Simple Summary
The subfamily Eumeninae comprises more than 3900 described species and eumenine mitochondrial analyses are almost absent. In order to provide further evidence toward understanding the relationships within the subfamily, the characteristics of 54 eumenine mitogenomes were comparatively analyzed, among which 52 mitogenomes are newly annotated. Meanwhile, using both Maximum-likelihood (ML) and Bayesian inference (BI), comprehensive phylogenetic relationship in the subfamily were investigated based on two mitochondrial datasets.
Abstract
The subfamily Eumeninae plays a significant role in the biological control of agricultural pests. However, the characteristics of eumenine mitogenomes that are important molecular markers for phylogenetics are not clearly revealed. Here, 52 eumenine mitogenomes are newly sequenced and annotated, and the phylogenetic relationships of the subfamily are comprehensively analyzed based on 87 vespid mitogenomes. Through the comparative analysis of the 54 eumenine mitogenomes, the gene compositions of about one half of the 54 species match with ancestral insect mitogenome, and remaining others contain two trnM which are highly similar, with 51.86% (Eumenes tripunctatus) to 90.65% (Pseumenes nigripectus) sequence identities, which is unique among the reported mitogenomes of the family Vespidae. Moreover, the translocation trnL1 upstream of nad1 is a common rearrangement event in all eumenine mitogenomes. The results of phylogenetic analyses support the paraphyly of the subfamily Eumeninae and the tribe Odynerini, respectively, and the monophyly of the tribe Eumenini, and verify that the tribe Zethini is a valid subfamily Zethinae. In this study, the relationships between some genera such as Allorhynchium and Pararrhynchium or the taxonomic status of the subgenera such as Eremodynerus and Dirhynchium are found to be confusing and there should be further inquiry with more samples.
1. Introduction
The subfamily Eumeninae containing more than 3900 described species is the biggest of the family Vespidae (Hymenoptera). It is the primary lineage of the Vespidae [1], which plays a significant role in the biological control of agricultural pests because of its cosmopolitan predation of the larvae of Lepidoptera (e.g., Geometridae, Tortricidae) and Coleoptera (e.g., Chrysomelidae and Curculionidae) [2,3]. Most of them are solitary wasps, using mud to partition the cells, while some are primitively social, burrowing in the soil or wood, many species in Zethini construct their nests by exploiting masticated and salivated plant material such as Zethus, Ischnocoelia, Elimus, Discoelius, and Protodiscoelius using vegetable matter for cell partition, and Psiliglossa and Raphiglossa using pith [4,5]. Additionally, the high morphological diversity and complexity of Eumeninae leads to some difficulties in its classification, and some other difficulties may be attributed to its troubled taxonomic history [6]. Hence, the early classifications of this subfamily underwent a radical transformation. Based on the morphology of the mouthparts and the general shape of the metasoma, Latreille began the generic classification of Eumeninae and divided the current species of Eumeninae into three genera: Eumenes, Odynerus, and Synagris [7]. Later, de Saussure divided ‘Euméniens’ into Anomaloptéres, Euptéres, and Mischoptéres, after that he separated Zethus, Calligaster, and Discoelius from the rest of his section ‘Euptéres’ of the ‘Eumhiens’ as the group ‘Zethites’ [8,9]. Thereafter, Richards proposed a classification for the “Eumenidae” with three subfamilies: Raphiglossinae, Discoeliinae (=Zethinae), and Eumeninae [10], while Carpenter did not recognize the names Zethinae and Raphiglossinae after investigating the relationships among the subfamilies of the Vespidae with a cladistic treatment [11]. Recently, Hermes et al. corroborated the monophyly of Eumeninae and proposed three tribes of this subfamily, namely Zethini, Eumenini, and Odynerini, which was the most comprehensive classification of the Eumeninae based on morphology [12]. Whether the tribe Zethini should be upgraded to the subfamily Zethinae and the subfamily Eumeninae is monophyletic are worthy of further exploration. Additionally, with the continuous enrichment of molecular data, controversies over the classifications of Eumeninae appear in the disagreement between morphological and molecular data.
In the published research on phylogenetic relationships in the subfamily Eumeninae, some molecular data have been utilized. The first molecular study showed that the solitary Eumeninae was a sister taxon to the Polistinae + Vespinae cluster leveraged on nuclear 28S rDNA and mitochondrial 16S rDNA of 12 species from the family Vespidae including 3 eumenine species [13]. Thereafter, based on the analysis of four nuclear gene fragments (18S rDNA, 28S rDNA, abdominal-A, and RNA polymerase II) from 27 Vespidae species (containing 11 eumenine species), Hines et al. supported the division of Eumeninae into two separate monophyletic clades: “Zethinae” and “Eumeninae” [5]. Neither of the two studies clarified the generic relationships of Eumeninae because only a few species of Eumeninae were contained. Later, with a total of 49 transcriptomes of vespid wasps (containing 40 eumenine species), Bank et al. suggested the subfamily Eumeninae was paraphyletic and the “Zethini” were divided into two clades: Raphiglossinae and Zethinae [14]. Then, Piekarski et al. also suggested that “Zethini” should be a valid subfamily Zethinae [15]. So far, several nuclear gene fragments, mitochondrial fragments, and transcriptomes have not completely resolved the phylogenetic relationships of Eumeninae due to insufficient and unrepresentative sampling of taxa. Thus, to understand the evolution of the various biologies exhibited by Eumeninae, robust investigations of phylogenetic relationships are still needed.
The mitogenome is a widely accepted molecular marker used in phylogenetic studies due to its maternal inheritance as well as the higher rate of nucleotide substitution compared with nuclear DNA [16,17]. To date, two mitogenomes of Eumeninae have been published, which is insufficient to explore phylogenetic relationships [18]. In China, which spans two faunal regions (Palearctic and Oriental Regions), there is a total of more than 310 known species and subspecies in 58 genera of the subfamily Eumeninae [19,20,21,22,23,24,25,26,27,28,29,30], which constitutes a quarter of the total genera of the world. To clarify the phylogenetic relationships within Eumeninae, especially the placement of Zethini, 52 new mitogenomes of 33 genera of Eumeninae from China are obtained and analyzed by combining 35 published vespid mitogenomes in this study. Given that gene rearrangements are very informative for phylogenetic analysis and are exhibited extensively in some insect orders [17], these eumenine mitogenomes are compared with the gene order of ancestral mitogenome to investigate their distinctive rearrangement models. Additionally, the characters of the eumenine mitogenomes are compared with other vespids, in order to identify any unique character to support the classification of Eumeninae.
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
The 52 species of the subfamily Eumeninae, which were firstly identified at least to genus level by taxonomic specialists, were collected and stored in 95% ethanol at −20 °C in Chongqing Normal University, Chongqing, China (Table 1). Thereafter, total DNA was isolated from the muscle tissues of the thorax using the DNeasy DNA Extraction kit (Qiagen) and according to its instructions. Finally, we followed the manufacturer’s protocol of the Qubit dsDNA high-sensitivity kit (Invitrogen) to determine the DNA concentration for each sample.

Table 1.
GenBank accession numbers for mitogenomes of Eumeninae newly sequenced and annotated in this study.
2.2. Whole-Genome Sequencing and Assembling
The Illumina TruSeq library, containing an average size of 350 bp, was sequenced using the Illumina Hiseq 2500 platform at BerryGenomics (Beijing, China). Then, high-quality reads (after deletion of low-quality reads) were used in de novo assembly with IDBA-UD by using the NGS QC Toolkit [31,32]. COX1 and srRNA were amplified by standard PCR reactions and were used to identify mitogenome assemblies with at least 98% similarity sequences in BLAST [33,34]. Finally, the accuracy of the assembly was investigated by mapping clean reads onto the obtained mitochondrial scaffold in each library using Geneious 10.1.3 (http://www.geneious.com/. Accessed date: 12 January 2022), which allowed for up to 2% mismatches, a maximum gap size of 3 bp, and a minimum overlap of 100 bp.
2.3. Mitogenome Annotation and Sequence Analysis
Annotation of the assembled mitochondrial sequences was identified using Clustal X 1.8 with homologous sequences against the publicly available Eumeninae mitogenomes [35]. Unrecognized tRNA genes were found by use of tRNA scan-SE version 2.0.2 and secondary structure modeling was completed using ARWEN version 1.2 [36,37]. The nucleotide composition, AT content, GC-skew, and the Relative Synonymous Codon Usage (RSCU) were calculated in MEGA X [38]. Effective Number of Codons (ENC) and GC of silent 3rd codon posit (GC3s) were computed in Codon W 1.4 and non-synonymous (Ka) and synonymous (Ks) substitution ratio (Ka/Ks) of PCGs were calculated in DnaSP 5.0 [39,40]. Then, the gene arrangement events were detected in CREx [41].
2.4. Phylogenetic Analyses
A total of 87 Vespidae mitogenomes containing 52 newly sequenced eumenine mitogenomes and 35 species of the subfamilies Eumeninae, Stenogastrinae (three species), Polistinae (19 species), and Vespinae (11 species) downloaded from GenBank were selected as ingroups, and four species from Apoidea (Hylaeus dilatatus, Andrena cineraria, Megachile sculpturalis, and Apis cerana) were selected as outgroups (Table S1). In total, 13 PCGs and 2 rRNAs were extracted by PhyloSuite v 1.2.2 [42]. The individual alignments of PCGs were performed using the L-INS-i strategy of the MAFFT algorithm executed in the TranslatorX online platform, and rRNA genes were aligned individually using the G-INS-i strategy implemented in MAFFT version 7.205 [43,44]. GBlocks v.0.91b was used to remove all ambiguously aligned sites from 13 PCGs and two rRNAs [45]. After that, MEGA X was used to check and correct all the alignments [38].
