Simple Summary
In the European Union, there is no registered product for the control of the honey bee endoparasite Nosema ceranae. Thus, researchers are looking for options for Nosema treatment. The aim of this study was to investigate the effect of a natural essential-oil ingredient (thymol) derived from Thymus vulgaris on honey bees infected with N. ceranae. Thymol exerted certain positive effects (increasing bee survival, immunity, and antioxidative protection), as well as positively affecting the spore loads in Nosema-infected bees. However, when applied to Nosema-free bees, thymol caused certain health disorders; therefore, beekeepers should be careful with its use.
Abstract
Nosema ceranae is the most widespread microsporidian species which infects the honey bees of Apis mellifera by causing the weakening of their colonies and a decline in their productive and reproductive capacities. The only registered product for its control is the antibiotic fumagillin; however, in the European Union, there is no formulation registered for use in beekeeping. Thymol (3-hydroxy-p-cymene) is a natural essential-oil ingredient derived from Thymus vulgaris, which has been used in Varroa control for decades. The aim of this study was to investigate the effect of thymol supplementation on the expression of immune-related genes and the parameters of oxidative stress and bee survival, as well as spore loads in bees infected with the microsporidian parasite N. ceranae. The results reveal mostly positive effects of thymol on health (increasing levels of immune-related genes and values of oxidative stress parameters, and decreasing Nosema spore loads) when applied to Nosema-infected bees. Moreover, supplementation with thymol did not induce negative effects in Nosema-infected bees. However, our results indicate that in Nosema-free bees, thymol itself could cause certain disorders (affecting bee survival, decreasing oxidative capacity, and downregulation of some immune-related gene expressions), showing that one should be careful with preventive, uncontrolled, and excessive use of thymol. Thus, further research is needed to reveal the effect of this phytogenic supplement on the immunity of uninfected bees.
1. Introduction
Nosemosis is a honey bee disease caused by microsporidia from the genus Nosema (N. apis and N. ceranae). It is the most widespread microsporidian infection of adult Apis mellifera individuals that leads to chronic infection, the weakening of honey bee colonies [1], and the decline of their productive and reproductive capacities [2,3,4].
This endoparasite survives in the infected colony throughout the year and reaches its maximum number before the end of winter and in early spring [5,6]. Diseased bees excrete a large amount of infectious agent in their feces, which easily reaches uninfected bees. In intensive beekeeping, forager bees have high metabolic requirements, which further potentiates the development of nutritional and energy stress [1,7]. The only registered product for nosemosis is the antibiotic fumagillin; however, in the European Union, there is no formulation registered for use in beekeeping [8,9,10]. The tendency to reduce the use of antibiotics in beekeeping has led to the continual search for natural alternatives in the treatment of diseases such as nosemosis, reviewed in [11] including dietary supplements [12,13,14,15,16,17,18,19,20,21,22].
Similar to Varroa ectoparasite control [23,24], organic chemicals or natural-based treatments are welcomed for Nosema treatment [25,26,27]. Thymol (3-hydroxy-p-cymene) is a natural compound as it is a component of essential oil, derived from Thymus vulgaris L., Lamiaceae, and many other plant species. The inhibitory effect of thymol on the growth of pathogenic bacteria and fungi, such as Escherichia coli, Streptococcus spp., Salmonella typhimurium, Staphylococcus aureus, Aspergillus flavus, and Cryptococcus neoformans, has been known for many years [28,29]. In beekeeping, thymol has been used for decades to control the honey bee mite Varroa destructor [30,31] with variable success [32]. The first studies of the potential effect of thymol in the control of Nosema infection in the hive were performed at the beginning of the 21st century [33,34], but to date, the anti-Nosema potential and effect of thymol on Nosema have not been fully elucidated. It was assumed that the mechanism of thymol action is based on penetration into the Nosema spore, interfering with the plasma membrane and preventing spore germination [28,33]. Thymol can be found naturally in low concentrations in honey [35,36], and it was thought not to leave residues in bee products [37]. However, recent studies revealed residues of thymol in honey [38] and beeswax [38,39].
Thymol is thought to interfere with the GABA signaling pathway in the central nervous system of insects [40], and there is therefore a legitimate concern that thymol also affects bees [41]. In addition, in a study by Bergougnoux et al. [42], thymol negatively affected the phototactic behavior of bees. Boncristiani et al. [43] reported that thymol had increased the susceptibility of bees to N. ceranae infection through the reduced expression of the Dscam and Basket genes, which are significant cellular and humoral immune factors, respectively, in defending bees from parasites [44,45]. In several studies, treatment with thymol (orally or topically) did not induce toxic effects on bees [42,46,47], and bees even lived longer compared with the control [47]. These data together with earlier observations on the low toxicity of thymol [48], as well as its importance for beekeeping, led to thymol’s approval by the European Union [49] for the control of the honey bee mite V. destructor in conventional and organic beekeeping [28,37].
An infection with N. ceranae induces oxidative stress in bees and compromises their immunity [13,14,15,16], particularly in combination with pesticides [50,51]. Thus, it could be important to identify the effects of a thymol-enriched diet on the biochemical and transcription levels of Nosema-infected bees. The aim of this study was to investigate the effect of thymol supplementation on bees’ spore loads, their expression of immune-related genes, and the parameters of oxidative stress, as well as the survival of bees infected with microsporidia N. ceranae.
2. Materials and Methods
2.1. Bees and Experimental Design
Colonies of Apis mellifera bees used as a bee brood source were located at the apiary of the Faculty of Veterinary Medicine, University of Belgrade. In accordance with good beekeeping practice, all colonies were without clinical symptoms of either adult bee diseases or brood diseases. The Varroa infestation was maintained at a minimum level, following the recommendations of the COLOSS BEEBOOK [52]. A sealed brood (prior to emergence) was taken from five randomly selected hives. The frames were placed in net bags to keep any emerging bees on the frame and left overnight in an incubator under controlled conditions (temperature 34 ± 1 °C, and humidity 66 ± 1%). After 12 h, the emerged bees were collected and placed randomly in cages that were specially designed for this purpose, following the method described by Glavinic et al. [13]. Each cage contained 80 randomly selected bees (15 for the RNA extraction, 15 for the analyses of oxidative stress, and 30 for counting Nosema spores, while the remaining 20 bees were used for survival monitoring). According to the experimental design (Table 1), the cages (experimental units) were divided into six experimental groups: bees in the non-infected control (NI), bees infected with N. ceranae (infected control—I), bees treated with thymol (treatment control—T), and three treatment groups—all infected with Nosema and treated with thymol from the first, third, and sixth day (I-T1, I-T3, and I-T6, respectively). The whole experiment was repeated, and the results were merged into a single dataset.

Table 1.
Experimental design.
