62% |
| 24 June 2021 | Malathion 5EC | 27 | 0.5 | 98% | 24 | 8 | 75% |
| 1 July 2021 | GF-120 | 23 | 1 | 96% | 16 | 4 | 80% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shikano, I.; Gutierrez-Coarite, R.; Streit, C.; Perez, E.; Fujitani, E.; Mau, R.F.L. Field Tests of Three Alternative Insecticides with Protein Bait for the Development of an Insecticide Rotation Program to Control Melon Flies, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). Insects 2022, 13, 629. https://doi.org/10.3390/insects13070629
Shikano I, Gutierrez-Coarite R, Streit C, Perez E, Fujitani E, Mau RFL. Field Tests of Three Alternative Insecticides with Protein Bait for the Development of an Insecticide Rotation Program to Control Melon Flies, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). Insects. 2022; 13(7):629. https://doi.org/10.3390/insects13070629
Chicago/Turabian StyleShikano, Ikkei, Rosemary Gutierrez-Coarite, Christian Streit, Edwin Perez, Earl Fujitani, and Ronald F. L. Mau. 2022. "Field Tests of Three Alternative Insecticides with Protein Bait for the Development of an Insecticide Rotation Program to Control Melon Flies, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae)" Insects 13, no. 7: 629. https://doi.org/10.3390/insects13070629
APA StyleShikano, I., Gutierrez-Coarite, R., Streit, C., Perez, E., Fujitani, E., & Mau, R. F. L. (2022). Field Tests of Three Alternative Insecticides with Protein Bait for the Development of an Insecticide Rotation Program to Control Melon Flies, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). Insects, 13(7), 629. https://doi.org/10.3390/insects13070629






