Flight Capability and the Low Temperature Threshold of a Chinese Field Population of the Fall Armyworm Spodoptera frugiperda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of the Flight Capability of a Natural Population of S. frugiperda
2.1.1. Collection and Feeding of S. frugiperda
2.1.2. Flight Mill and Tethering
2.1.3. Data Analysis
2.2. Determination of the Low Temperature Threshold for Flight of S. frugiperda
2.2.1. Collection and Feeding of S. frugiperda
2.2.2. Flight Mill and Tethering
2.2.3. Data Analysis
3. Results
3.1. Flight Capability of a Field Population of S. frugiperda
3.2. Flight Capability of S. frugiperda Moths in the First 12 h
3.3. Flight Capability of S. frugiperda Moths under Low Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luginbill, P. The Fall Armyworm; USDA Technology Bulletin: Washington, DC, USA, 1928; pp. 1–8.
- Sparks, A.N. A review of the biology of the fall armyworm. FL. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Li, X.J.; Wu, M.F.; Ma, J.; Gao, B.Y.; Wu, Q.L.; Chen, A.D.; Liu, J.; Jiang, Y.Y.; Zhai, B.P.; Early, R.; et al. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest. Manag. Sci. 2020, 76, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Casmuz, A.; Juárez, M.L.; Socías, M.G.; Murúa, M.G.; Prieto, S.; Medina, S.; Willink, E.; Gastaminza, G. Review of the host plants of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Soc. Entomol. Argent. 2010, 69, 209–231. [Google Scholar]