The bees were fed ad libitum with 50% (w/v) sucrose solution. The tested substance was thymol (Sigma-Aldrich, St. Louis, MO, USA, CAS 89-83-8). The feeding solution was prepared in a concentration of 0.1 mg/g (0.1 g/kg) of syrup, according to Costa et al. [47]. The syrup volume was measured before and after the bees had been fed for 24 h to ascertain syrup consumption [16]. Further, the average consumption per bee per day was calculated. The dead bees were removed from cages daily, and the number of dead bees per cage was recorded.
2.2. Experimental Infection with N. ceranae Spores
On the third day of the experiment, the bees from the infected control group (I) and the treatment groups (I-T1, I-T3, and I-T6) were infected according to the experimental design (Table 1), with inoculum freshly prepared according to a previously published procedure [15]. The final concentration of inoculum was 1 × 106 spores/mL, while the presence of N. ceranae and absence of N. apis was confirmed by species-specific PCR tests [53]. The food was removed from the cages two hours before the infection was performed, in order to starve the bees and ensure better consumption of the inoculum.
2.3. Counting of Nosema Spores
The number of spores per bee was estimated according to the methodology adopted by Glavinic et al. [13]. Briefly, the abdomen of a single bee was placed in a 1.5 mL tube with 1 mL of dH2O and homogenate in TissueLyser II (Qiagen, Germany) for 1 min at 25 Hz. The suspension was observed using a hemocytometer according to the OIE guidelines [54].
2.4. Gene Expression Analyses
RNA was extracted from five bees from each cage, using a Quick-RNA MiniPrep Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. For cDNA synthesis, 1000 ng of RNA per sample were reverse-transcribed using the RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Vilnius, Lithuania).
The expression levels of abaecin, hymenoptaecin, defensin, apidaecin, and vitellogenin (immune-related genes) were determined by the methodology described in our previous studies [15,16]. The 2-ddCt method was used, while β-actin was an internal control gene [55]. The median value of the non-infected group served as a calibrator.
2.5. Oxidative Stress Analyses
The spectrophotometric analyses described in our previous study [15] were used for oxidative stress-parameter measurements: activities of the antioxidative enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferase (GST), and the concentrations of malondialdehyde (MDA). Pools of five bees collected from every cage on each sampling day (6, 9, and 15) were used and analyzed on a UV/VIS Spectrophotometer BK-36 S390 (Biobase Jinan, Shandong, China,).
2.6. Statistical Analyses
The Kaplan–Meier survival function was used for the survival dynamic presentation. To compare the difference in survival between two or more independent groups, a log-rank test was used. To ensure heterogeneity of the data for gene expression levels and spore loads, we used the Mann–Whitney U test in order to determine the significance of the difference between medians of two samples. We used ANOVA to maintain homogeneity of the oxidative stress data when determining the significance in the differences between three or more means. Moreover, the Tukey’s test was used to test the difference between the means of sample pairs.
All conclusions were made by comparing the level of significance of the realized value of the sample test statistics, p, with standard levels of significance, 0.05 and 0.01.
The statistical analyses of the results were done with Statistica Software (StatSoft Inc., Tulsa, OK, USA).
3. Results and Discussion
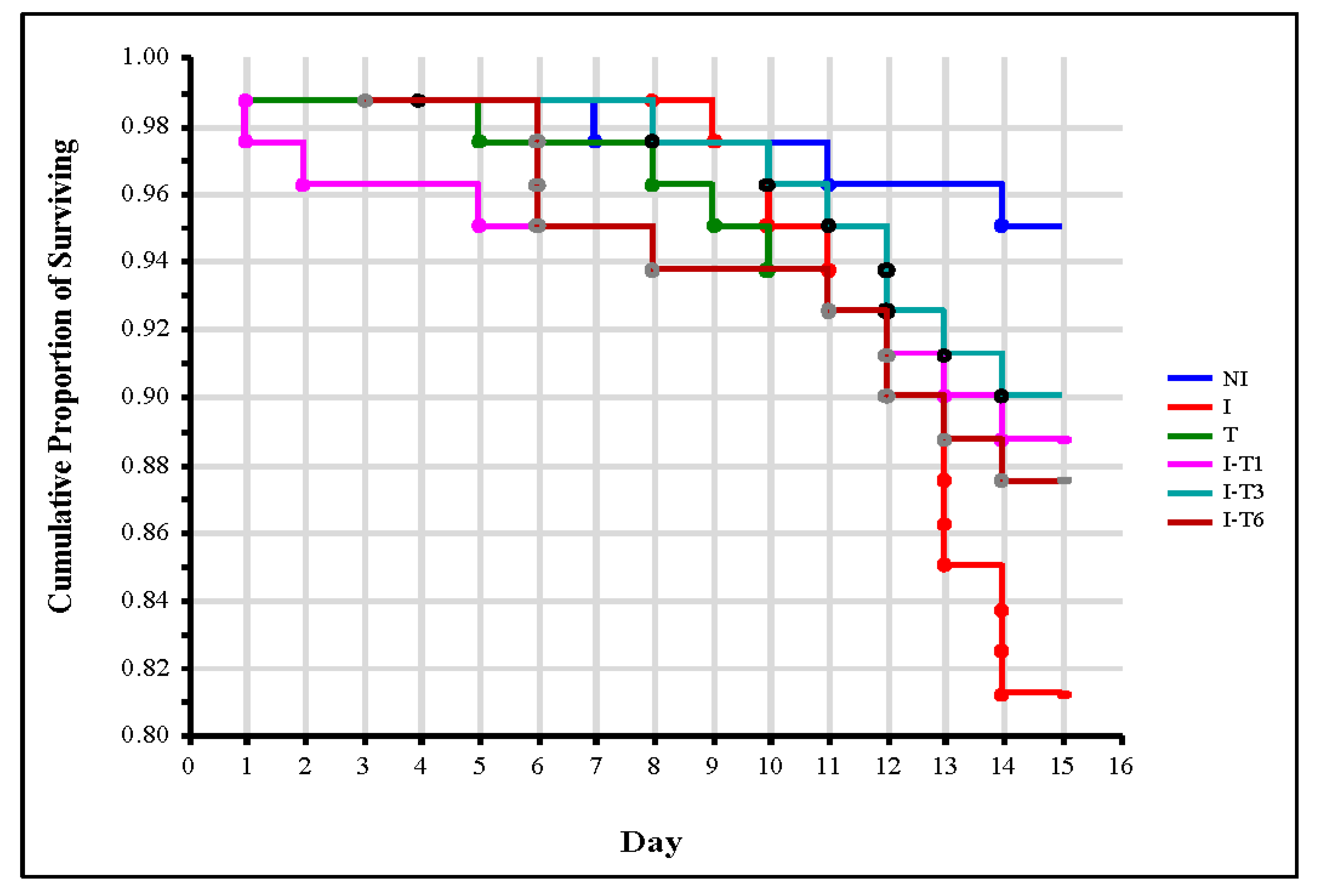
Nosema ceranae infection induced significant bee mortality (p = 0.008) in the infected control (I) group compared with the non-infected control (NI) group, confirming a negative impact of Nosema infection on bees’ lifespan [13,14,15,16]. When simultaneously analyzing the number of dead bees in the control group I and in all the thymol-treated groups, the log-rank test found an absence of significant differences (χ2 = 5.173; p = 0.270). Furthermore, the log-rank test for the two groups revealed the absence of statistically significant differences in the number of dead bees between control group I and each group treated with thymol (p ≥ 0.126) (Figure 1). The same Nosema- and thymol-induced mortality was reported by Maistrello et al. [28]. Bee mortality induced with thymol could be explained by the toxic potential of thyme essential oils being classified as a moderately toxic product [28]. On the other hand, in this experiment (Figure 1), there was no significant differences (log-rank test: χ2 = 3.048; p = 0.550) observed in the survival dynamic of the control group NI and groups treated with thymol. The number of dead bees between the control group NI and each group treated with thymol was not statistically significantly different (p ≥ 0.095). This result is in accordance with those of Ebert et al. [46], Costa et al. [47], and Bergougnoux et al. [42], who found that thymol was not toxic to bees. Nevertheless, according to EU Regulation 834/2007 on organic production [49], thymol is authorized for use in Varroa control in organic beekeeping. Keeping in mind that the presence of V. destructor reduces the potential of bees to combat N. ceranae [56], a great advantage of thymol could be its potential to control both pathogens.
