Fumigant Activity of Ethyl Formate against the Chestnut Weevil, Curculio sikkimensis Heller
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Insects and Chestnuts
2.2. Chemicals
2.3. Quantitative Analysis Using Gas Chromatography
2.4. Fumigation Bioassay for EF in Laboratory Conditions
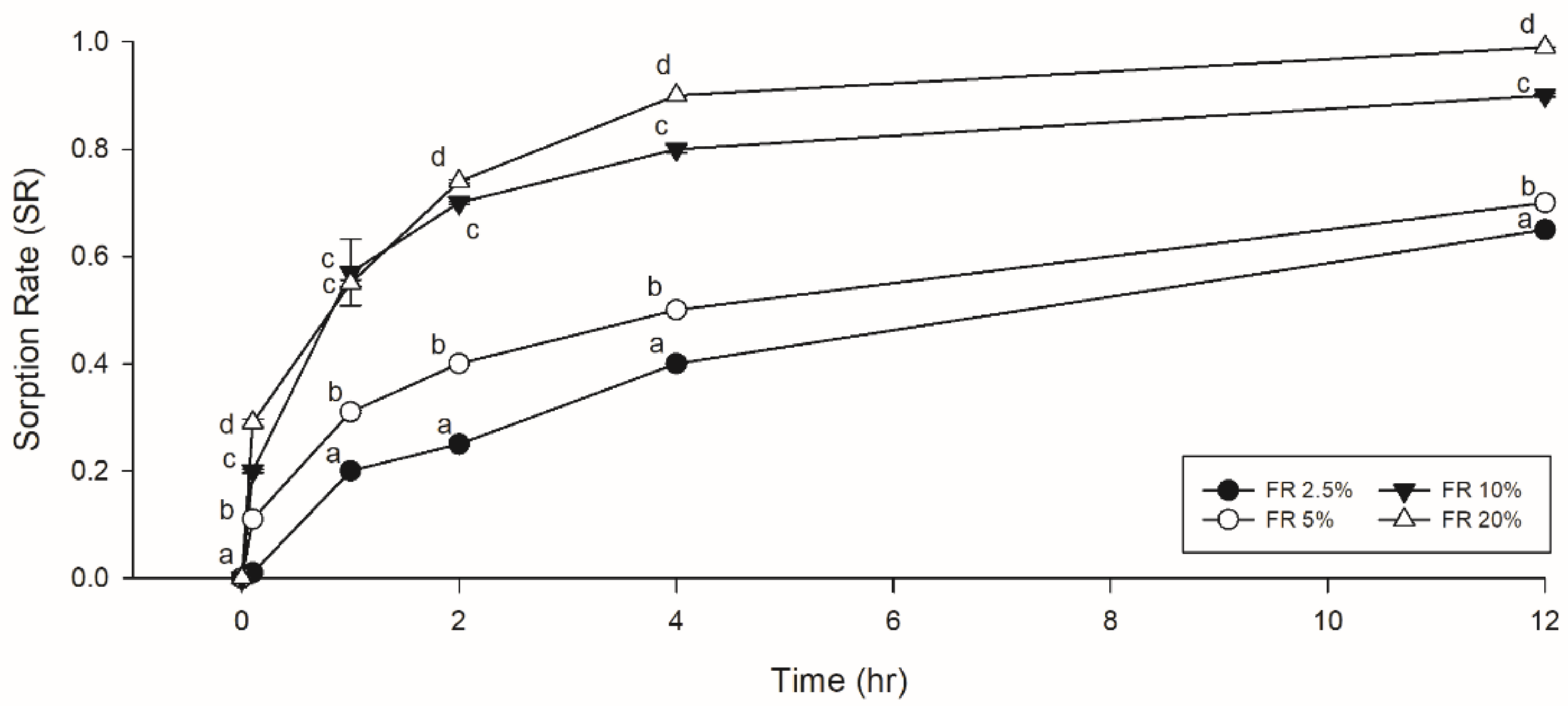
2.5. Sorption Test
2.6. Fumigation and Immersion Tests
2.7. Depression (Reduced Pressure) Fumigation of Ethyl Formate
2.8. Small-Scale (2 m3) Fumigation Using Ethyl Formate
2.9. Statistical Analysis
3. Results
3.1. Fumigation Bioassay Using EF in Laboratory Conditions
3.2. Sorption Test
3.3. Fumigation and Immersion Methods
3.4. Depression (Reduced Pressure) Fumigation of Ethyl Formate
3.5. Small-Scale (2 m3) Fumigation Using Ethyl Formate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barstow, M. Castanea crenata Siebold & Zucc. Available online: https://www.iucnredlist.org/species/62004433/62004435 (accessed on 2 April 2022).