Phylogenetic trees were inferred from two sets of data: (1) PCGR: 13 PCGs and 2 rRNAs; (2) PCG: 13 PCGs. Before the construction of trees, PartitionFinder version 2.1.1 [46] was used to simultaneously choose the best partition schemes and substitution models for each matrix with the Akaike Information Criterion (AIC) and greedy search algorithm (Table S2). A Bayesian inference (BI) tree was constructed in MrBayes v.3.2.7, approximately 10,000,000 generations were conducted for the matrix, with the average deviation of split frequencies below 0.01 which suggests that runs reach convergence and were sampled every 1000 generations with a burn-in of 25% [47]. Maximum likelihood (ML) was constructed on the PhyML online web server (http://www.atgc-montpellier.fr/phyml/. Accessed date: 12 January 2022) and the node support values were evaluated via a bootstrap test with 100 replicates [48]. In addition, for (maximum parsimony) MP, the matrix was analyzed through the use of Winclada slaving TNT [49,50]. New technology search algorithms were used with the default settings, except ratchet 200 iterations, with up:down perturbation 8:4; hits to minimum length 25. Bootstrapping was via traditional search, with 100 replicates.
3. Results and Discussion
3.1. Mitochondrial Genome Organization
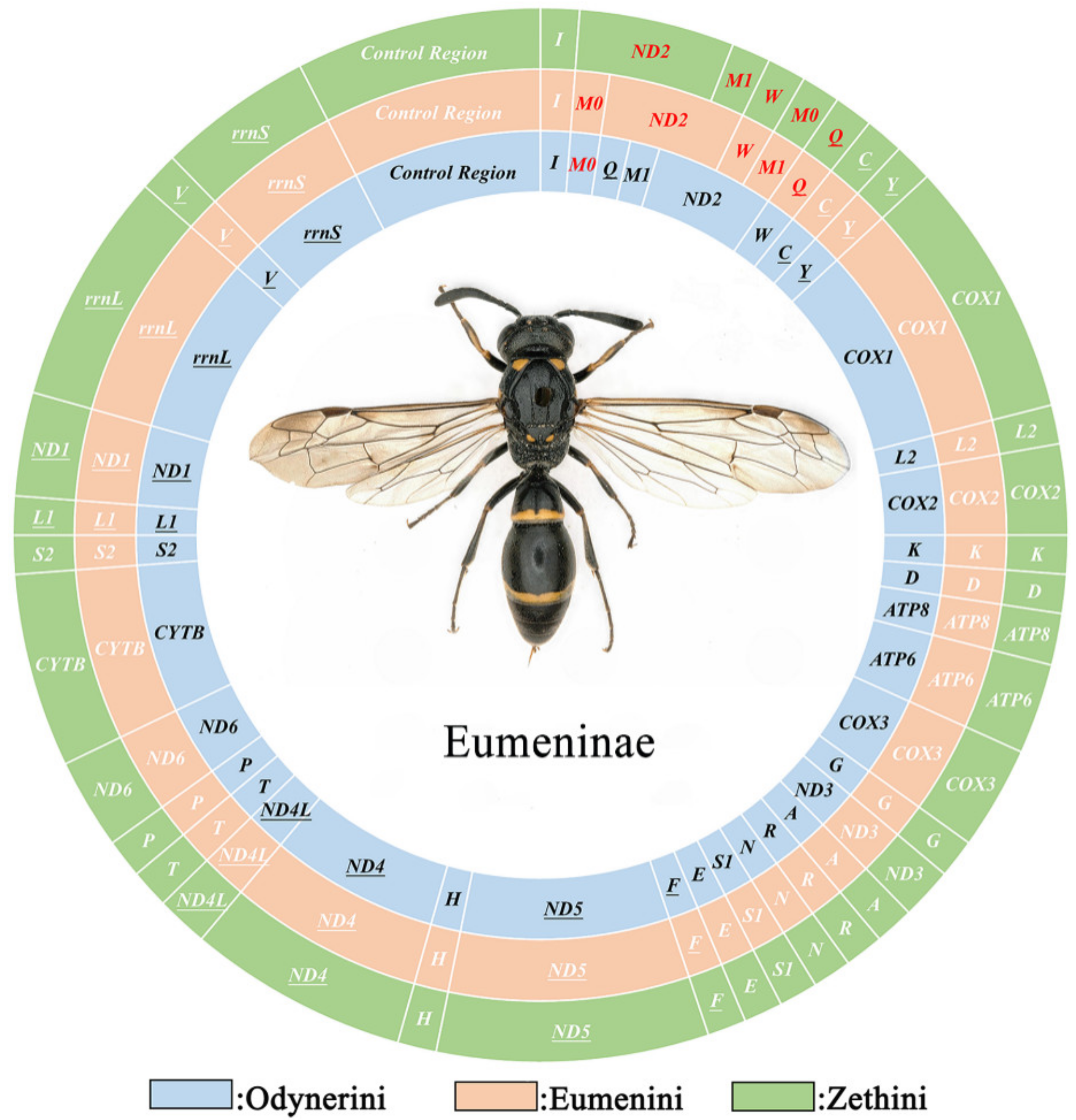
We obtained 52 complete or partial mitogenomes, which were deposited in GenBank (Table 1). Most of them include 13 protein-coding genes (PCGs), 2 rRNA genes (rRNAs), a control region, and 22 or 23 tRNA genes (tRNAs), with a size from 14,241 (Leptochilus sp.) to 23,251 bp (Rhynchium brunneum brunneum) and some of the entire A+T rich regions as well as three tRNA genes (trnI, trnQ and trnM) were unable to be amplified in 23 mitogenomes (Figure S1). The composition of 29 complete mitogenomes are significantly biased toward adenine and thymine, with high A+T content from 78.6% (Subancistrocerus camicrus) to 84.7% (Eumenes pomiformis) which is similar to other hymenopteran mitogenomes [51] and the AT skews are from −0.09 (Pararrhynchium striatum) to 0.19 (Antepipona sp.) (Table S3).
Typically, the mitogenomes of metazoan animals are double-strand circular DNA composed of 37 genes including 13 PCGs, 22 tRNAs, 2 rRNAs, and a control region, and most genes are located on the J-strand (major strand), the remaining being on the N-strand [52]. In this study, some mitogenomes of Eumeninae generally match that of the inferred mitogenomes except for some trnM duplications (Figure 1).
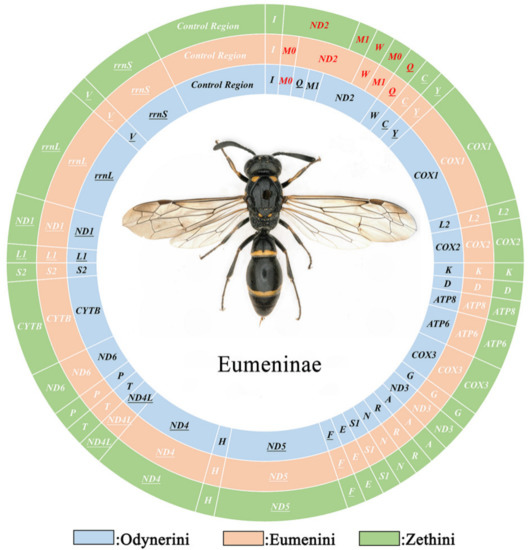
Figure 1.
The mitochondrial genomes of Eumeninae. Circles of different colors indicate different tribes of Eumeninae. The red gene means its position is inconsistent with the ancestor insect.