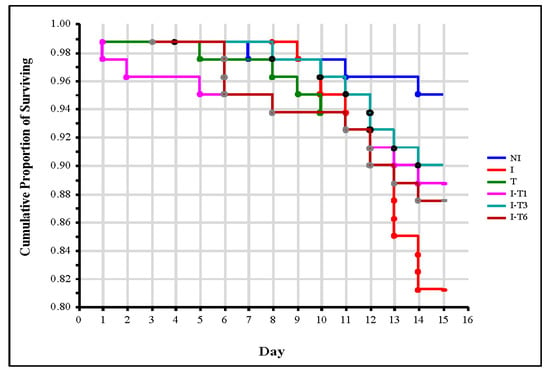
Figure 1.
Survival curves of bees from control groups and from groups treated with thymol. Group infected with N. ceranae (I), group that was non-infected but treated with thymol (T), groups infected with N. ceranae and treated with thymol from day 1 (I-T1), day 3 (I-T3), and day 6 (I-T6), and non-infected (NI) group.
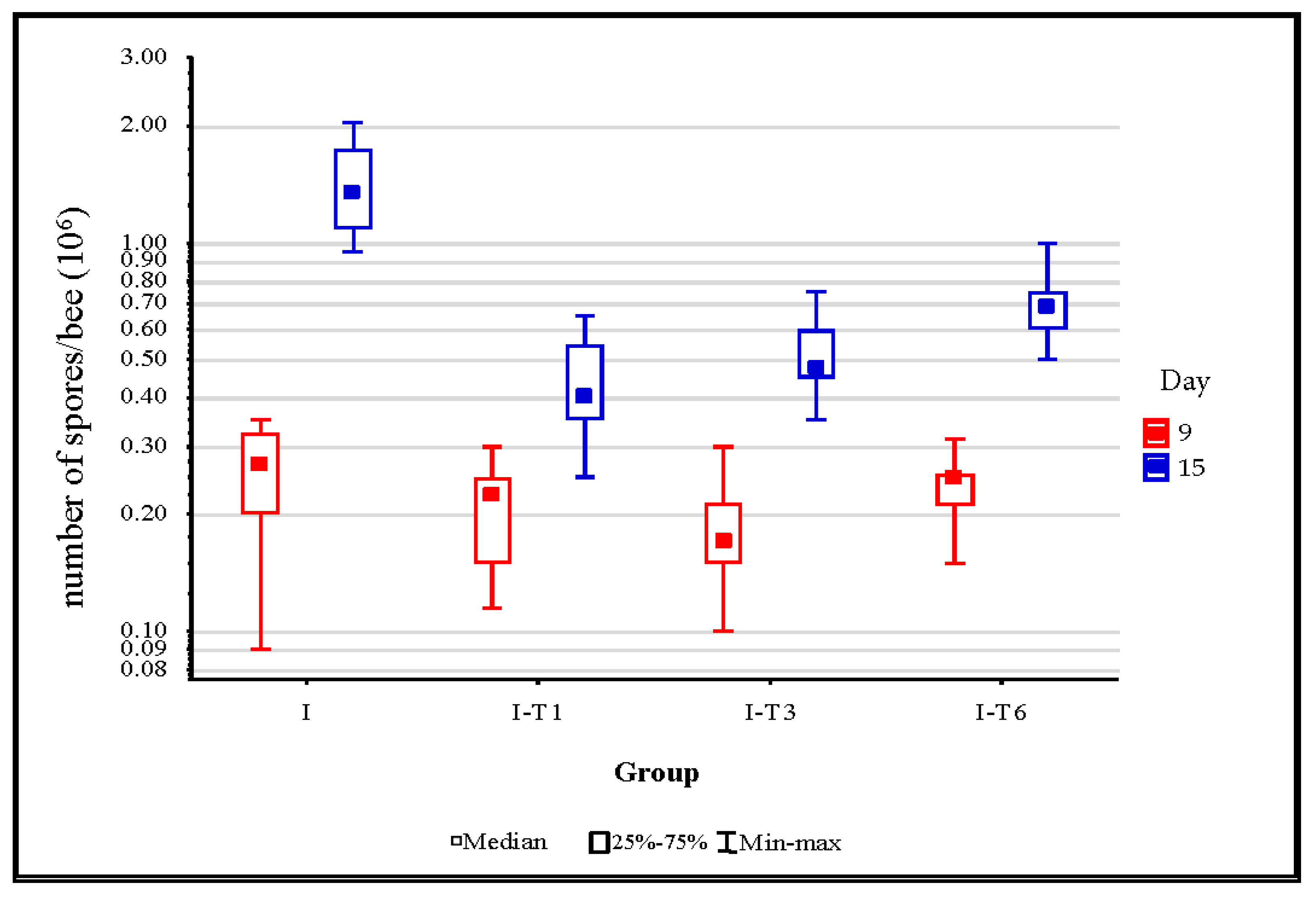
According to the Mann–Whitney U test, no significant differences (p > 0.05) between the control group I and groups infected and treated with thymol (I-T1, I-T3, and I-T6) were noticed in the number of Nosema spores in bees collected on day 9 (semi-logarithmic diagram, Figure 2). On day 15, the number of spores were significantly higher (p < 0.01), and the numbers varied between the experimental groups from 0.2 to 2 × 106/bee (semi-logarithmic diagram, Figure 2), which is similar to some previous studies [15,16,47,57,58]. A significantly higher (p < 0.001) Nosema spore load was detected in the infected control group compared with bees collected on day 15 from the I-T1, I-T3, and I-T6 groups. This difference could be due to thymol’s inhibitory effect on Nosema development, which resulted in lower Nosema spore loads in the latter stages of the experiment. Maistrello et al. [28] assumed that the mechanism of thymol’s anti-Nosema effect is based on its interaction with the Nosema spore by interfering with the plasma membrane and preventing spore germination [28,33]. Similar to our results, Costa et al. [47] detected significantly lower Nosema spore loads in bees supplemented with thymol, while Van den Heever et al. [59] reported that thymol decreased spore load by 40%. Keeping in mind the described effects of thymol consumption, especially its potential in Nosema control, we further investigated its impact on immune-related genes and the oxidative stress in bees infected with Nosema.