- Jiang, Y.Y.; Liu, J.; Wu, Q.L.; Ciren, Z.; Zeng, J. Investigation on winter breeding and overwintering areas of Spodoptera frugiperda in China. Plant Prot. 2021, 47, 212–217. [Google Scholar]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2016, 60, 255–267. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.P.; Wu, M.F.; Gao, B.Y.; Liu, J.; Lee, G.S.; Otuka, A.; Hu, G. High risk of the fall armyworm invading Japan and the Korean Peninsula via overseas migration. J. Appl. Entomol. 2019, 143, 911–920. [Google Scholar] [CrossRef]
- Chen, H.; Wu, M.F.; Liu, J.; Shen, A.D.; Jiang, Y.Y.; Hu, G. Migratory routes and occurrence divisions of the fall armyworm S. frugiperda in China. Acta Phytophy Sin. 2020, 47, 747–757. [Google Scholar]
- Wu, M.F.; Qi, G.J.; Chen, H.; Ma, J.; Liu, J.; Jiang, Y.Y.; Lee, G.S.; Otuka, A.; Hu, G. Overseas immigration of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) invading Korea and Japan in 2019. Insect Sci. 2022, 29, 505–520. [Google Scholar] [CrossRef]
- Hogg, D.B.; Pitre, H.N.; Anderson, R.E. Assessment of early-season phenology of the fall armyworm (Lepidoptera: Noctuidae) in Mississippi. Environ. Entomol. 1982, 11, 705–710. [Google Scholar] [CrossRef]
- Ge, S.S.; He, L.M.; He, W.; Xu, R.B.; Sun, X.T.; Wu, K.M. Determination on moth flight capacity of Spodoptera frugiperda. Plant Prot. 2019, 45, 28–33. [Google Scholar]
- Xie, D.J.; Zhang, L.; Cheng, Y.X.; Jiang, Y.Y.; Liu, J.; Jiang, X.F. Effect of temperature on flight capacity of the fall armyworm, Spodoptera frugiperda. Plant Prot. 2019, 45, 13–17. [Google Scholar]
- Snyder, T.P.; Switzer, K.M.; Keen, R.E. Allozymic variability in toxicity-testing strains of Ceriodaphnia dubia and in natural populations of Ceriodaphnia. Environ. Toxical. Chem. 2010, 10, 1045–1049. [Google Scholar] [CrossRef]