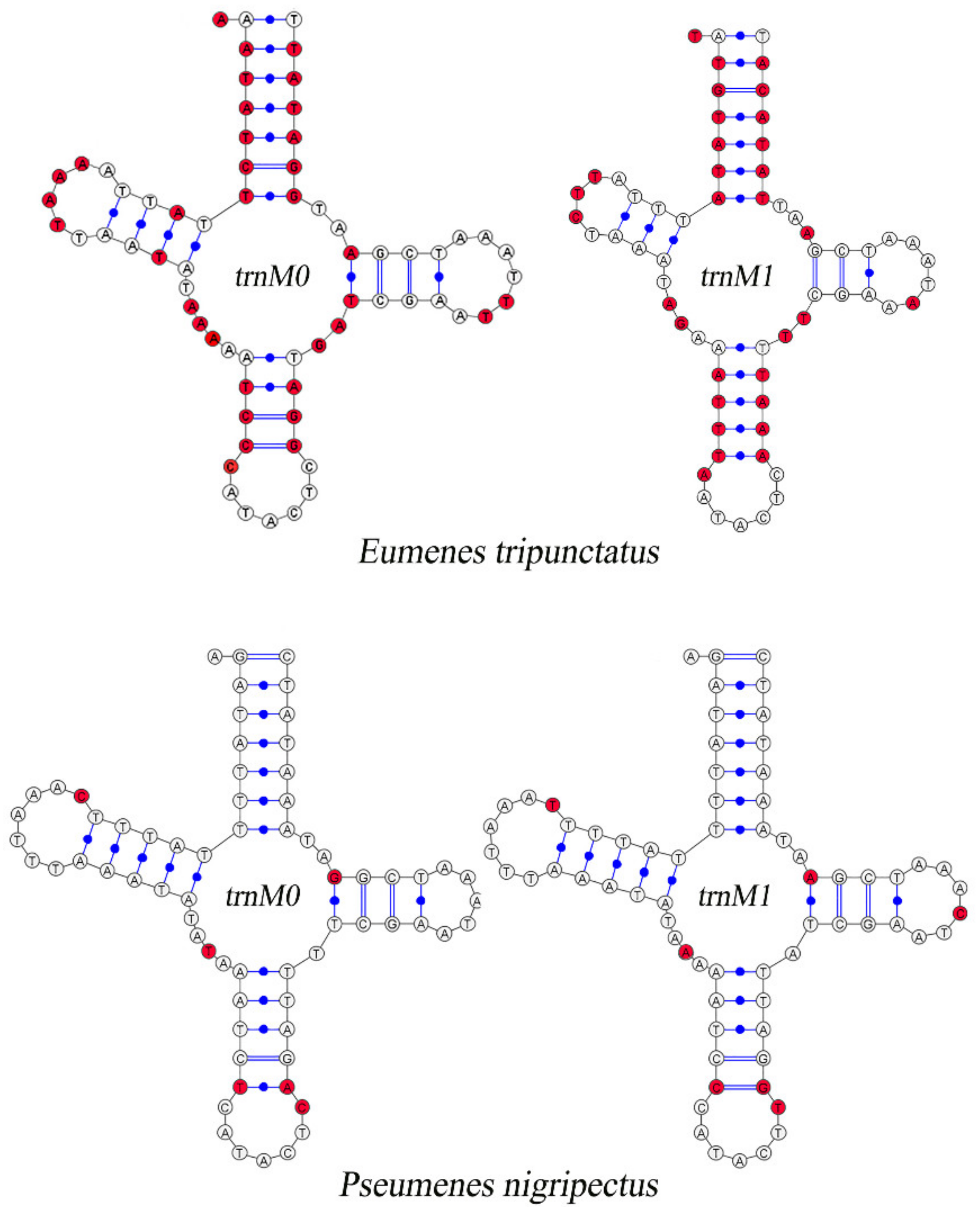
Compared with other Vespidae, there are 26 in total in the 52 newly assembled mitogenomes of Eumeninae containing two trnM genes which are highly similar, with 51.86% (Eumenes tripunctatus) to 90.65% (Pseumenes nigripectus) sequence identities (Figure S2). The substitutions between trnM0 and trnM1 are identified in the Amino Acid acceptor (AA) arm, TψC (T) arm, Variable (V) loop, Anticodon (AC) arm, and the dihydorouridine (DHU) arm (Figure 2). The positions of trnM0 and trnM1 are different: some are connected and others are separated by trnQ and trnW. The duplication event is unique in the subfamily Eumeninae among the reported mitogenomes of Vespidae; meanwhile, it was reported in the mitogenomes of both Ibalia leucospoides (Hymenoptera: Cynipoidea) containing three trnM with 92–97% sequence identities and the genus Pachycephus (Hymenoptera: Cephidae) [18,53,54]. Moreover, there is another duplication of trnL2 within the Eumeninae such as three regions of noncoding DNA containing four copies of trnL2 in Abispa ephippium [55]. According to the existing reports, the duplication of tRNA is common in Hymenoptera; for instance, the copies of trnD, trnA, and trnE in the family Cephidae (Hymenoptera) and Trigonalyoidea (Hymenoptera), respectively [18,53]. Therefore, within the family Vespidae, the duplication of trnM may be one of the features to indicate whether a species belongs to the subfamily Eumeninae.
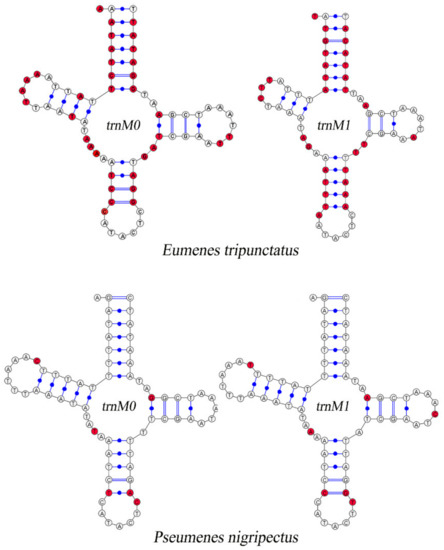
Figure 2.
Inferred secondary structures of duplicated trnM. The substitutions in trnM0 and trnM1 compared with each other are indicated by red color.
3.2. Protein-Coding Genes and Codon Usage Patterns
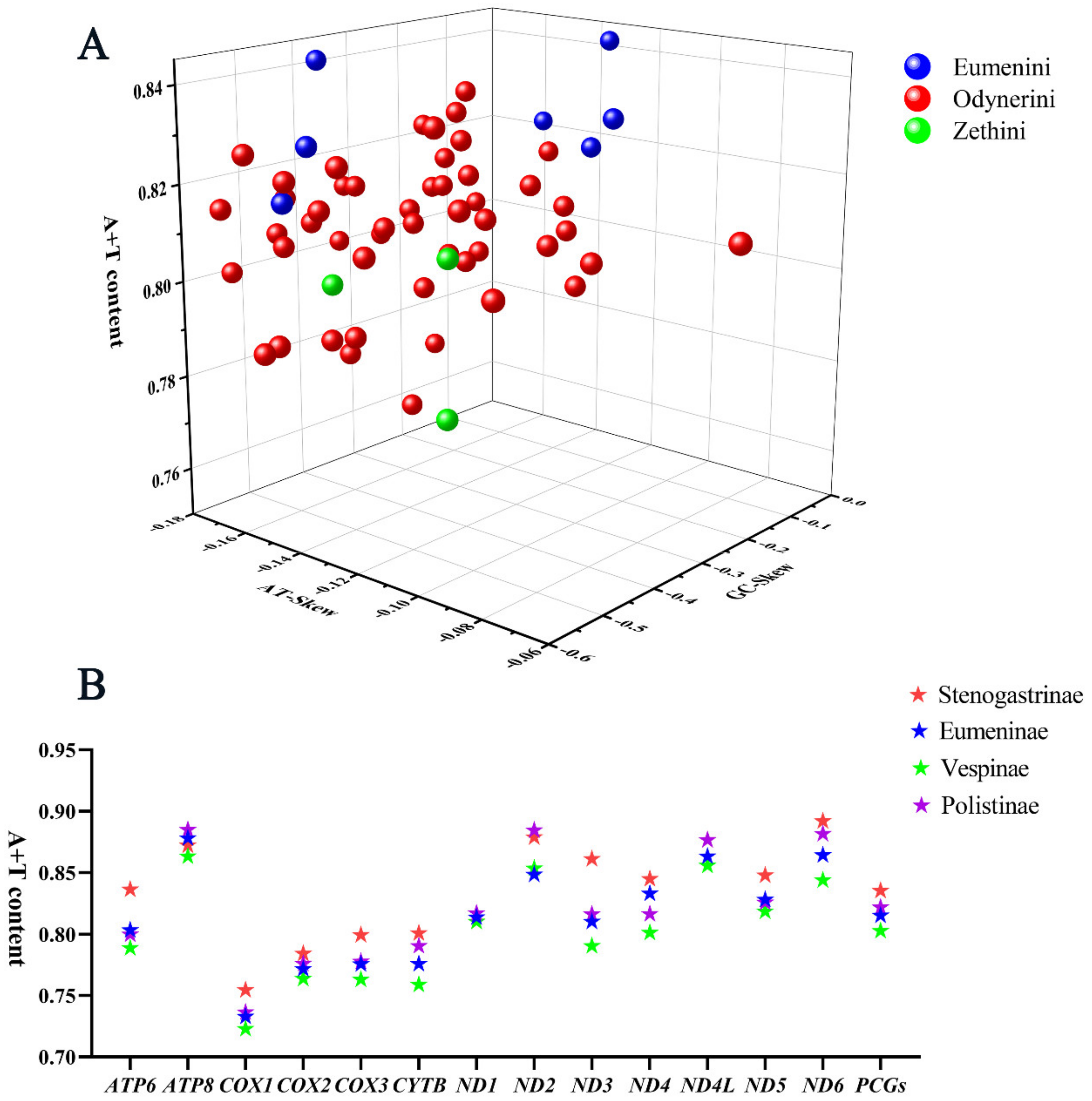
All the PCGs start with the typical ATA, ATG, or ATT codons and stop with the complete TAA or TAG or truncate TA- or T -- termination codons. The composition of PCGs is significantly biased toward adenine and thymine, with high A+T content from 75.9% to 84.4%, and the AT skews are always negative from −0.16 to −0.095 (Figure 3A). The A+T content of PCGs in other subfamilies of Vespidae was computed, showing that the value of A+T content in Stenogastrinae is higher than in three other subfamilies and in Vespinae it is the minimum (Figure 3B).
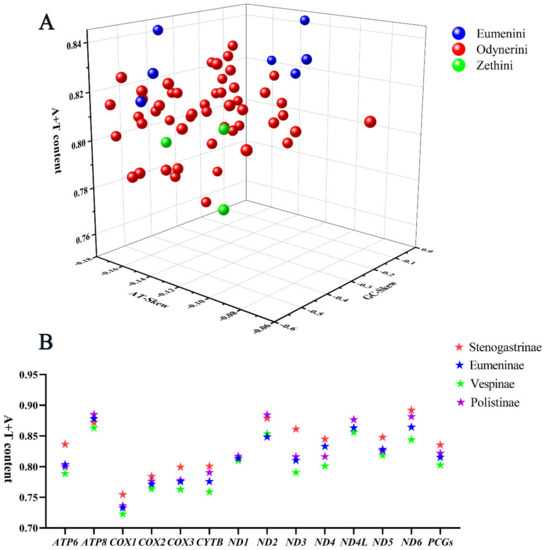
Figure 3.
A+T content, AT-Skew, and GC-Skew of PCG in vespid mitogenomes. (A) The A+T content, AT-Skew, and GC-Skew of PCG in Eumeninae; (B) the A+T content of PCG in four subfamilies of Vespidae.