- KFRI [Korea Forest Research Institute]. Coloured Woody Plants of Korea; KFRI: Seoul, Korea, 1987.
- KFS [Korea Forest Service]. Statistical Yearbook of Foresty; Korea Forest Service: Daejun, Korea, 2020; p. 448.
- Keesey, I.W.; Barrett, B.A. Seasonal occurrence and soil distribution of the lesser chestnut weevil, Curculio sayi (Coleoptera: Curculionidae) in Mid-Missouri. J. Kansas Entomol. Soc. 2008, 81, 345–354. [Google Scholar] [CrossRef]
- Paparatti, B.; Speranza, S. Management of chestnut weevil (Curculio spp.) 1, insect key-pest in central Italy. Acta Hortic. 2005, 93, 551–556. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, Y.-J.; Yoon, C.-M.; Shin, S.-C.; Choi, K.-S.; Kim, G.-H. Seasonal occurrence of the larvae and adults of chestnut weevil, Curculio sikkimensis (Coleoptra: Curculionidae). Korean J. Appl. Entomol. 2008, 47, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Okabe, N.; Takaeda, M. Ecology and control of Curculio dentipes Roelofs in Ishikawa prefecture. Plant Prot. 1993, 47, 301–304. [Google Scholar]
- Xiao, G.G. Forest Insects of China, 2nd ed.; China Forestry Publishing House: Beijing, China, 1991. [Google Scholar]
- Shin, S.C.; Choi, K.S.; Lee, S.M.; Moon, I.S.; Boo, K.S.; Jung, J.K.; Han, K.S.; Jung, C.H.; Park, J.W. Development of Attractant(s) for the Chestnut Weevils, Curculio spp.; Ministry of Agriculture and Forest: Gwacheon, Korea, 1998; p. 81.
- Kwon, J.-H.; Kwon, Y.-J.; Byun, M.-W.; Kim, K.-S. Competitiveness of gamma irradiation with fumigation for chestnuts associated with quarantine and quality security. Radiat. Phys. Chem. 2004, 41, 41–44. [Google Scholar] [CrossRef]
- RDA [Rural Development Administration]. Pesticide Safety Information System. Available online: www.psis.rda.go.kr (accessed on 30 March 2022).
- Kim, Y.-J.; Kim, H.K.; Lee, K.-S.; Kim, G.-H. Effects of immersion temperatures and times on chestnut fruit and mortality of the chestnut weevil, Curculio sikkimensis Heller. Korean J. Appl. Entomol. 2014, 53, 339–346. [Google Scholar] [CrossRef]
- WHO [World Health Organization]. Evaluation of Certain Food Additives and Contaminants: Forthy-Sixty Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO: Geneva, Switzerland, 1997. [Google Scholar]
- Muthu, M.; Rajendran, S.; Krishnamurthy, T.S.; Narasimhan, K.S.; Rangaswamy, J.R.; Jayaram, M.; Majumder, S.K. Ethyl formate as a safe general fumigant. Dev. Agric. Eng. 1984, 5, 369–393. [Google Scholar]
- Haritos, V.S.; Dojckinov, G. Cytochrome c oxidase inhibition in the rice weevil Sitophilus oryzae (L.) by formate, the toxic metabolite of volatile alkyl formates. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2003, 136, 135–143. [Google Scholar] [CrossRef]
- Desmarchelier, J.M.; Allen, S.E.; Ren, Y.L.; Moss, R.; Vu, L.T. Commerclal Scale Trials on the Application of Ethyl Formate, Carbonyl Sulphide, and Carbon Disulphide to Wheat; CISRO Entomology: Canberra, Australia, 1998. [Google Scholar]
- Ducom, P.J.F. The Return of the Fumigants. In Proceedings of the 9th International Working Conference on Stored Product Protection, Sao Paulo, Brazil, 15–18 October 2006; Lorini, I., Bacaltchuk, B., Beckel, H., Deckers, D., Sundfeld, E., Dos Santos, J.P., Biagi, J.D., Celaro, J.C., Faroni, L.R.D.A., Bortolini, L.d.O.F., et al., Eds.; Brazilian Post-Harvest Association: São Paulo, Brazil, 2006; pp. 510–516. [Google Scholar]