- Leppla, N.C.; Huettel, M.D.; Chambers, D.L.; Ashley, T.R.; Miyashita, D.H.; Wong, T.T.Y.; Harris, E.J. Strategies for colonization and maintenance of the mediterranean fruit fly. Entomol. Exp. Appl. 1983, 33, 89–96. [Google Scholar] [CrossRef]
- Norris, D.E.; Shurtleff, A.C.; Toure, Y.T.; Lanzaro, G.C. Microsatellite DNA polymorphism and heterozygosity among field and laboratory populations of Anopheles gambiae s.s. (Diptera: Culicidae). J. Med. Entomol. 2001, 38, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, A.P.; Zhang, W.; Mian, M.A. Genetic diversity and differentiation among laboratory and field populations of the soybean aphid, Aphis glycines. Bull. Entomol. Res. 2010, 100, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Francuski, L.; Djurakic, M.; Ludoški, J.; Hurtado, P.; Pérez-Bañón, C.; Ståhls, G.; Rojo, S.; Milankov, V. Shift in phenotypic variation coupled with rapid loss of genetic diversity in captive populations of eristalis tenax (Diptera: Syrphidae): Consequences for rearing and potential commercial use. J. Econ. Entomol. 2014, 107, 821–832. [Google Scholar] [CrossRef]
- Mckibben, G.H.; Grodowitz, M.J.; Villavaso, E.J. Comparison of flight ability of native and two laboratory-reared strains of boll weevils (Coleoptera: Curculionidae) on a flight mill. Environ. Entomol. 1988, 17, 852–854. [Google Scholar] [CrossRef]
- Dingle, H.; Drake, V.A. What Is Migration? BioScience 2007, 57, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Riley, J.R.; Cheng, X.N.; Zhang, X.X.; Reynolds, D.R.; Xu, G.M.; Smith, A.D.; Cheng, J.Y.; Bao, A.D.; Zhai, B.P. The long-distance migration of Nilaparvata lugens (Stål) (Delphacidae) in China: Radar observations of mass return flight in the autumn. Ecol. Entomol. 1991, 16, 471–489. [Google Scholar] [CrossRef]