Figure 2.
N. ceranae spore loads on day 9 and day 15 in bees from the control group and from groups infected and treated with thymol. Group infected with N. ceranae (I) and groups infected with N. ceranae and treated with thymol from day 1 (I-T1), day 3 (I-T3), and day 6 (I-T6).
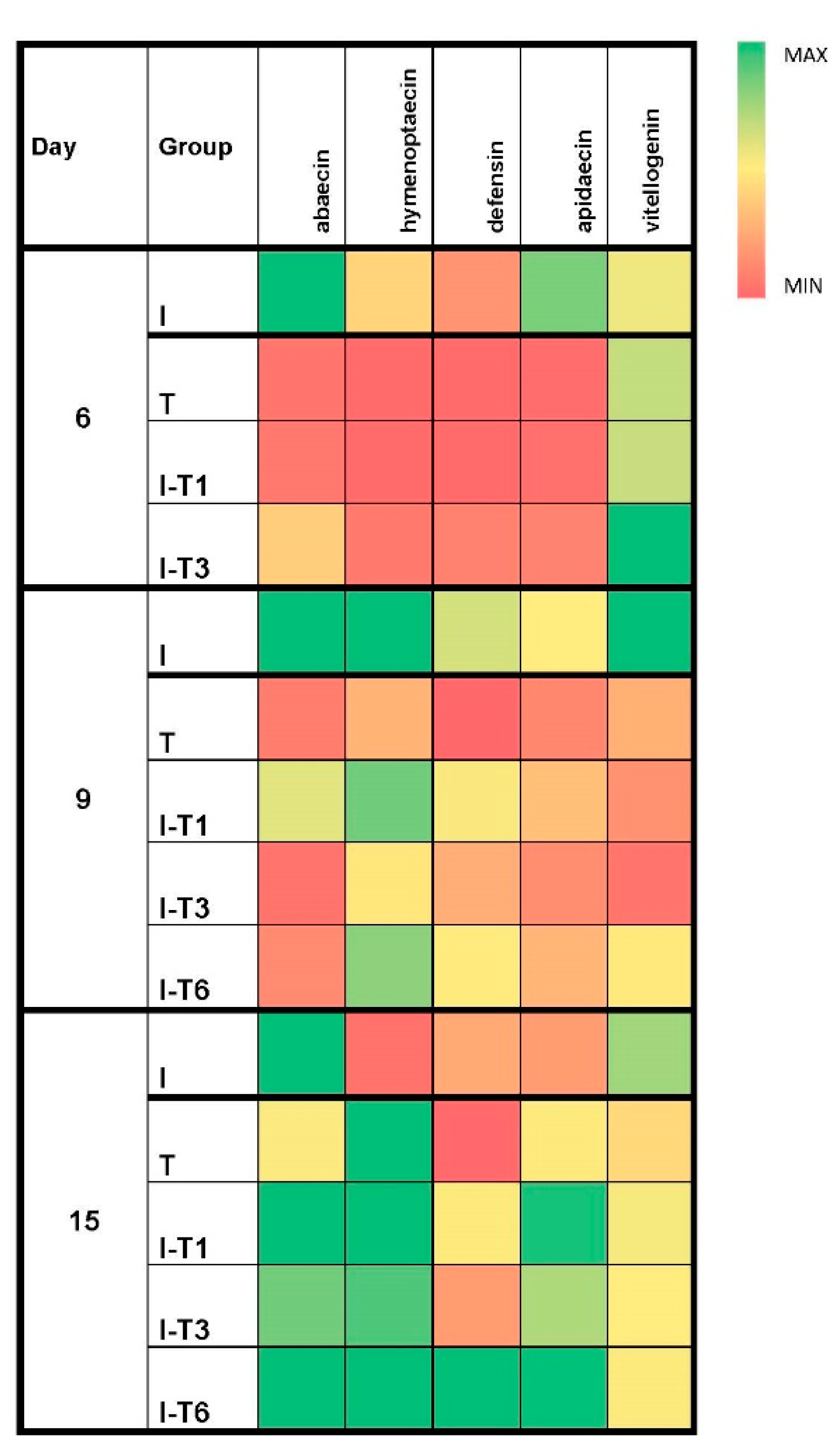
The expression levels of the abaecin gene on day 9 were significantly higher in the infected (I) group (Mann–Whitney U test: p ≤ 0.012) compared with all groups treated with thymol (Figure 3 and Figure S1). Therefore, thymol decreased abaecin’s gene expression more than the Nosema infection, which is in accordance with findings of Maistrello et al. [28] and Costa et al. [47], who identified that thymol worked best after longer usage (at the end of the experiment), contrary to Nosema whose negative effects increased over time [13,47]. Moreover, the oxidative stress parameters were inconsistent and without a clear pattern (Figure 4) in bees collected on day 9, which is in line with the detected number of spores on day 9 (that did not differ significantly among groups), because bees were struggling to overcome the negative impact of the endoparasite (N. ceranae) through the production of ROS (Reactive Oxygen Species) and by activating antioxidant (protective) mechanisms in order to prevent tissue damage caused by the ROS [14].
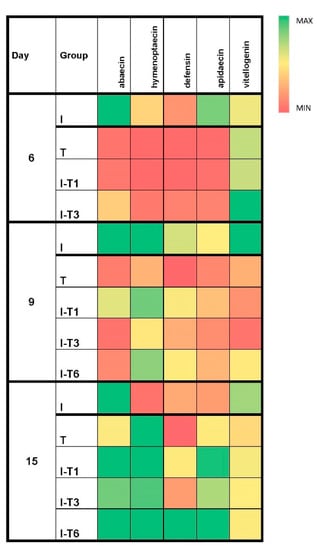
Figure 3.
Heat map of median values for the relative genes’ expression levels (abaecin, hymenoptaecin, defensin, apidaecin, and vitellogenin) at different time points (day 6, 9, and 15) in the experimental groups. Group infected with N. ceranae (I), group that was non-infected but treated with thymol (T), groups infected with N. ceranae and treated with thymol from day 1 (I-T1), day 3 (I-T3), and day 6 (I-T6).