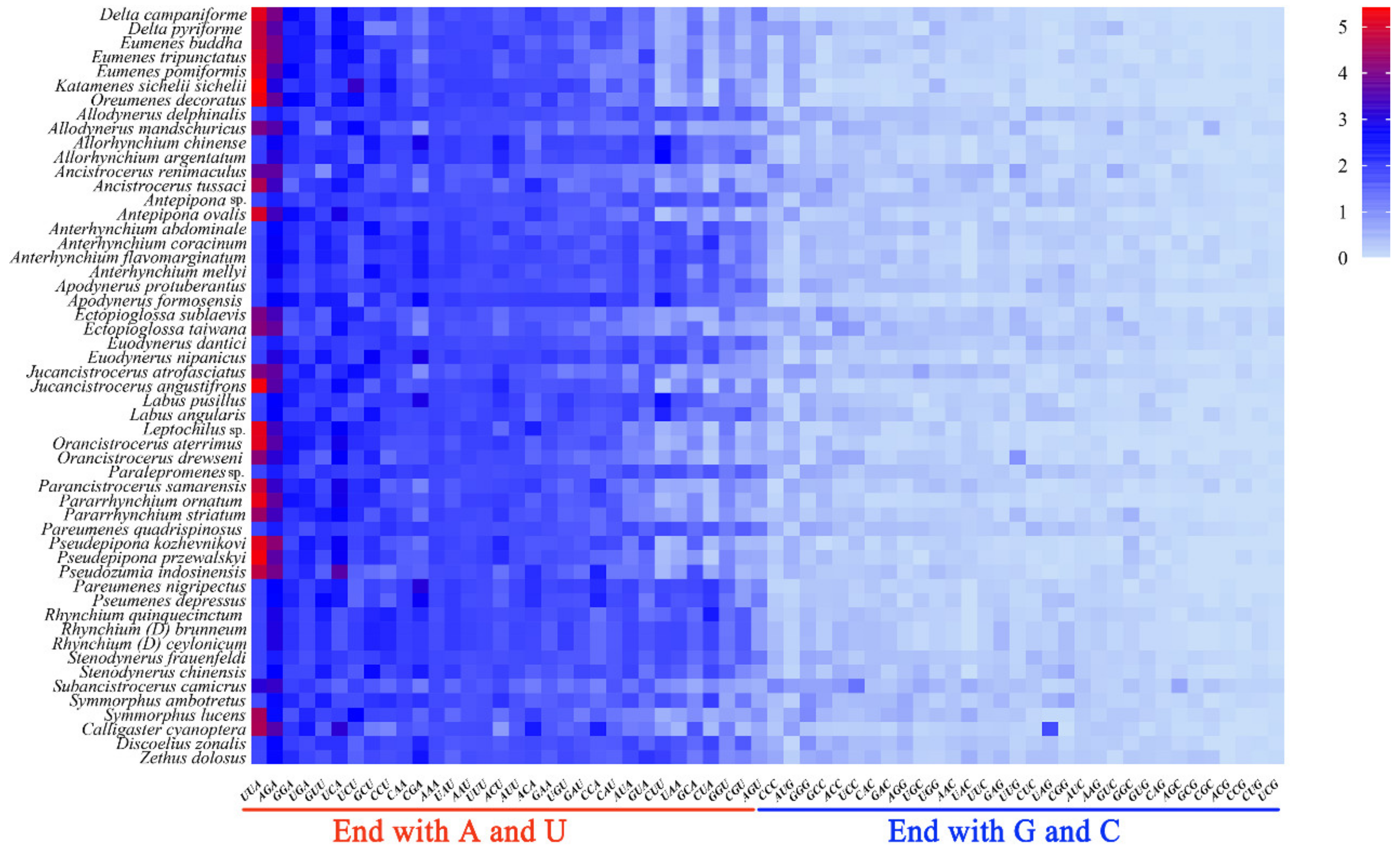
The Relative Synonymous Codon Usage (RSCU) values of codons such as UUA, GUU which ended with A or U, are all greater than 1.3 and those ending with G or C are all less than 1 (Figure 4). The RSCU value can directly reflect the frequency of codon usage: the RSCU value equivalent to 1 indicates that the codon has no preference, or the RSCU value greater than 1 illustrates that the frequency of the codon is relatively higher [56,57]. As a result, the optimal codons among PCGs of eumenine mitogenomes are codons ending with A or U, and accordingly, the third position of the codon in PCGs is significantly biased toward adenine and thymine with 90.5% A+T content. Additionally, the optimal codons of eumenine mitogenomes are consistent with those of Vespidae which frequently used UUU, UUA, AUU, and AUA, and among them, UUA (Leu2) is the one with the highest RSCU value. In addition, both UAA and AGA are the stop codons of the eumenine mitogenomes, of which UAA with the higher RSCU is the eumenine preferred codon.
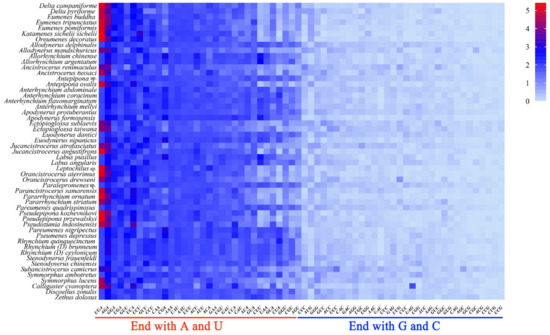
Figure 4.
Relative synonymous codon usage (RSCU) of the mitogenomes of Eumeninae.
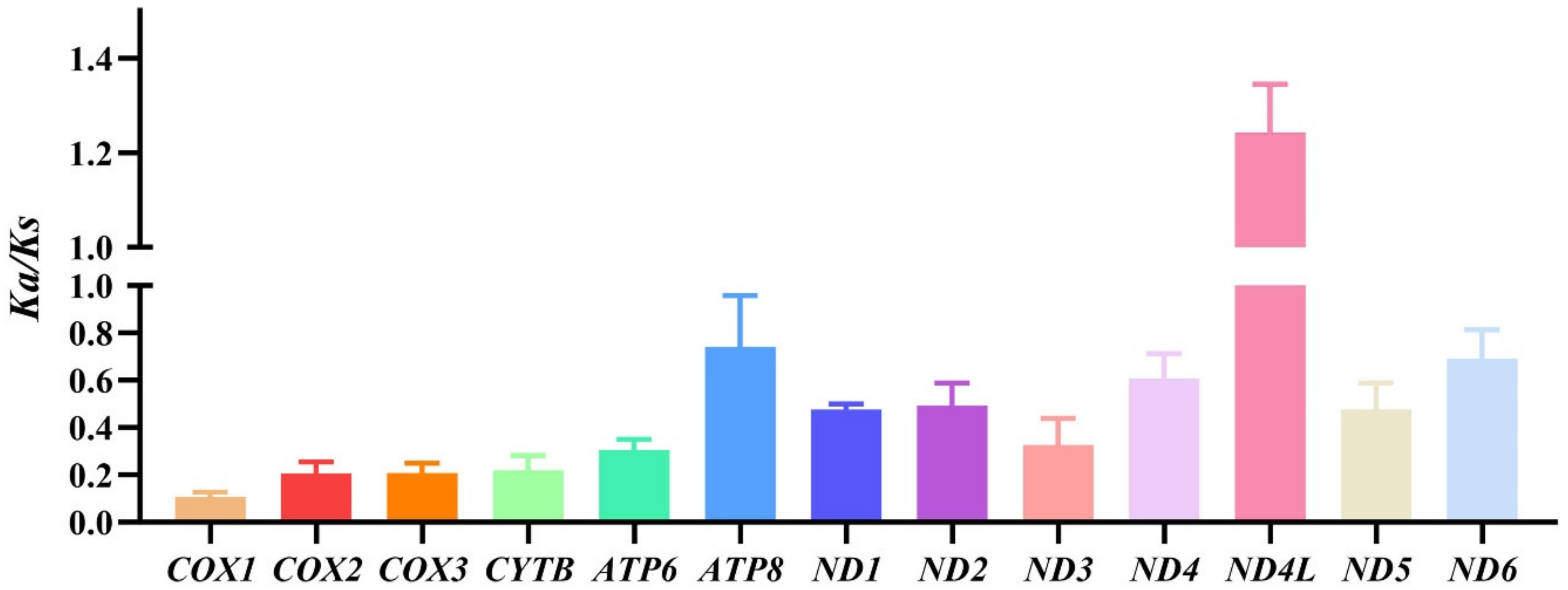
The synonymous codon usage bias is influenced by mutation pressure and natural selection, and an effective number of codons (ENC) standard curve can indicate that the determinant of codon preference is mutation pressure or natural selection [58]. Our result shows that all the points lie under the standard curve, which indicates that the codon usage bias is influenced by selection pressure (Figure S3). Ka/Ks is the ratio of the number of nonsynonymous substitutions per nonsynonymous site (Ka) to the number of synonymous substitutions per synonymous site (Ks), which could indicate something about the selective forces acting on the protein [59]. Thus, we computed the Ka/Ks value of PCGs from eumenine mitogenomes, and the result shows that all the Ka/Ks of PCGs except ND4L are less than 1, which indicates that only ND4L is under a positive selection and evolves rapidly, and other PCGs are under a purifying selection. Moreover, the lowest Ka/Ks value of COX1 (0.11) indicates that it is conservative under environmental selection pressure and suitable for molecular barcoding (Figure 5).
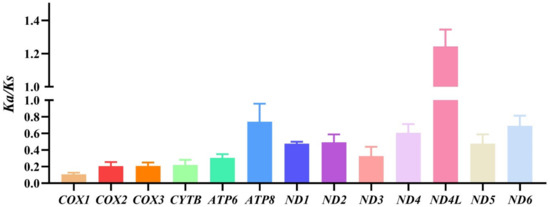
Figure 5.
The Ka/Ks values of the subfamily Eumeninae are based on each PCG.