- Gao, Y.B.; Sun, Y.J.; Zhang, Q.; Sun, W.; Zhou, J.C. The spring migration behavior of the oriental armyworm, Mythimna separata, in northeastern China. J. Appl. Entomol. 2014, 51, 906–913. [Google Scholar]
- Wu, Y.; Li, X.J.; Chen, X.; Hu, G.; Hu, Y.Y.; Xiong, K.; Zhang, G.; Zhu, J.; Chen, S.L.; Lu, M.H.; et al. The influence of the topography of the Ailao mountains on congregated landings of airborne Sogatella furcifera (Hemiptera: Delphacidae) populations. Environ. Entomol. 2017, 46, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.B.; Zhai, B.P. Active temperature selection of flying Helicoverpa armigera (Leidoptera: Noctuidae). Acta Entomol. Sin. 2010, 53, 540–548. [Google Scholar]
- Chen, H.; Yang, X.L.; Chen, A.D.; Li, Y.C.; Wang, D.H.; Liu, J.; Hu, G. Immigration timing and origin of the first fall armyworms (Spodoptera frugiperda) detected in China. J. Appl. Entomol. 2020, 57, 1270–1278. [Google Scholar]
- Jones, C.M.; Papanicolaou, A.; Mironidis, G.K.; Vontas, J.; YiHua, Y.; Lim, K.S.; Oakeshott, J.G.; Bass, C.; Chapman, J.W. Genomewide transcriptional signatures of migratory flight activity in a globally invasive insect pest. Mol. Ecol. 2015, 24, 4901–4911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minter, M.; Pearson, A.; Lim, K.S.; Wilson, K.; Chapman, J.W.; Jones, C.M. The tethered flight technique as a tool for studying life-history strategies associated with migration in insects. Ecol. Entomol. 2018, 43, 397–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayar, J.K.; Van Handel, E. Flight performance and metabolism of the moth Spodoptera frugiperda. J. Insect. Physiol. 1971, 17, 2475–2479. [Google Scholar] [CrossRef]