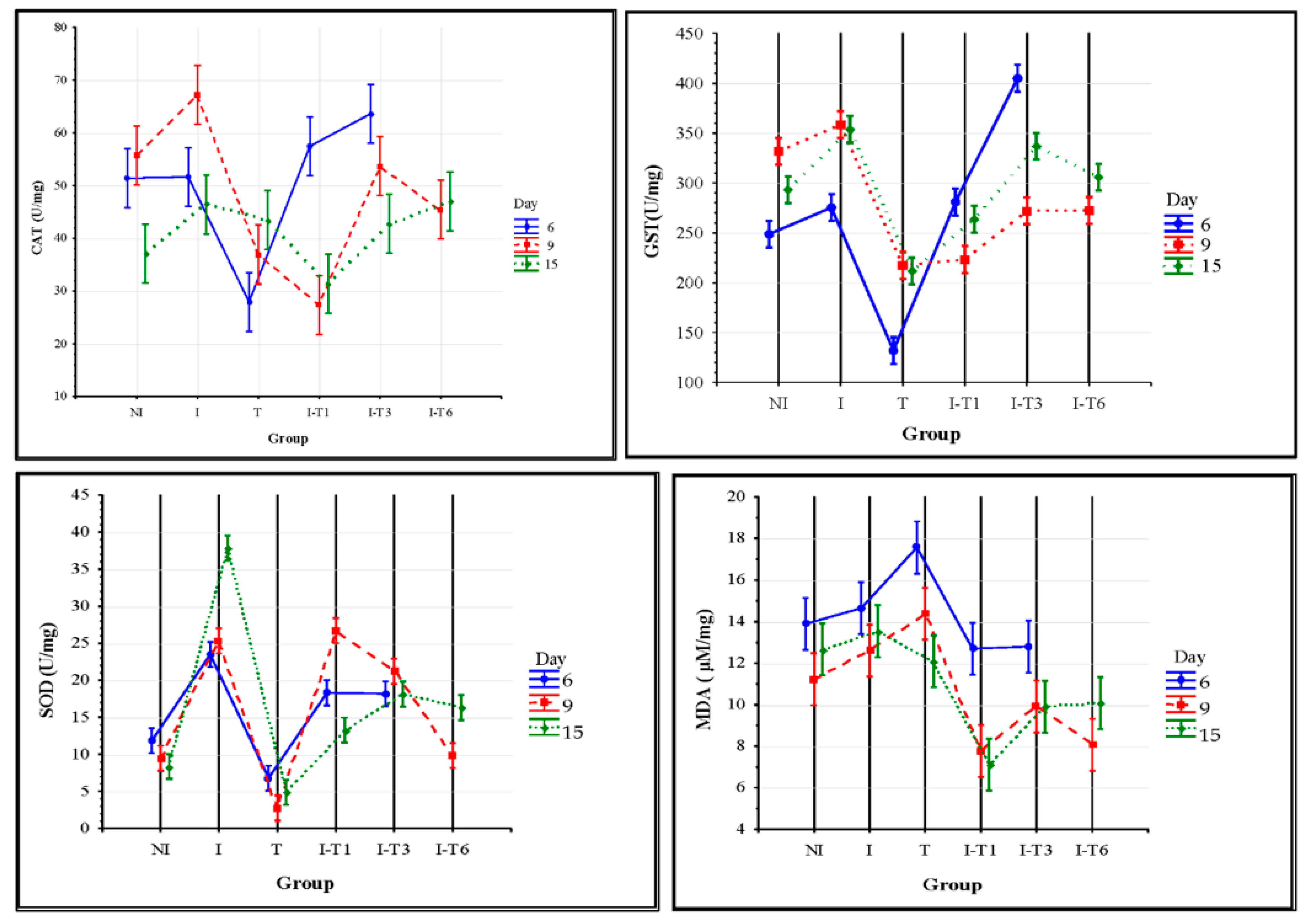
Figure 4.
Activities of superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferase (GST) and malondialdehyde (MDA) concentration at different time points in experimental groups. Non-infected control (NI) group, N. ceranae-infected control (I) group, thymol-treatment control group (T), and groups infected and supplemented with thymol from day 1 (I-T1), day 3 (I-T3), and day 6 (I-T6).
At the end of the experiment (on day 15), the thymol consumed through the sucrose syrup exerted the best anti-Nosema effect (observed through the number of spores, Figure 2). Moreover, the results of the activity and concentrations of monitored oxidative stress parameters revealed the best effect of thymol consumption on day 15 (Figure 4). The activities of all antioxidative enzymes (SOD, CAT, and GST) and MDA concentrations were significantly higher according to Tukey’s test (p < 0.05) in the infected (I) group compared with the majority of the other groups (Figure 4). The lowest activities of antioxidative enzymes (SOD, GST, and CAT) were detected in the groups supplemented with thymol from the first day of experiment (T and I-T1), which were lower than the majority of the other groups (Tukey’s test: p < 0.05). The reason for these findings could be the balance in the redox potential of thymol-fed bees and in the bees’ success in controlling the antioxidative response. In the infected control (I) group (which did not receive thymol), N. ceranae successfully induced oxidative stress, which was detected by increased levels of SOD, CAT, GST, and MDA at the end of the experiment (Figure 4). Oxidative stress induced by different stressors, such as N. ceranae [15,60] and environmental pollutants [61,62], was also reported in some previous studies.
The expression levels of the apidaecin and hymenoptaecin genes on day 15 were significantly higher (Mann–Whitney U test: p ≤ 0.021) in all thymol-treated groups compared with the infected control (I) group. Apidaecin-gene expression levels were lower (p ≤ 0.036) in the T group (thymol-treated and Nosema-free) compared with the infected and thymol-treated bees, while for hymenoptaecin expression levels, no difference was defected (p > 0.05). Bees from the T group had lower values for abaecin and defensin gene expression compared with groups infected and treated with thymol (I-T1, I-T3, and I-T6). The defensin levels in group T were lower (p ≤ 0.022) even when compared with the infected control group I (Figure 3 and Figure S1).
The number of Nosema spores at the end of the experiment (Figure 2) indicates an evident anti-Nosema effect of thymol in our cage experiment. Accordingly, the results of the gene expression levels showed that thymol prevented the suppressive effect of N. ceranae on the expression of immune-related genes. Moreover, thymol treatment reduced the activity of SOD, CAT, and GST, as well as concentration of MDA (Figure 4). The suppression of certain genes in the thymol-treated and Nosema-free group (T) indicates the potential immunosuppressive effect of thymol when given preventively to uninfected bees. The negative impact of thymol on some other insect species has been known; thus, thymol was used for the suppression of the development and survival of adult mosquitoes [63,64] and cockroaches [65]. Gene expression levels for all genes except vitellogenin continuously increased over the experiment throughout the three sampling times in all groups infected with Nosema and treated with thymol (I-T1, I-T3, and I-T6). These findings could be explained by the anti-Nosema activity of thymol [28,47], which subsequently mitigated the immunosuppression caused by Nosema.