- Van Epenhuijsen, C.W.; Hedderley, D.K.; Somerfield, K.G.; Brash, D.W. Efficacy of ethyl formate and ethyl acetate for the control of onion thrips (Thrips tabaci). N. Z. J. Crop Hortic. Sci. 2007, 35, 267–274. [Google Scholar] [CrossRef]
- Pupin, F.; Bikoba, V.; Biasi, W.B.; Pedroso, G.M.; Ouyang, Y.; Grafton-Cardwell, E.E.; Mitcham, E.J. Postharvest control of western flower thrips (Thysanoptera: Thripidae) and California red scale (Hemiptera: Diaspididae) with ethyl formate and its impact on citrus fruit quality. J. Econ. Entomol. 2013, 106, 2341–2348. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, E.M.; Newman, J.; Coupland, G.T.; Thomas, M.; van der Merwe, J.; Ren, Y.; McKirdy, S.J. Commercial trials evaluating the novel use of ethyl formate for in-transit fumigation of shipping containers. J. Environ. Sci. Health-B Pestic. Food Contam. Agric. Wastes 2019, 54, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Redpath, S.P.; Wilson, A.; Jamieson, L.E.; Page-Weir, N.E.M.; Grifin, M.J.; Chhagan, A.; Hamilton, B. Postharvest management of New Zealand flower thrips on export apricots using ethyl formate. N. Z. Plant Prot. 2014, 67, 103–108. [Google Scholar] [CrossRef]
- Kim, B.S.; Shin, E.M.; Park, Y.J.; Yang, J.O. Susceptibility of the cigarette beetle Lasioderma serricorne (Fabricius) to phosphine, ethyl formate and their combination, and the sorption and desorption of fumigants on cured tobacco leaves. Insects 2020, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.L.; Lee, B.H.; Padovan, B. Penetration of methyl bromide, sulfuryl floride, ethanedinitrile and phosphine into timber blocks and the sorption rate fthe fumigants. J. Stored Prod. Res. 2011, 47, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Püntener, W. Manual for Field Trials in Plant Protection, 2nd ed.; Agricultural Division, Ciba-Geigy, Ltd.: Basle, Switzerland, 1981. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971; p. 333. [Google Scholar]
- Kwon, T.H.; Park, C.G.; Lee, B.-H.; Zarders, D.R.; Roh, G.H.; Kendra, P.E.; Cha, D.H. Ethyl formate fumigation and ethyl formate plus cold treatment combination as potential phytosanitary quarantine treatments of Drosophila suzukii in blueberries. J. Asia Pac. Entomol. 2021, 24, 129–135. [Google Scholar] [CrossRef]
- Chiluwal, K.; Lee, B.H.; Kwon, T.H.; Kim, J.; Bae, S.D.; Roh, G.H.; Ren, Y.; Li, B.; Park, C.G. Synergistic effect of fumigation with ethyl formate and methyl salicylate on mortality of life stages of adzuki bean beetle, Callosobruchus chinensis (L.). J. Asia Pac. Entomol. 2020, 23, 483–491. [Google Scholar] [CrossRef]
- Agarwal, M.; Ren, Y.; Newman, J.; Learmonth, S. Ethyl formate: A potential disinfestation treatment for eucalyptus weevil (Gonipterus platensis)(Coloptera: Curculionidae) in Apples. J. Econ. Entomol. 2015, 108, 2566–2571. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Song, J.-E.; Park, J.S.; Park, Y.; Shin, E.-M.; Yang, J.O. Insecticidal effects of fumigatns (EF, MB, and PH3) towards phosphine-susceptibleand and -resistant Sitophilus oryzae (Coloptera: Curculionidae). Insects 2019, 10, 327. [Google Scholar] [CrossRef] [Green Version]