3.3. Gene Arrangement
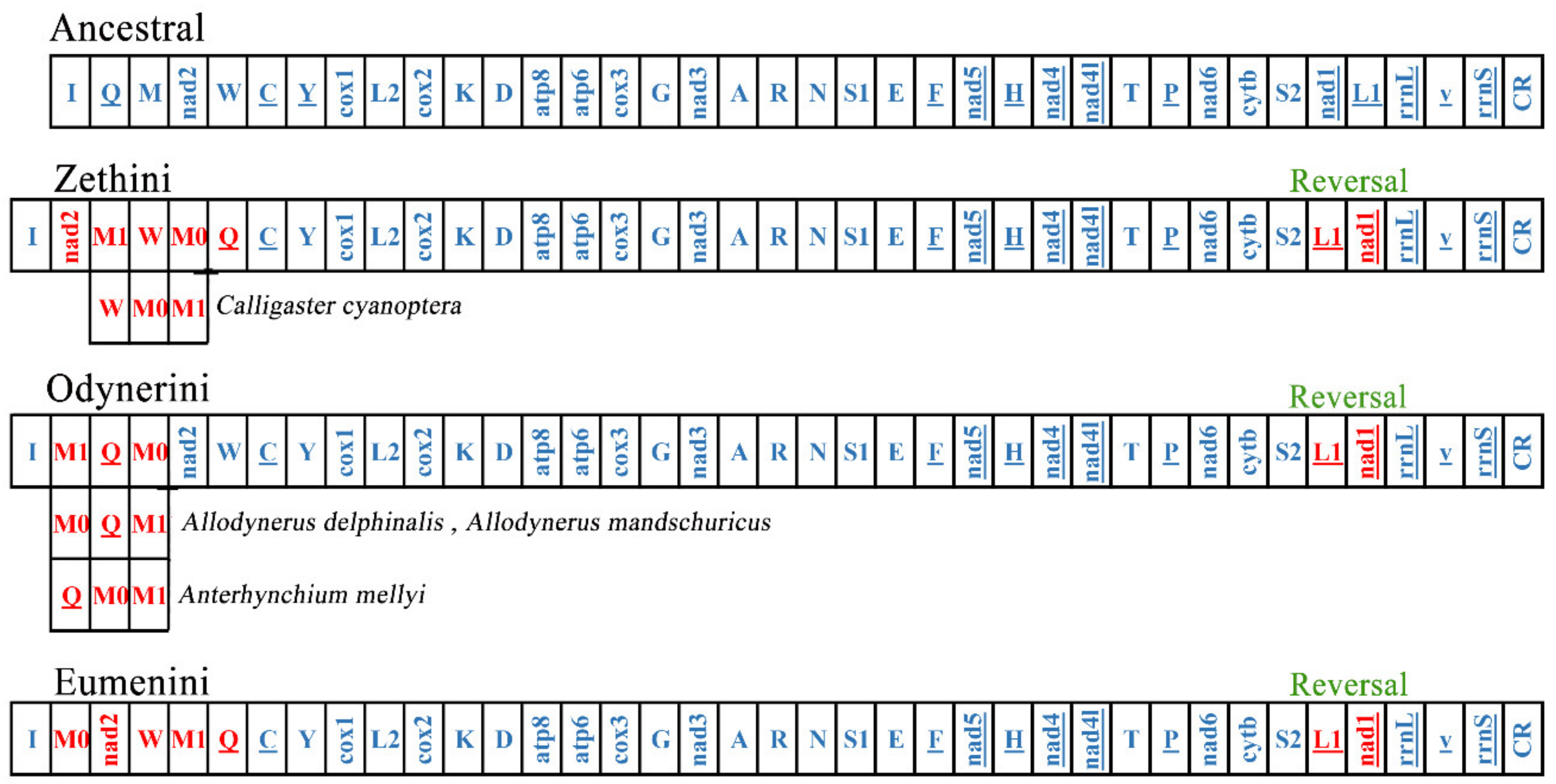
Mitogenomes are usually stable in composition and gene arrangement is relatively conservative; therefore, recombination rarely occurs in the evolutionary history of insects [52,60]. As more and more mitogenomes of insects are reported, the rates of mitogenome rearrangement in Hymenoptera are accelerated [53,61]. The subfamily Eumeninae is the primary lineage of the Vespidae, and its gene rearrangement events are still poorly studied. Some eumenine mitogenomes contain a duplication of trnM and the positions of the two trnM are different, which means that the different mechanisms occurred in the gene rearrangement of Eumeninae. We investigated more rearrangement events of 54 eumenine mitogenomes and found that all eumenine mitogenomes contain a translocation trnL1 upstream of nad1 (Figure 6). Because gene duplications are not allowed in CREx, the rearrangement events in cluster trnQ-trnM-ND2-trnW are inferred as three patterns compared with the ancestral mitochondrial gene order (Figure S4): the tandem duplication of trnM occurs in all three clusters of Eumeninae, and then the distinct recombination occurs in the three clusters, respectively. In the tribe Zethini, the recombination occurs in trnQ-trnM0 and trnM1-ND2 after trnM duplicated to trnM0-trnM1, and subsequently, it occurs between trnM0-trnQ and ND2-trnM1-trnW, and there is another rearrangement type: from the ancestral order trnQ-trnM-ND2-trnW to ND2-trnW-trnM0-trnM1 in Calligaster cyanoptera. In the tribe Odynerini, the recombination between trnM1 and trnQ-trnM0 occurs in most species, and the recombination trnQ-trnM0 after trnM duplicated to trnM0-trnM1 occurs in Allodynerus delphinalis and Allodynerus mandschuricus. In the same way, the recombination occurs in trnQ-trnM1 after trnM duplicated to trnM0-trnM1, and then the recombination occurs between trnM1 trnQ and trnM0-ND2-trnW in most species of the tribe Eumenini. As mentioned above, the three tribes of the subfamily Eumeninae possess their distinctive rearrangement pattern. Our results provided additional evidence that the majority of mitogenome rearrangements occur in tRNAs in hymenopteran insects and also showed that the gene block trnI-trnQ-trnM-ND2 may be the hot spot of rearrangement in Hymenoptera because the rearrangement events of this block are found in many hymenopteran lineages, such as the rearrangement events of the gene block CR-trnI-trnQ-trnM-ND2-trnW-trnC-trnY in all the Icheumonoid lineages and rearrangement of CR-trnI-trnQ-trnM in the mitochondrial genome of Allantus luctifer [62,63].
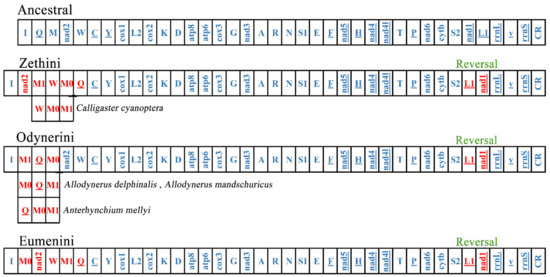
Figure 6.
The rearrangement event in three tribes of the subfamily Eumeninae. The red genes represent its’ position changes compared with ancestral mitogenomes.
3.4. Phylogenetic Relationship of Vespidae
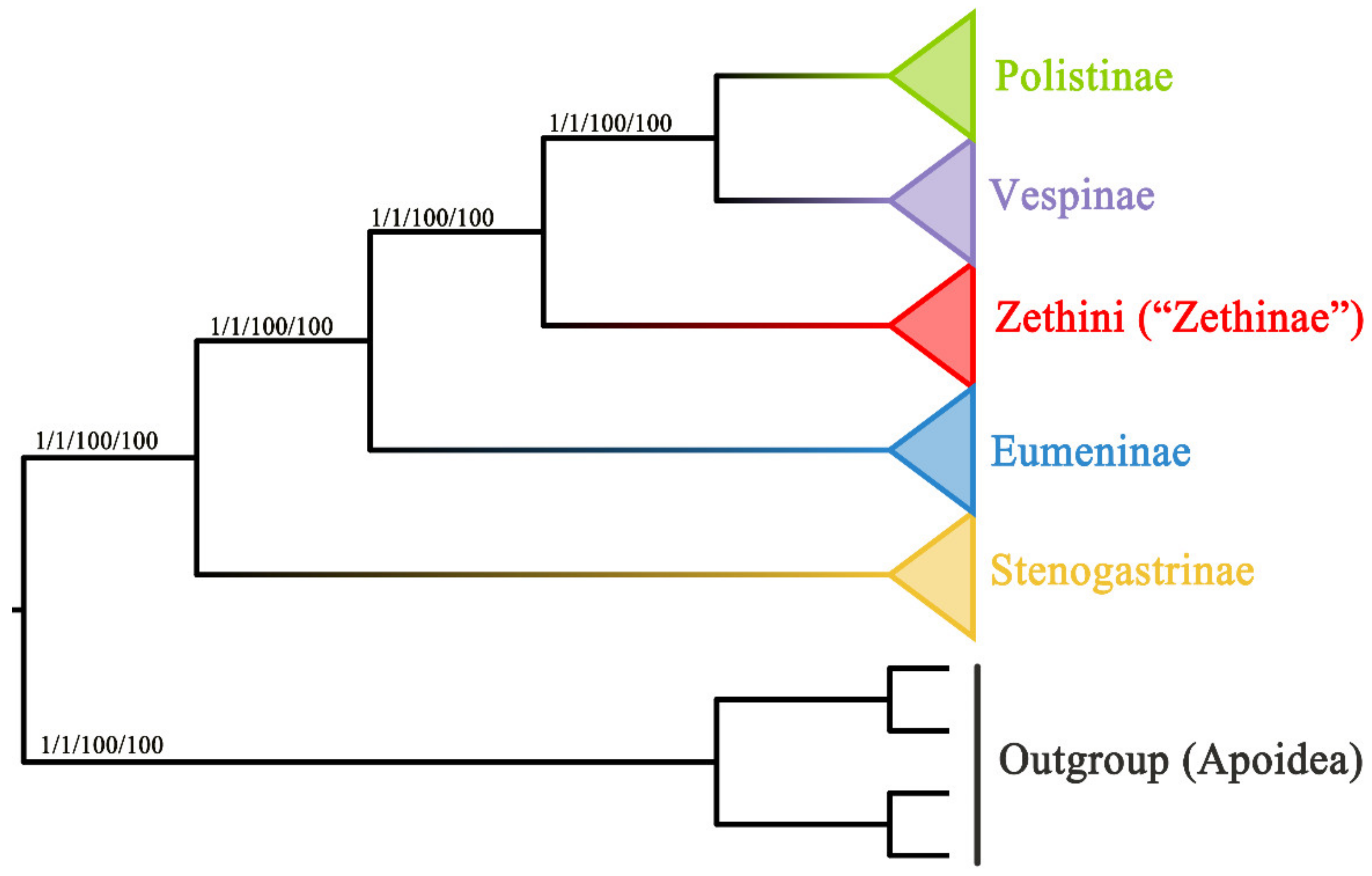
The results of substitution saturation show that Iss > Iss.c and p = 0.0000 within both PCG and PCGR (Table S4), which indicates that the sequences are not saturated and can be used for phylogenetic analysis. In this study, phylogenetic analyses of two concatenated nucleotides (PCG and PCGR) were conducted, both representing four subfamilies (Stenogastrinae, Eumeninae, Polistinae, and Vespinae) of Vespidae and the outgroup (Hylaeus dilatatus, Andrena cineraria, Megachile sculpturalis, and Apis cerana). The two concatenated nucleotides were subjected to Bayesian inference (BI) and maximum likelihood (ML) analyses, resulting in four trees where the positions of the four subfamilies are congruent (Figure 7). In these trees, the phylogenetic relationships of the Vespidae are as follows: Stenogastrinae + (“Eumeninae” + (Zethini + (Polistinae + Vespinae))). According to our results, the subfamilies Stenogastrinae, Polistinae, and Vespinae are undoubtedly monophyletic, but nevertheless, the subfamily Eumeninae excluding Zethini is monophyletic. Additionally, Stenogastrinae is a sister lineage to other subfamilies of Vespidae with high bootstrap support values (BS) and Bayesian posterior probabilities values (PP) (BS = 100, PP = 1), which is consistent with some recent phylogenomic studies [64,65]. The study reveals that the tribe Zethini of the subfamily Eumeninae is an independent branch and more closely related to Polistinae and Vespinae, which is similar to previous studies [5,13,15]. As the tree shows in Figure 7, the position of the Zethini (“Zethinae”) is between solitary Eumeninae and eusocial Vespinae + Polistinae. The genus Calligaster and the subgenus Zethoides of genus Zethus in Zethini (“Zethinae”) have been cited as exemplifying the critical evolutionary stages of subsocial and communal behavior which connects solitary and eusocial wasps because it is reported that some species of both Zethus and Calligaster construct their nests with plant material rather than the typical eumenine nest construction with mud [66,67].