The vitellogenin levels in bees collected on day 15 (Figure 3 and Figure S1) were the least altered. There was no difference in vitellogenin expression levels between the infected group (I) and the thymol-treated group (T) with to the other experimental groups (p > 0.05). This indicates that the level of vitellogenin was similar in the group that received thymol and in the groups that did not. Therefore, N. ceranae inhibited the expression of the vitellogenin gene equally to thymol. In addition, no synergistic effect (Nosema or thymol) on the vitellogenin gene expression was noted, keeping in mind that the same level of vitellogenin gene expression was obtained in the groups treated with thymol and infected with Nosema (I-T1, I-T3, and I-T6). Thymol used in Varroa treatment in the study of Boncristiani et al. [43] led to the downregulation of the vitellogenin gene, as well as other genes important for the detoxification and immunity of bees, which were not monitored in our experiment. Changes in the vitellogenin gene expression levels under the influence of thymol has been linked to possible modifications of bee-specific traits that are significantly influenced by vitellogenin [66]. It is also worth noting the rapid effect of thymol application on gene expression in the brain of the honey bee: significant upregulation of the transient-receptor-potential-like (TRPL) gene and downregulation of the octopamine receptor OA1 gene Amoa1 [67].
Initial studies of the effect of thymol on bees infected with Varroa mites [31,46,68] did not report negative effects of thymol on bees. However, further research reported some negative effects of thymol on bees [43,69,70,71], which was one of the reasons for our research.
Our study revealed the positive effects of thymol on the health of Nosema-infected bees without producing negative effects. The proven anti-Nosema effect of thymol and subsequent prevention of Nosema’s negative effects could be beneficial on bees’ health. Moreover, our results indicate that in Nosema-free bees, thymol itself could cause certain disorders (side effects such as inducing oxidative stress, immunosuppression of the monitored genes, and reduction in bee longevity). Keeping in mind the obtained results, one should be careful with the preventive, uncontrolled, and excessive use of thymol. Further research should be conducted in order to determine the possible mechanisms of thymol activity when applied to infected and uninfected bees.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13070574/s1, Figure S1: Expression levels of abaecin, hymenoptaecin, defensin, apidaecin and vitellogenin at different time points (day 6, 9 and 15) in experimental groups. N. ceranae infected control (I) and groups infected and supplemented with thymol from day 1 (I-T1), day 3 (I-T3) and day 6 (I-T6).
Author Contributions
Conceptualization, U.G., J.S., and Z.S.; data curation, U.G., N.L., and M.M.; formal analysis, U.G., J.B., and M.R.; investigation, U.G., J.B., and M.R.; methodology, U.G., M.R., N.L., and Z.S.; resources, Z.S.; software, N.L. and M.M.; supervision, J.S. and Z.S.; validation, U.G.; writing—original draft, U.G. and J.B.; writing—review and editing, J.S., M.M., and Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Ministry of Education of the Science and Technological Development of the Republic of Serbia (Contract no. 451-03-68/2022-14/200143 for the project led by Zoran Stanimirovic).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the study of invertebrates only (honey bees are exempted from ethical review).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to the excessive data size.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Higes, M.; Martín-Hernández, R.; Martínez-Salvador, A.; Garrido-Bailón, E.; González-Porto, A.V.; Meana, A.; Bernal, J.L.; Del Nozal, M.J.; Bernal, J. A preliminary study of the epidemiological factors related to honey bee colony loss in Spain. Environ. Microbiol. Rep. 2010, 2, 243–250. [Google Scholar] [CrossRef]
- Botias, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet. Res. 2013, 44, 25. [Google Scholar] [CrossRef]
- Higes, M.; Meana, A.; Bartolomé, C.; Botías, C.; Martín-Hernández, R. Nosema ceranae (Microsporidia), a controversial 21st century honey bee pathogen. Environ. Microbiol. Rep. 2013, 5, 17–29. [Google Scholar] [CrossRef]
- Simeunovic, P.; Stevanovic, J.; Cirkovic, D.; Radojicic, S.; Lakic, N.; Stanisic, L.J.; Stanimirovic, Z. Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony. J. Apic. Res. 2014, 53, 545–554. [Google Scholar] [CrossRef]
- Stevanovic, J.; Simeunovic, P.; Gajic, B.; Lakic, N.; Radovic, D.; Fries, I.; Stanimirovic, Z. Characteristics of Nosema ceranae infection in Serbian honey bee colonies. Apidologie 2013, 44, 522–536. [Google Scholar] [CrossRef]
- Gisder, S.; Schueler, V.; Horchler, L.L.; Groth, D.; Genersch, E. Long-term temporal trends of Nosema spp. infection prevalence in Northeast Germany: Continuous spread of Nosema ceranae, an emerging pathogen of honey bees (Apis mellifera), but no general replacement of Nosema apis. Front. Cell. Infect. Microbiol. 2017, 7, 301. [Google Scholar] [CrossRef]
- Taric, E.; Glavinic, U.; Vejnovic, B.; Stanojkovic, A.; Aleksic, N.; Dimitrijevic, V.; Stanimirovic, Z. Oxidative stress, endoparasite prevalence and social immunity in bee colonies kept traditionally vs. those kept for commercial purposes. Insects 2020, 11, 266. [Google Scholar] [CrossRef]
- Stanimirovic, Z.; Stevanovic, J.; Bajic, V.; Radovic, I. Evaluation of genotoxic effects of fumagillin (dicyclohexylamine) by citogenetic tests in vivo. Mutat. Res. 2006, 628, 1–10. [Google Scholar]
- Stevanovic, J.; Stanimirovic, Z.; Radakovic, M.; Stojic, V. In vitro evaluation of the clastogenicity of fumagillin. Environ. Mol. Mutagen. 2008, 49, 594–601. [Google Scholar] [CrossRef]
- Van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Fumagillin: An overview of recent scientific advances and their significance for apiculture. J. Agric. Food Chem. 2014, 62, 2728–2737. [Google Scholar] [CrossRef]
- Chaimanee, V.; Kasem, A.; Nuanjohn, T.; Boonmee, T.; Siangsuepchart, A.; Malaithong, W.; Sinpoo, C.; Disayathanoowat, T.; Pettis, J.S. Natural extracts as potential control agents for Nosema ceranae infection in honeybees, Apis mellifera. J. Invertebr. Pathol. 2021, 186, 107688. [Google Scholar] [CrossRef]