- Rose, A.H.; Silversides, R.H.; Lindquist, O.H. Migration flight by an aphid, Rhopalosiphum maidis (Hemiptera: Aphididae), and a noctuid, S. frugiperda (Lepidoptera: Noctuidae). Can. Enol. 1975, 107, 567–576. [Google Scholar] [CrossRef]
- Wang, F.Y.; Zhang, X.X.; Zhai, B.P. Flight and re-migration capacity of the rice leaf folder moth, Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Crambidae). Acta Entomol. Sin. 2010, 53, 1265–1272. [Google Scholar]
- Luo, L.; Johnson, J.; Hammond, A.M.; Lopez, J.D.; Geaghan, J.P.; Beerwinkle, K.R.; Westbrook, J.K. Determination and consideration of flight potential in a laboratory population of True armyworm (Lepidoptera: Noctuidae). Environ. Entomol. 2002, 31, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Cai, B.; Luo, L.; Cao, Y.; Liu, Y. Influences of temperature and humidity synthesize on flight capacity in the moth s of Oriental armyworm, Mythimna separata (Walker). Acta Ecol. Sin. 2003, 23, 738–743. [Google Scholar]
- Zhang, L.; Jiang, X.F.; Luo, L.Z. Determination of sensitive stage for switching migrant Oriental Armyworms into residents. Environ. Entomol. 2008, 37, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Early, R.; Gonzalez-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef] [Green Version]
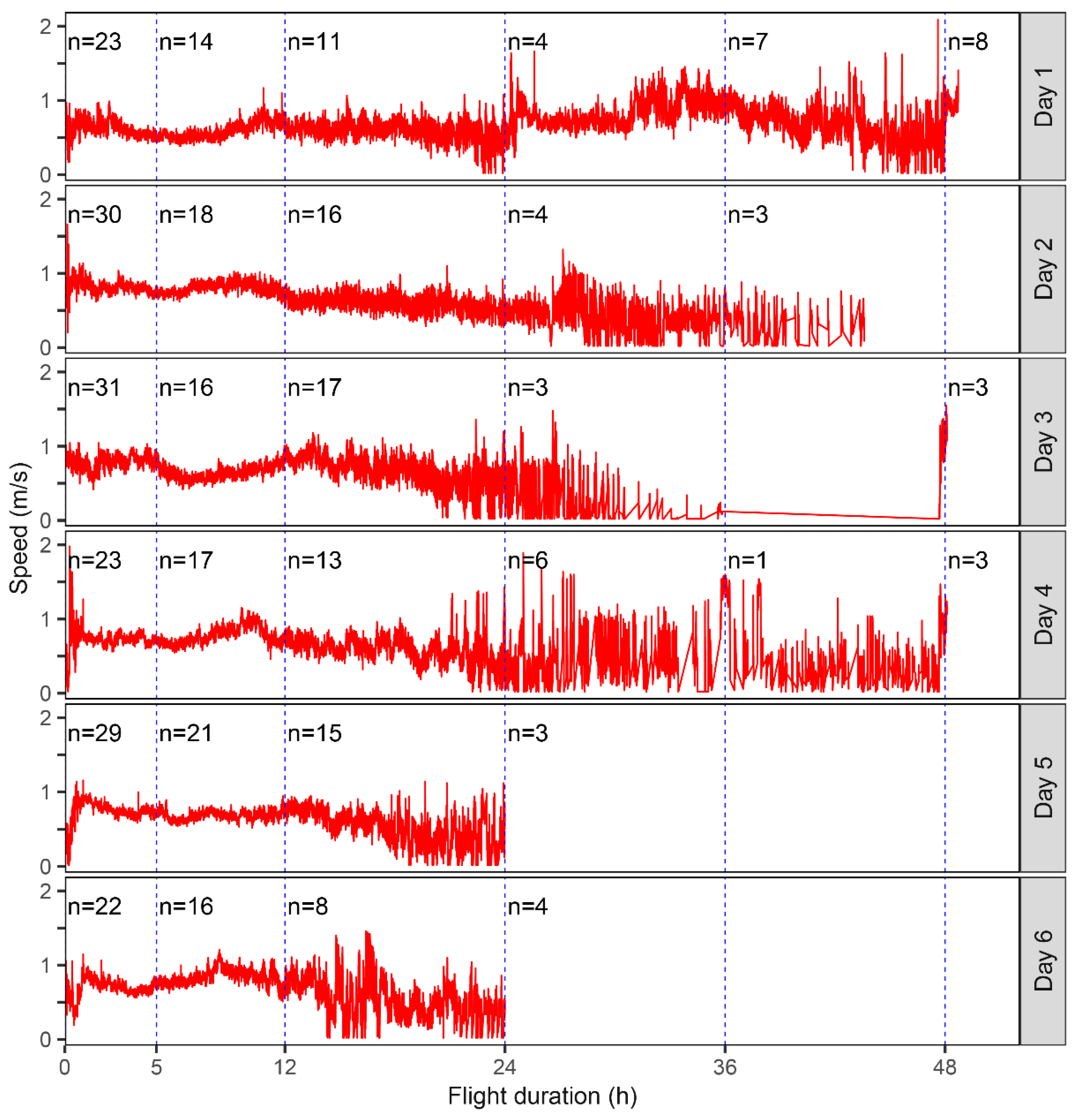
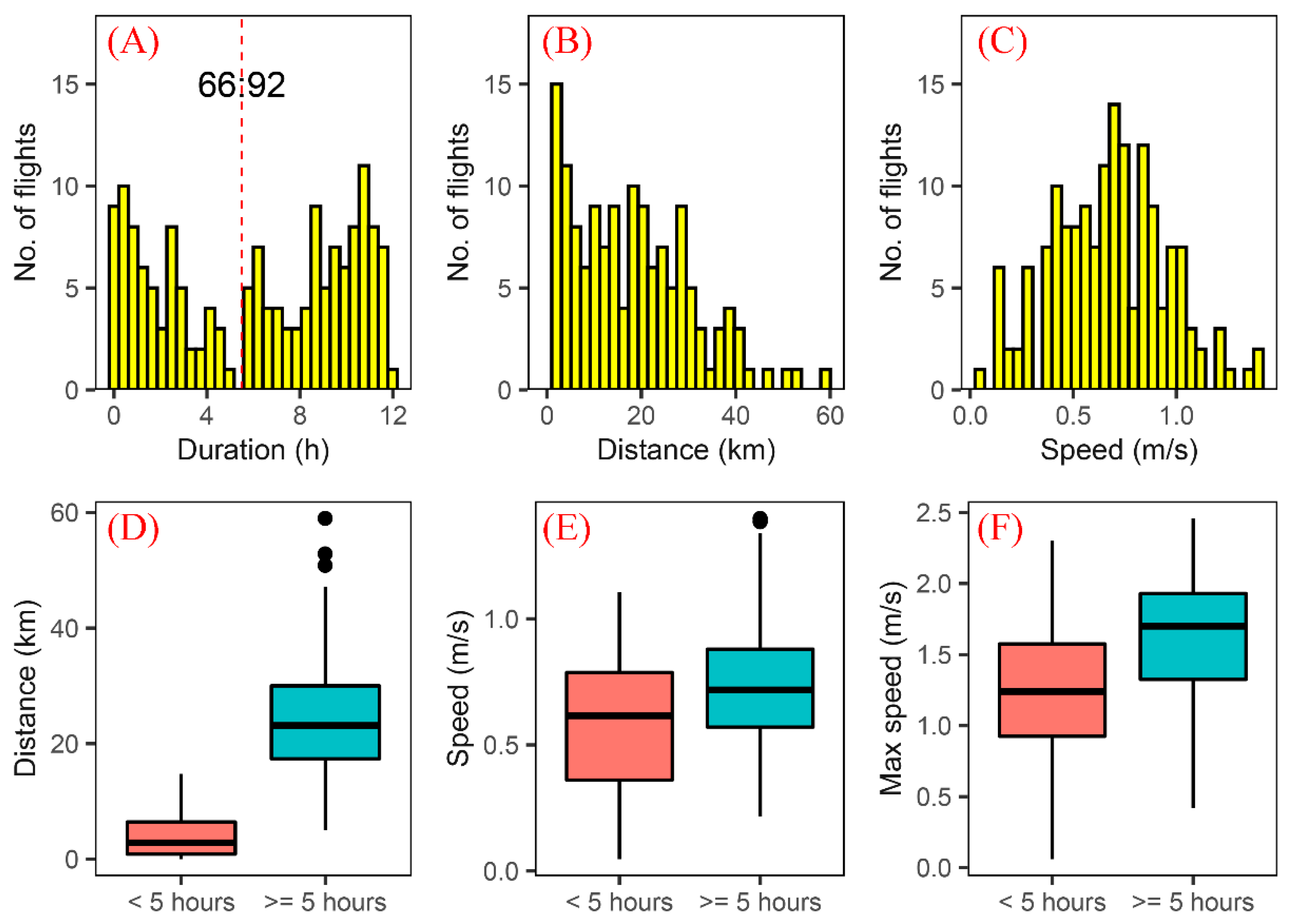
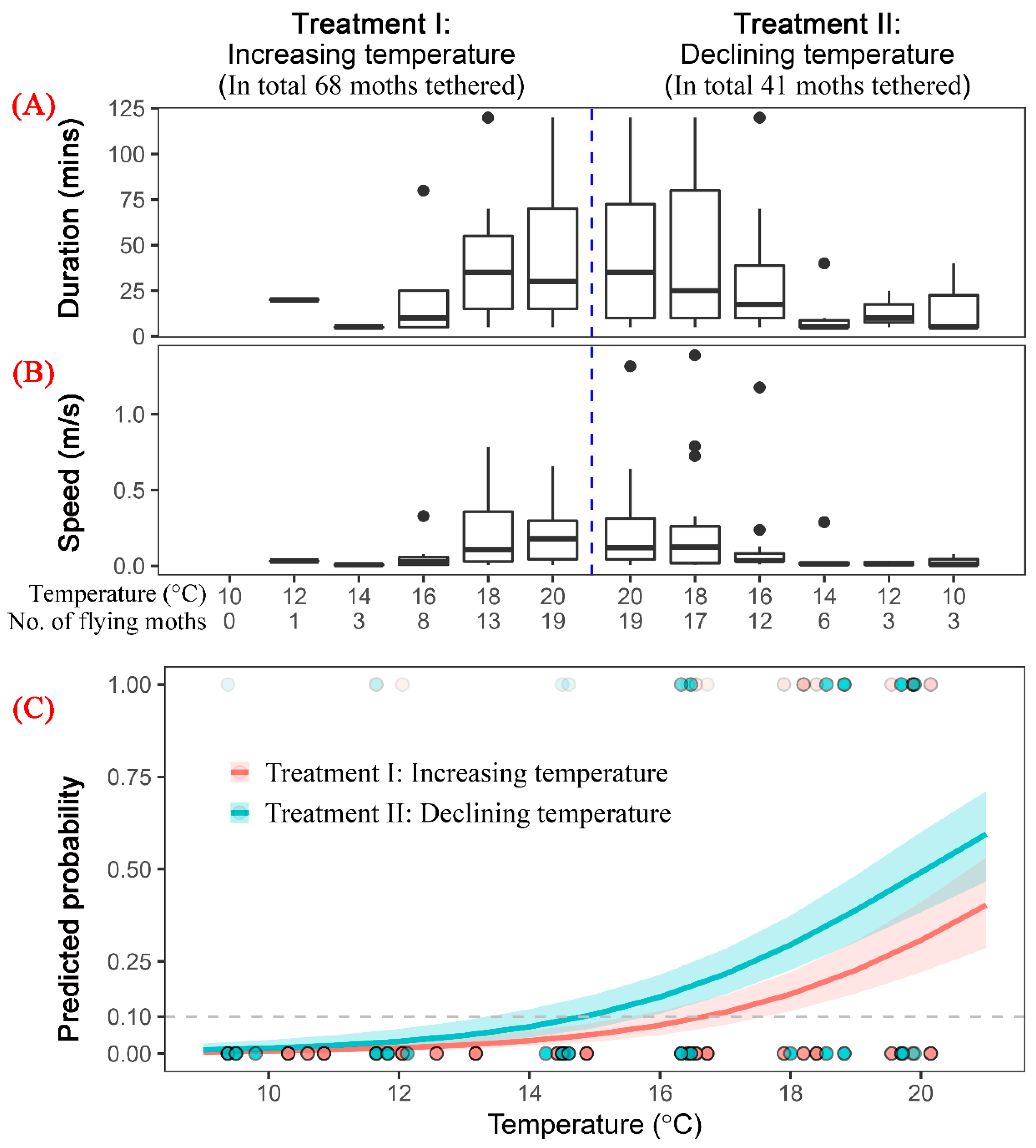
| Age | No. of Moths (Total/Female) | Tethering Mode | Max/Min Test Duration (h) | Max/Min Flight Duration (h) |
|---|---|---|---|---|
| 1 | 23/13 | Tethered until death | 49.61/26.32 | 36.51/8.86 |
| 2 | 30/16 | Tethered until death | 43.60/24.52 | 33.77/7.32 |