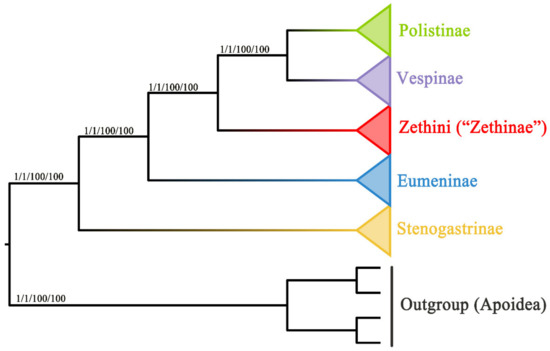
Figure 7.
Phylogenetic trees of the Vespidae inferred from PCG and PCGR by ML and BI. Each node shows the Bayesian posterior probabilities (PP)/maximum likelihood bootstrap support (BS) values.
3.5. Phylogenetic Relationship within Eumeninae
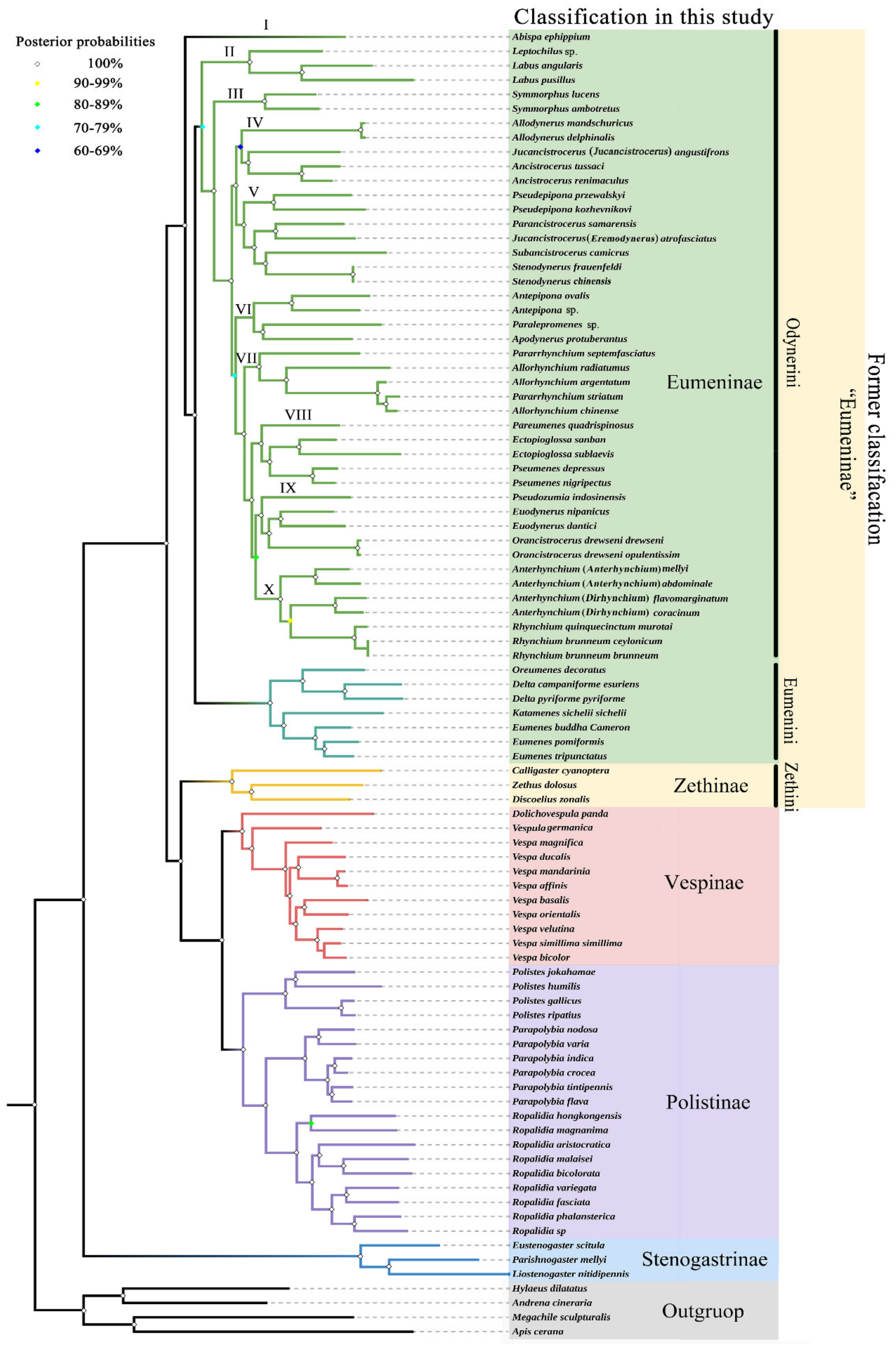
The ingroup relationships of the family Vespidae are congruent in the obtained trees with the same methods, respectively, and the notable difference between the obtained tree topologies with ML and BI methods is that Pseudozumia indosinensis belongs to clade VIII in ML trees with low bootstrap support value (BS = 39, 38), while in BI trees it belongs to clade IX with high Bayesian posterior probability (PP = 1,1) (Figure S5). That the BS of a branch is lower than 50 means that the relationship has not been supported. In order to verify the accuracy of the obtained trees, a MP analysis was also performed, and the results were (of course) similar to the BI trees (Figure S6). Therefore, with the high Bayesian posterior probabilities, it is more likely that Pseudozumia indosinensis belongs to clade IX in BI trees. Of course, it may be because the differing placement in the ML trees is an artifact of bootstrap values below 50. The placement of the genus Pseudozumia in BI trees is also consistent with the result of Piekarski et al. In their research, a maximum-likelihood tree of Vespidae inferred from 235 selected loci obtained also shows the genus Pseudozumia as a sister group to Orancistrocerus which belongs to clade IX in this study [15]. Furthermore, the illustration in Figure 8 is identical to the results from analyzing the data of PCG and PCGR with the BI method. Both ML and BI reveal that the tribe Eumenini is monophyletic and the tribe Odynerini is paraphyletic containing 10 clades. The results also consistently indicate that the clade II to clade X is the sister group to the tribe Eumenini.
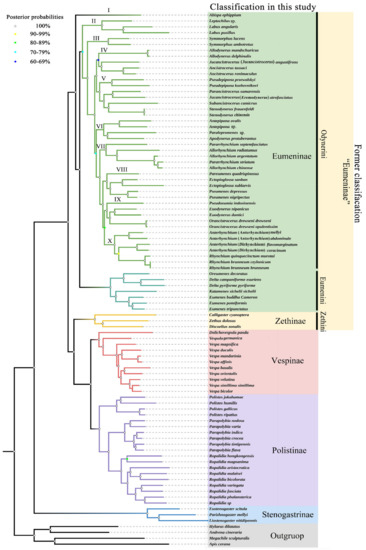
Figure 8.
Phylogenetic tree of Vespidae inferred from PCG and PCGR by BI. Each node shows the Bayesian posterior probability (PP) values.
Within the tribe Eumenini, the sister relationship of (Oreumenes + Delta) + (Katamenes + Eumenes) is strongly supported by all datasets in this study (BS = 100; PP = 1). Hermes et al. found that the genera Oreumenes, Delta, Katamenes, and Eumenes all belong to their clade 3 of Eumenini, and the genus Eumenes was recovered as a sister to the remaining taxa of Eumenini [6]. The differences in phylogenetic relationships of the four genera between our study and Hermes et al. might be attributed to our limited generic sample, which is not enough to clarify the comprehensive relationships of all genera of the tribe Eumenini. Therefore, to clearly understand the phylogenetic relationships within the tribe Eumenini, more data are needed.