- Braglia, C.; Alberoni, D.; Porrini, M.P.; Garrido, M.P.; Baffoni, L.; Di Gioia, D. Screening of Dietary Ingredients against the Honey Bee Parasite Nosema ceranae. Pathogens 2021, 10, 1117. [Google Scholar] [CrossRef]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar] [CrossRef]
- Glavinic, U. The Effects of Various Antimicrobials and Supplements on the Expression of Immune-Related Genes, Oxidative Stress and Survival of Honey Bee Apis mellifera Infected with Microsporidium Nosema ceranae. Ph.D. Thesis, Faculty of Veterinary Medicine, University of Belgrade, Belgrade, Serbia, 2019. [Google Scholar]
- Glavinic, U.; Stevanovic, J.; Ristanic, M.; Rajkovic, M.; Davitkov, D.; Lakic, N.; Stanimirovic, Z. Potential of Fumagillin and Agaricus blazei Mushroom Extract to Reduce Nosema ceranae in Honey Bees. Insects 2021, 12, 282. [Google Scholar] [CrossRef]
- Glavinic, U.; Rajkovic, M.; Vunduk, J.; Vejnovic, B.; Stevanovic, J.; Milenkovic, I.; Stanimirovic, Z. Effects of Agaricus bisporus Mushroom Extract on Honey Bees Infected with Nosema ceranae. Insects 2021, 12, 915. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Delic, B.; Vejnovic, B.; Aleksic, N.; Mladjan, V.; Stanimirovic, Z. Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance. Prev. Vet. Med. 2021, 190, 105322. [Google Scholar] [CrossRef]
- Schulz, M.; Łoś, A.; Grzybek, M.; Ścibior, R.; Strachecka, A. Piperine as a new natural supplement with beneficial effects on the life-span and defence system of honeybees. J. Agric. Sci. 2019, 157, 140–149. [Google Scholar] [CrossRef]
- Cristina, R.T.; Kovačević, Z.; Cincović, M.; Dumitrescu, E.; Muselin, F.; Imre, K.; Militaru, D.; Mederle, N.; Radulov, I.; Hădărugă, N.; et al. Composition and efficacy of a natural phytotherapeutic blend against Nosemosis in honey bees. Sustainability 2020, 12, 5868. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Załuski, D. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. roots: A new hope against honeybee death caused by nosemosis. Molecules 2020, 25, 4452. [Google Scholar] [CrossRef]
- Shumkova, R.; Balkanska, R.; Hristov, P. The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An alternative therapy for N. ceranae infection and its effects on honey bee strength and production traits. Pathogens 2021, 10, 234. [Google Scholar] [CrossRef]
- Shumkova, R.; Balkanska, R.; Koynarski, T.; Hristov, P. Application of the natural products NOZEMAT HERB and NOZEMAT HERB PLUS can decrease honey bee colonies losses during the winter. Diversity 2021, 13, 228. [Google Scholar] [CrossRef]
- Jack, C.J.; Ellis, J.D. Integrated pest management control of Varroa destructor (Acari: Varroidae), the most damaging pest of (Apis mellifera L. (Hymenoptera: Apidae)) Colonies. J. Insect Sci. 2021, 21, 6. [Google Scholar] [CrossRef]
- Stanimirovic, Z.; Glavinic, U.; Jovanovic, N.M.; Ristanic, M.; Milojković-Opsenica, D.; Mutic, J.; Stevanovic, J. Preliminary trials on effects of lithium salts on Varroa destructor, honey and wax matrices. J. Apicult. Res. 2022, 61, 375–391. [Google Scholar] [CrossRef]
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; Martín-Hernández, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite N. ceranae by methanolic extracts from Chilean native plants and propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of Api-Bioxal® and ApiHerb® treatments against N. ceranae Infection in Apis mellifera investigated by two qPCR methods. Vet. Sci. 2020, 7, 125. [Google Scholar] [CrossRef]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Kladar, N.; Cabarkapa, I.; Radinovic, M.; Maletic, M.; Erdeljan, M.; Bozin, B. New Perspective of Origanum vulgare L. and Satureja montana L. Essential Oils as Bovine Mastitis Treatment Alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef]
- Chiesa, F.; D’agaro, M. Effective control of varroatosis using powdered thymol. Apidologie 1991, 22, 135–145. [Google Scholar] [CrossRef][Green Version]
- Imdorf, A.; Kilchenmann, V.; Bogdanov, S.; Bachofen, B.; Beretta, C. Toxizität von thymol, campher, menthol und eucalyptol auf Varroa jacobsoni oud und Apis mellifera L. im labortest (Toxic effects of thymol, camphor, menthol and eucalyptol on Varroa jacobsoni Oud and Apis mellifera L. in a laboratory test.). Apidologie 1995, 26, 27–31. [Google Scholar] [CrossRef]
- Stanimirovic, Z.; Glavinic, U.; Ristanic, M.; Aleksic, N.; Jovanovic, N.M.; Vejnovic, B.; Stevanovic, J. Looking for the causes of and solutions to the issue of honey bee colony losses. Acta Vet. 2019, 69, 1–31. [Google Scholar] [CrossRef]
- Rice, R.N. Nosema Disease in Honeybees: Genetic Variation and Control. Report n. 01/46; Australian Government, Rural Industries Research and Development Corporation: Barton, Australia, 2001.
- Yücel, B.; Doğaroğlu, M. The impact of Nosema apis Z. infestation of honey bee (Apis mellifera L.) colonies after using different treatment methods and their effects on the population levels of workers and honey production on consecutive years. Pak. J. Biol. Sci. 2005, 8, 1142–1145. [Google Scholar] [CrossRef][Green Version]
- Palmer-Young, E.C.; Tozkar, C.Ö.; Schwarz, R.S.; Chen, Y.; Irwin, R.E.; Adler, L.S.; Evans, J.D. Nectar and pollen phytochemicals stimulate honey bee (Hymenoptera: Apidae) immunity to viral infection. J. Econ. Entomol. 2017, 110, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Česnik, H.B.; Kmecl, V. Investigation on amitraz, coumaphos and thymol concentrations in honey produced by Slovenian beekeepers in 2020. Acta Agric. Slov. 2021, 117, 1–7. [Google Scholar] [CrossRef]
- Tihelka, E. Effects of synthetic and organic acaricides on honey bee health: A review. Slov. Vet. Res. 2018, 55, 114–140. [Google Scholar] [CrossRef]
- Sánchez, L.M.; Ramos, M.J.G.; del Mar Gómez-Ramos, M.; Vazquez, P.P.; Flores, J.M. Presence, persistence and distribution of thymol in honeybees and beehive compartments by high resolution mass spectrometry. Environ. Adv. 2021, 5, 100085. [Google Scholar] [CrossRef]
- Kast, C.; Kilchenmann, V.; Charrière, J.D. Long-term monitoring of lipophilic acaricide residues in commercial Swiss beeswax. Pest Manag. Sci. 2021, 77, 4026–4033. [Google Scholar] [CrossRef]
- Price, K.L.; Lummis, S.C. An atypical residue in the pore of Varroa destructor GABA-activated RDL receptors affects picrotoxin block and thymol modulation. Insect Biochem. Mol. Biol. 2014, 55, 19–25. [Google Scholar] [CrossRef]