| 3 | 31/10 | 20 adults tethered until death (7 females); remainder tethered for 24 h | 49.00/23.26 | 22.29/6.19 |
| 4 | 23/7 | 3 adults tethered until death (2 females); remainder tethered for 24 h | 24.00(49.00)/23.69 | 21.76(22.00)/9.45 |
| 5 | 29/12 | Tethered for 24 h | 24.00/23.00 | 19.60/9.31 |
| 6 | 22/4 | Tethered for 24 h (5 died before 24 h) | 24.00/18.09 | 16.46/10.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, Y.; Huang, L.; Xu, C.-F.; Li, J.-H.; Wang, F.-Y.; Cheng, W.; Gao, B.-Y.; Chapman, J.W.; Hu, G. Flight Capability and the Low Temperature Threshold of a Chinese Field Population of the Fall Armyworm Spodoptera frugiperda. Insects 2022, 13, 422. https://doi.org/10.3390/insects13050422
Chen H, Wang Y, Huang L, Xu C-F, Li J-H, Wang F-Y, Cheng W, Gao B-Y, Chapman JW, Hu G. Flight Capability and the Low Temperature Threshold of a Chinese Field Population of the Fall Armyworm Spodoptera frugiperda. Insects. 2022; 13(5):422. https://doi.org/10.3390/insects13050422
Chicago/Turabian StyleChen, Hui, Yao Wang, Le Huang, Chuan-Feng Xu, Jing-Hui Li, Feng-Ying Wang, Wei Cheng, Bo-Ya Gao, Jason W. Chapman, and Gao Hu. 2022. "Flight Capability and the Low Temperature Threshold of a Chinese Field Population of the Fall Armyworm Spodoptera frugiperda" Insects 13, no. 5: 422. https://doi.org/10.3390/insects13050422
APA StyleChen, H., Wang, Y., Huang, L., Xu, C.-F., Li, J.-H., Wang, F.-Y., Cheng, W., Gao, B.-Y., Chapman, J. W., & Hu, G. (2022). Flight Capability and the Low Temperature Threshold of a Chinese Field Population of the Fall Armyworm Spodoptera frugiperda. Insects, 13(5), 422. https://doi.org/10.3390/insects13050422