The tribe Odynerini is the biggest one within the subfamily Eumeninae [6]. Here, we investigated 24 genera of Odynerini to illustrate their phylogenetic relationships. The results show that Odynerini comprising 10 major clades (I-X) is paraphyletic, and clade I (the genus Abispa) is a sister group to all remaining Eumeninae. Bank et al. reported that the genus Alastor (clade A) is inferred as a sister lineage to all remaining Eumeninae based on transcriptomes of 49 vespid wasps [14]. Our study does not contain any species in the genus Alastor, and Bank et al.’s study did not contain any species in the genus Abispa, whereas that of Piekarskis et al. containing both Abispa and Alastor, is consistent with the standpoint of Bank et al. [14,15]. In clade II, the genus Leptochilus is inferred as a sister lineage to the genus Labus. In succession, the genus Symmorphus is an independent clade III and sister group to clades IV-X. In clades IV-X, there is a sister-group relationship between clades IV-V and VI-X. Within clades IV-V, clade IV is a sister group to clade V, while Jucancistrocerus (Jucancistrocerus) angustifrons and Jucancistrocerus (Eremodynerus) atrofasciatus are located at clades IV and V, respectively, which may support subgenera Eremodynerus being a valid genus [68]. Of course, more morphological evidence of more species should be investigated to confirm our results in further research. Within clades VI-X, clade VI is a sister group to clades VII-X and is composed of Antepipona + (Paralepromenes + Apodynerus). Then, clade VII is a sister group to clades VIII-X, composed of Pararrhynchium and Allorhynchium, while Pararrhynchium striatum is located within the genus Allorhynchium. The misidentification of Pararrhynchium striatum is eliminated by the examination of specimens, so Pararrhynchium striatum should be transferred to the genus Allorhynchium, or these two genera are synonymized. Likewise, there is a sister relationship between clade VIII and clades IX-X, and the phylogenetic relationships of clade VIII are as follows: (Pareumenes + (Ectopioglossa + Pseumenes)). Finally, within these two clades IX and X, they are sisters to each other, and the phylogenetic relationships of clade IX are as follows: (Pseudozumia + (Euodynerus + Orancistrocerus)), and of clade X they are (Anterhynchium (Anterhynchium) + (Anterhynchium (Dirhynchium) + Rhynchium)). As the results show, with high Bayesian posterior probabilities (PP = 1), the subgenus Dirhynchium of Anterhynchium is more closely related to the genus Rhynchium than the nominate subgenus Anterhynchium, which means the subgenus Dirhynchium should be upgraded to a valid genus. Again, further investigation is needed.
4. Conclusions
To sum up, the mitogenomes of Eumeninae are commonly found to contain two trnM, which differs remarkably from the gene orders of other Vespidae. This study based on mitogenomes further supports previously proposed relationships among Vespidae [5,14,68], especially the placement of the tribe Zethini and some genera of the subfamily Eumeninae, indicating that the tribe Zethini should be raised to Zethinae and that the tribe Eumenini is monophyletic and Odynerini is paraphyletic. Meanwhile, some issues have not been clearly resolved in this study. First, stable generic morphological characters are needed to support these two subgenera Eremodynerus and Dirhynchium as valid genera. Additionally, although Pararrhynchium striatum is proposed to be moved to Allorhynchium, it is possible that the relationship between these two genera is confused, which requires more species sampling and morphological characteristics to elucidate. Second, considering that only one limited mitogenome in some genera of Eumeninae, such as Abispa, Apodynerus, Leptochilus, Parancistrocerus, Paralepromenes, Pareumenes, Pseudozumia, and Subancistrocerus, is presented in our analyses, the taxonomic status of these genera may be unstable and uncertain. In the end, the relationships of these taxa in this study still need to be verified by morphological and biological information. Therefore, to further advance the research on the systematic relationships of the subfamily Eumeninae, more taxon sampling and information about the morphological characteristics, molecular data, and biological behaviors are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13060529/s1, Supplementary Figure S1. The gene order of eumenine mitogenomes used in this study; Supplementary Figure S2. Inferred secondary structures of duplicated trnM. The substitutions in trnM0 and trnM1 compared with each other are indicated by red color; Supplementary Figure S3. ENC-GC3S plot of total PCGs in 54 eumenine mitogenomes, the black curve shows the relationship between ENC values and GC3S under random codon usage assumption; Supplementary Figure S4. The hypothesized pathway of the translocated inversion derived by recombination and duplication in three tribes of the subfamily Eumeninae. The red genes represent their positions; Supplementary Figure S5. Phylogenetic trees of Vespidae were inferred from PCG and PCGR by ML. Each node shows the bootstrap support values; Supplementary Figure S6. Phylogenetic trees of Vespidae. (A): Phylogenetic tree of Vespidae inferred from PCG by MP. (B): Phylogenetic tree of Vespidae inferred from PCGR by MP. Each nod shows the bootstrap support values; Supplementary Table S1. Mitochondrial genomes used for phylogenetic analysis in this study; Supplementary Table S2. The best partitioning scheme selected by PartitionFinder for different data matrices; Supplementary Table S3. Base composition, total length (bp), and AT-skew of complete Eumeninae mitogenomes; Supplementary Table S4. Substitution saturation test results.
Author Contributions
L.L. completed all the analyses and wrote the manuscript. J.M.C. provided data analysis, reviewed the manuscript, and approved the final version to be published. B.C. contributed design of the work, gave important comments on this study, and reviewed the manuscript. T.L. is responsible for the implementation of the entire project and revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos: 31772490, 31372247, 31000976).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Mitochondrial genome sequences are accessible on GenBank and accession numbers are contained within Table 1.
Acknowledgments
We are extremely grateful to Song Fan of China Agricultural University for his important comments on this study and to Shu-Lin He of Chongqing Normal University for his help with the analysis method of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cowan, D.P. The Social Biology of Wasps; Cornell University Press: Ithaca, NY, USA, 1991; pp. 33–73. [Google Scholar]
- Jennings, D.T.; Houseweart, M.W. Predation by Eumenid Wasps (Hymenoptera: Eumenidae) on Spruce Budworm (Lepidoptera: Tortricidae) and Other Lepidopterous Larvae in Spruce-Fir Forests of Maine. Ann. Entomol. Soc. Am. 1984, 49, 39–45. [Google Scholar] [CrossRef]
- Pannure, A.; Belavadi, V.V.; Carpenter, J.M. Taxonomic studies on potter wasps (Hymenoptera: Vespidae: Eumeninae) of south India. Zootaxa 2016, 4171, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Bohart, R.M.; Stange, L.A. A Revision of the Genus Zethus in the Western Hemisphere (Hymenoptera, Eumenidae); University of California Press: Oakland, CA, USA, 1965; Volume 40, pp. 1–208. [Google Scholar]
- Hines, H.M.; Hunt, J.H.; O’Connor, T.K.; Gillespie, J.J.; Cameron, S.A. Multigene phylogeny reveals eusociality evolved twice in vespid wasps. Proc. Natl. Acad. Sci. USA 2007, 104, 3295–3299. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.M.; Cumming, J.M. A character analysis of the North American potter wasps (Hymenoptera: Vespidae; Eumeninae). J. Nat. Hist. 1985, 19, 877–916. [Google Scholar] [CrossRef]
- Latreille, P.A. Histoire Naturelle, Générale et Particulière des Crustacés et des Insects: Ouvrage Faisant Suite aux Oeuvres de Leclerc de Buffon, et Partie du Cours Complet D’historie Naturelle Rédigé par C.S.; Sonnini, F., Ed.; Dufart: Paris, France, 1802. [Google Scholar] [CrossRef]
- de Saussure, H.F. Etudes sur la Famille des Vespides 1. Monographie des Guepes Solitaires ou de la Tribe des Eumeniens; Masson, V., Cherbuliez, J., Eds.; Victor Masson: Paris, France, 1852–1853; pp. 1–286. [Google Scholar]
- de Saussure, H.F. Etudes sur la Famille des Vespides 3. La Monographie des Masariens et un Supplement a la Monographie des Eumeniens; Masson, V., Cherbuliez, J., Eds.; Victor Masson: Paris, France, 1854–1856; pp. 1–352. [Google Scholar]
- Richards, O.W. A Revisional Study of the Masarid Wasps (Hymenoptera, Vespoidea); British Museum (Natural History): London, UK, 1962; pp. 1–294. [Google Scholar]
- Carpenter, J.M. The phylogenetic relationships and natural classification of the Vespoidea (Hymenptera). Syst. Entomol. 1982, 7, 11–38. [Google Scholar] [CrossRef]
- Hermes, M.G.; Melo, G.; Carpenter, J.M. The higher-level phylogenetic relationships of the Eumeninae (Insecta, Hymenoptera, Vespidae), with emphasis onEumenessensu lato. Cladistics 2013, 30, 453–484. [Google Scholar] [CrossRef]
- Schmitz, J.; Moritz, R. Molecular Phylogeny of Vespidae (Hymenoptera) and the Evolution of Sociality in Wasps. Mol. Phylogenetics Evol. 1998, 9, 183–191. [Google Scholar] [CrossRef][Green Version]