- Colin, T.; Plath, J.A.; Klein, S.; Vine, P.; Devaud, J.M.; Lihoreau, M.; Meikle, W.G.; Barron, A.B. The miticide thymol in combination with trace levels of the neonicotinoid imidacloprid reduces visual learning performance in honey bees (Apis mellifera). Apidologie 2020, 51, 499–509. [Google Scholar] [CrossRef]
- Bergougnoux, M.; Treilhou, M.; Armengaud, C. Exposure to thymol decreased phototactic behaviour in the honeybee (Apis mellifera) in laboratory conditions. Apidologie 2012, 44, 82–89. [Google Scholar] [CrossRef]
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Aronstein, K.A.; Chen, Y.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.S.; Evans, J.D. Single and mixed-species trypanosome and microsporidia infections elicit distinct, ephemeral cellular and humoral immune responses in honey bees. Dev. Comp. Immunol. 2013, 40, 300–310. [Google Scholar] [CrossRef]
- Ebert, T.A.; Kevan, P.G.; Bishop, B.L.; Kevan, S.D.; Downer, R.A. Oral toxicity of essential oils and organic acids fed to honey bees (Apis mellifera). J. Apicult. Res. 2007, 46, 220–224. [Google Scholar] [CrossRef]
- Costa, C.; Lodesani, M.; Maistrello, L. Effect of thymol and resveratrol administered with candy or syrup on the development of Nosema ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 2010, 41, 141–150. [Google Scholar] [CrossRef]
- Lenga, R.E. The Sigma-Aldrich Library of Chemical Safety Data; Sigma-Aldrich Corporation: St. Louis, MO, USA, 1988. [Google Scholar]
- EC (2007) Council Regulation, no. Regulation (EEC) No 834/2007 of the European Parliament and of the Council of 28 June 2007 on organic production and labeling of organic products and repealing Regulation (EEC) No 2454/93 2092/91. Off. J. Eur. Union 2007, 89, 23. [Google Scholar]
- Glavinic, U.; Tesovnik, T.; Stevanovic, J.; Zorc, M.; Cizelj, I.; Stanimirovic, Z.; Narat, M. Response of adult honey bees treated in larval stage with prochloraz to infection with Nosema ceranae. PeerJ 2019, 7, e6325. [Google Scholar] [CrossRef] [PubMed]
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, P.D.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apicult. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Martín-Hernandez, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microb. 2007, 73, 6331–6338. [Google Scholar] [CrossRef]
- OIE–Office International Des Epizooties. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Chapter 2.2.4. Nosemosis of Honey Bees. 2018. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.02.04_NOSEMOSIS_FINAL.pdf (accessed on 4 May 2022).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Martín-Hernández, R.; Meana, A.; Higes, M. Critical aspects of the Nosema spp. diagnostic sampling in honey bee (Apis mellifera L.) colonies. Parasitol. Res. 2011, 110, 2557–2561. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, P.; Guzman-Novoa, E.; Goodwin, P.H. Effect of immune inducers on Nosema ceranae multiplication and their impact on Honey Bee (Apis mellifera L.) survivorship and behaviors. Insects 2020, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Control of the microsporidian parasite Nosema ceranae in honey bees (Apis mellifera) using nutraceutical and immuno-stimulatory compounds. PLoS ONE 2020, 15, e0227484. [Google Scholar] [CrossRef]
- Van den Heever, J.P.; Thompson, T.S.; Otto, S.J.G.; Curtis, J.M.; Ibrahim, A.A.; Pernal, S.F. Evaluation of Fumagilin-B® and other potential alternative chemotherapies against Nosema ceranae-infected honeybees (Apis mellifera) in cage trial assays. Apidologie 2016, 47, 617–630. [Google Scholar] [CrossRef]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.L.; Texier, C.; Biron, D.G.; Blot, N.; Alaoui, H.E.; et al. Exposure to Sublethal Doses of Fipronil and Thiacloprid Highly Increases Mortality of Honeybees Previously Infected by Nosema ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar]
- Nikolić, T.V.; Purać, J.; Orčić, S.; Kojić, D.; Vujanović, D.; Stanimirović, Z.; Grzetic, I.; Ilijevic, K.; Sikoparija, B.; Blagojević, D.P. Environmental effects on superoxide dismutase and catalase activity and expression in honey bee. Arch. Insect Biochem. Physiol. 2015, 90, 181–194. [Google Scholar] [CrossRef]
- Orčić, S.; Nikolić, T.; Purać, J.; Šikoparija, B.; Blagojević, D.P.; Vukašinović, E.; Plavsa, N.; Stevanovic, J.; Kojić, D. Seasonal variation in the activity of selected antioxidant enzymes and malondialdehyde level in worker honey bees. Entomol. Exp. Appl. 2017, 165, 120–128. [Google Scholar] [CrossRef]
- Pavela, R.; Vrchotová, N.; Tříska, J. Mosquitocidal activities of thyme oils (Thymus vulgaris L.) against Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2009, 105, 1365–1370. [Google Scholar] [CrossRef]
- Tabanca, N.; Bernier, U.R.; Tsikolia, M.; Becnel, J.J.; Sampson, B.; Werle, C.; Demirci, B.; Baser, K.H.C.; Blythe, E.K.; Pounders, C.; et al. Eupatorium capillifolium essential oil: Chemical composition, antifungal activity, and insecticidal activity. Nat. Prod. Commun. 2010, 5, 1934578X1000500913. [Google Scholar] [CrossRef]
- Phillips, A.K.; Appel, A.G.; Sims, S.R. Topical toxicity of essential oils to the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 2010, 103, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, G.; Vidau, C.; Ferdy, J.B.; Tabart, J.; Vetillard, A. Lethal and sub-lethal effects of thymol on honeybee (Apis mellifera) larvae reared in vitro. Pest Manag. Sci. 2014, 70, 140–147. [Google Scholar] [CrossRef]
- Bonnafé, E.; Drouard, F.; Hotier, L.; Carayon, J.L.; Marty, P.; Treilhou, M.; Armengaud, C. Effect of a thymol application on olfactory memory and gene expression levels in the brain of the honeybee Apis mellifera. Environ. Sci. Pollut. Res. 2015, 22, 8022–8030. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Otis, G.W.; Daley, J.; Schulz, T. Trials of apiguard, a thymol-based miticide part 2. Non-target effects on honey bees. Am. Bee J. 2000, 140, 68–70. [Google Scholar]
- Mutinelli, F.; Baggio, A. Use of medical drugs against varroosis. Apiacta 2004, 39, 53–62. [Google Scholar]
- Gashout, H.A.; Guzmán-Novoa, E. Acute toxicity of essential oils and other natural compounds to the parasitic mite, Varroa destructor, and to larval and adult worker honey bees (Apis mellifera L.). J. Apic. Res. 2009, 48, 263–269. [Google Scholar] [CrossRef]
- Toomemaa, K. The synergistic effect of weak oxalic acid and thymol aqueous solutions on Varroa mites and honey bees. J. Apic. Res. 2019, 58, 37–52. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).