- Asimah, H.; Albert, L.; Idris, A.B. Application of ethyl formate as a postharvest fumigatn for dry cocoa beans under a semi commercial trial. J. Entomol. 2015, 12, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Park, M.-G.; Park, C.-G.; Yang, J.-O.; Kim, G.-H.; Ren, Y.; Lee, B.-H.; Cha, D.H. Ethyl formate as a methyl bromide alternative for phytosanitary disinfestation of imported banana in Korea with logistical considerations. J. Econ. Entomol. 2020, 113, 1711–1717. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.; Kyung, Y.; Park, G.-H.; Lee, B.-H.; Yang, J.H.; Koo, H.-N.; Kim, G.-H. Fumigation activity of ethyl formate and phosphine against Tetranychus urticae (Acari: Tetranychidae) on imported sweet pumpkin. J. Econ. Entomol. 2018, 111, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Misumi, T.; Ogawa, N.; Yamada, K.; Shukuya, T. Susceptibilities of five species of scales (Diaspididae and Coccidae) and mealybugs (Pseudococcidae) to fumigation with a gas mixture of ethyl formate and carbon dioxide under normal atmospheric pressure or vacuum. Res. Bull. Plant Prot. Jpn. 2013, 49, 1–9. [Google Scholar]
- Stewart, J.K.; Mon, T.R. Ethyl formate for postharvest control of the green peach apid (Homoptera: Aphididae) on film-wrapped lettuce. J. Econ. Entomol. 1984, 77, 569–573. [Google Scholar] [CrossRef]
| Concentration of EF (g/m3) | Number of Larvae | Mortality (Mean ± SE, %) | CT Value of EF (g·h/m3) |
|---|---|---|---|
| 0.0 | 60 | 0.0 ± 0.0 | 0.0 |
| 10.0 | 60 | 0.0 ± 0.0 | 64.5 |
| 20.0 | 60 | 13.3 ± 1.7 | 151.6 |
| 30.0 | 60 | 21.7 ± 1.7 | 248.6 |
| 40.0 | 60 | 41.7 ± 1.7 | 359.5 |
| 60.0 | 60 | 55.0 ± 2.9 | 597.5 |
| 80.0 | 60 | 76.7 ± 3.3 | 729.9 |
| 90.0 | 60 | 86.7 ± 1.7 | 820.4 |
| 110.0 | 60 | 91.7 ± 1.7 | 964.3 |
| 130.0 | 60 | 96.7 ± 1.7 | 1120.8 |
| 160.0 | 60 | 100.0 ± 0.0 | 1221.9 |
| 180.0 | 60 | 100.0 ± 0.0 | 1360.3 |
| CT Value (g·h/m3) | Weight Change (%) | Color Values 1 | Firmness (kgf) | Decay Degree 2 | |
|---|---|---|---|---|---|
| Outer | Inside | ||||
| 0 | 1.7 ± 0.8 a | 46.3 ± 1.6 a | 23.5 ± 1.3 a | 6.5 ± 1.1 a | 0.0 ± 0.0 a |
| 1064.7 | 1.5 ± 0.5 a | 49.3 ± 0.7 a | 24.6 ± 1.1 a | 6. 4 ± 0.6 a | 0.0 ± 0.0 a |
| Treatment | Time (h) | CT (g·h/m3) | Mortality (Mean ± SE, %) | Numbers of Larvae |
|---|---|---|---|---|
| Immersion | 12 | - | 1.6 ± 0.3 b | 309 |
| Fumigation 1 | 12 | 838.6 | 78.8 ± 4.2 a | 302 |
| Fumigation 1 and immersion | 12/12 | 838.6 | 76.9 ± 8.2 a | 315 |
| Control | - | - | 0.0 b | 301 |
| Treatment | Number of Larvae | Mortality (Mean ± SE, %) | Control Value (%) |
| EF 160.0 g/m3 | 963 | 91.42 ± 1.05 | 91.4 |
| EF 180.0 g/m | 900 | 100.0 | 100.0 |
| Control | 911 | 0.11 ± 0.11 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, T.H.; Lee, B.; Kim, J. Fumigant Activity of Ethyl Formate against the Chestnut Weevil, Curculio sikkimensis Heller. Insects 2022, 13, 630. https://doi.org/10.3390/insects13070630
Kwon TH, Lee B, Kim J. Fumigant Activity of Ethyl Formate against the Chestnut Weevil, Curculio sikkimensis Heller. Insects. 2022; 13(7):630. https://doi.org/10.3390/insects13070630
Chicago/Turabian StyleKwon, Tae Hyung, Byungho Lee, and Junheon Kim. 2022. "Fumigant Activity of Ethyl Formate against the Chestnut Weevil, Curculio sikkimensis Heller" Insects 13, no. 7: 630. https://doi.org/10.3390/insects13070630
APA StyleKwon, T. H., Lee, B., & Kim, J. (2022). Fumigant Activity of Ethyl Formate against the Chestnut Weevil, Curculio sikkimensis Heller. Insects, 13(7), 630. https://doi.org/10.3390/insects13070630