- Bank, S.; Sann, M.; Mayer, C.; Meusemann, K.; Donath, A.; Podsiadlowski, L.; Kozlov, A.; Petersen, M.; Krogmann, L.; Meier, R.; et al. Transcriptome and target DNA enrichment sequence data provide new insights into the phylogeny of vespid wasps (Hymenoptera: Aculeata: Vespidae). Mol. Phylogenetics Evol. 2017, 116, 213–226. [Google Scholar] [CrossRef]
- Piekarski, P.K.; Carpenter, J.M.; Lemmon, A.R.; Lemmon, E.M.; Sharanowski, B.J. Phylogenomic Evidence Overturns Current Conceptions of Social Evolution in Wasps (Vespidae). Mol. Biol. Evol. 2018, 35, 2097–2109. [Google Scholar] [CrossRef]
- Heraty, J.; Ronquist, F.; Carpenter, J.M.; Hawks, D.; Schulmeister, S.; Dowling, A.P.; Murray, D.; Munro, J.; Wheeler, W.C.; Schiff, N.; et al. Evolution of the hymenopteran megaradiation. Mol. Phylogenetics Evol. 2011, 60, 73–88. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.-J.; Li, W.-H. Complete Mitochondrial Genome of Suwallia teleckojensis (Plecoptera: Chloroperlidae) and Implications for the Higher Phylogeny of Stoneflies. Int. J. Mol. Sci. 2018, 19, 680. [Google Scholar] [CrossRef]
- Mao, M.; Gibson, T.; Dowton, M. Higher-level phylogeny of the Hymenoptera inferred from mitochondrial genomes. Mol. Phylogenetics Evol. 2014, 84, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-J.; Chen, B. Descriptions of four new species of Pararrhynchium de Saussure (Hymenoptera: Vespidae: Eumeninae) from China, with one newly recorded species and a key to Chinese species. Orient. Insects 2018, 52, 175–189. [Google Scholar] [CrossRef]
- Li, T.-J.; Barthélémy, C.; Carpenter, J.M. The Eumeninae (Hymenoptera, Vespidae) of Hong Kong (China), with description of two new species, two new synonymies and a key to the known taxa. J. Hymenopt. Res. 2019, 72, 127–176. [Google Scholar] [CrossRef]
- Tan, J.-L.; Carpenter, J.M.; Van Achterberg, C. An illustrated key to the genera of Eumeninae from China, with a checklist of species (Hymenoptera, Vespidae). ZooKeys 2018, 740, 109–149. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.-L.; Carpenter, J.M.; Van Achterberg, C. Most northern Oriental distribution of Zethus Fabricius (Hymenoptera, Vespidae, Eumeninae), with a new species from China. J. Hymenopt. Res. 2018, 62, 1–13. [Google Scholar] [CrossRef]
- Wang, H.-C.; Chen, B.; Li, T.-J. A new species and a new record of the genus Discoelius Latreille, 1809 (Hymenoptera: Vespidae: Eumeninae) from China. Zootaxa 2019, 4686, 297–300. [Google Scholar] [CrossRef]
- Wang, H.-C.; Chen, B.; Li, T.-J. Three new species of the genus Zethus Fabricius, 1804 (Hymenoptera, Vespidae, Eumeninae) from China, with an updated key to the Oriental species. J. Hymenopt. Res. 2019, 71, 209–224. [Google Scholar] [CrossRef]
- Wang, H.-C.; Chen, B.; Li, T.-J. Taxonomy of the genus Ectopioglossa Perkins from China, with two new species and an updated key to the Oriental species (Hymenoptera: Vespidae: Eumeninae). J. Asia-Pacific Entomol. 2020, 23, 253–259. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Li, T.-J. Taxonomy of the genus Epsilon from China, with a new species and an updated key to the Oriental species (Hymenoptera, Vespidae, Eumeninae). ZooKeys 2020, 910, 131–142. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Li, T.-J. A taxonomic revision of Allodynerus Blüthgen (Hymenoptera: Vespidae: Eumeninae) from China. Zootaxa 2020, 4750, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chen, B.; Li, T.-J. A new species and two new records of the genus Pseudepipona de Saussure, 1856 (Hymenoptera, Vespidae, Eumeninae) from China, with a key to the Chinese species. J. Hymenopt. Res. 2021, 82, 285–304. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, B.; Li, T.-J. Two newly recorded genera Malayepipona Giordani Soika and Megaodynerus Gusenleitner, with eight new species from China (Hymenoptera, Vespidae, Eumeninae). Zootaxa 2021, 5060, 371–391. [Google Scholar] [CrossRef]
- Li, T.-J.; Bai, Y.; Chen, B. A revision of the genus Jucancistrocerus Blüthgen, 1938 from China, with review of three related genera (Hymenoptera: Vespidae: Eumeninae). Zootaxa 2022, 5105, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Leung, H.C.M.; Yiu, S.M.; Chin, F.Y.L. IBDA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Mukesh, J.; Liu, Z. NGS QC Toolkit: A Toolkit for Quality Control of Next Generation Sequencing Data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Buckley, T.R.; Frati, F.; Stewart, J.B.; Beckenbach, A.T. Incorporating Molecular Evolution into Phylogenetic Analysis, and a New Compilation of Conserved Polymerase Chain Reaction Primers for Animal Mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 545–579. [Google Scholar] [CrossRef]
- Altschup, S.; Gish, W.; Miller, W.; Myers, E.; Lipman, D. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Search and contextual analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Laslett, D.; Canbäck, B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.-S.; Cheng, A.-C.; Wang, M.-S.; Zhao, L.-C. Characterization of Synonymous Codon Usage Bias in the Duck Plague Virus UL35 Gene. Karger 2009, 52, 266–278. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Bernt, M.; Merkle, D.; Ramsch, D.; Fritzsch, G.; Perseke, M.; Bernhard, D.; Schlegel, M.; Stadler, P.F.; Middendorf, M. CREx: Inferring genomic rearrangements based on common intervals. Bioinformatics 2007, 23, 2957–2958. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlićc, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Abascal, F.; Zardoya, R.; Telford, M.J. TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010, 38, W7–W13. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of Phylogenies after Removing Divergent and Ambiguously Aligned Blocks from Protein Sequence Alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Mark, P.; Ayres, D.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.; Huelsenbeck, J. MrBayes 3.2: Efcient Bayesian phyloge netic inference and model choice across a large model space. Syst. Biol. 2015, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Nixon, K.C. Winclada, version 1.98; Published by the author: Ithaca, NY, USA, 2015.
- Tang, P.; Zhu, J.-C.; Zheng, B.-Y.; Wei, S.-J.; Sharkey, M.; Chen, X.-X. Mitochondrial phylogenomics of the Hymenoptera. Mol. Phylogenetics Evol. 2018, 131, 8–18. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Valerio, A.; Austin, A.D.; Dowton, M.; Johnson, N.F. The first mitochondrial genome for the wasp superfamily Platygastroidea: The egg parasitoid Trissolcus basalis. Genome 2012, 55, 194–204. [Google Scholar] [CrossRef]
- Korkmaz, E.M.; Dogan, O.; Durel, B.S.; Budak, M.; Basibuyuk, H.H. Mitogenome organization and evolutionary history of the subfamily Cephinae (Hymenoptera: Cephidae). Syst. Entomol. 2018, 43, 606–618. [Google Scholar] [CrossRef]
- Cameron, S.L.; Dowton, M.; Castro, L.R. Mitochondrial genome organization and phylogeny of two vespid wasps. Genome 2008, 51, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Li, W.-H. An evolutionary perspective on synonymous codon usage in unicellular organisms. J. Mol. Evol. 1986, 24, 28–38. [Google Scholar] [CrossRef]
- Yu, T.; Li, J.; Yang, Y.; Qi, L. Codon usage patterns and adaptive evolution of marine unicellular cyanobacteria Synechococcus and Prochlorococcus. Mol. Phylogenetics Evol. 2012, 61, 206–213. [Google Scholar] [CrossRef]
- Wei, L.; He, J.; Jia, X.; Qi, Q.; Liang, Z.; Zheng, H. Analysis of codon usage bias of mitochondrial genome in Bombyx moriand its relation to evolution. BMC Evol. Biol. 2014, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486. [Google Scholar] [CrossRef]
- Barr, C.M.; Neiman, M.; Taylor, D.R. Inheritance and recombination of mitochondrial genomes in plants, fungi and animals. New Phytol. 2005, 168, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Dowton, M.; Austin, A.D. Evolutionary dynamics of a mitochondrial rearrangement "hot spot" in the Hymenoptera. Mol. Biol. Evol. 1999, 16, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.B.; Wu, Y.F.; Yang, C.; Gu, X.H.; Wilson, J.J.; Li, H.; Cai, W.Z.; Yang, H.L.; Song, F. Evolution of tRNA gene rearrangement in the mitochondrial genome of ichneumonoid wasps (Hymenoptera: Ichneumonoidea). Int. J. Biol. Macromol. 2020, 164, 540–547. [Google Scholar] [CrossRef]
- Wei, S.-J.; Niu, F.-F.; Du, B.-Z. Rearrangement oftrnQ-trnMin the mitochondrial genome ofAllantus luctifer(Smith) (Hymenoptera: Tenthredinidae). Mitochondrial DNA 2014, 27, 856–858. [Google Scholar] [CrossRef]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Niehuis, O. Evolutionary History of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef]
- Huang, P.; Carpenter, J.M.; Chen, B.; Li, T.-J. The first divergence time estimation of the subfamily Stenogastrinae (Hymenoptera: Vespidae) based on mitochondrial phylogenomics. Int. J. Biol. Macromol. 2019, 137, 767–773. [Google Scholar] [CrossRef]
- Ducke, A. Uber Phylogenie und Klassifikation der socialen Vespiden. Zool. Jahr. Abt. Syst. Geogr. Biol. Tiere 1914, 36, 303–330. [Google Scholar]
- Rau, P. The Jungle Bees and Wasps of Barro Colorado Island. Ann. Entomol. Soc. Am. 1933, 2, 376. [Google Scholar] [CrossRef]
- Blüthgen, P. Beiträge zur Kenntnis der paläarktischen und einiger athiopischer Faltenwespen (Hym. Vespidae). Veröffentlichungen Aus Dem Dtsch. Kolon. Und Übersee Mus. Bremen 1939, 2, 233–267. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).