Morphology of Nasonov and Tergal Glands in Apis mellifera Rebels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Morphological Analyses of the Gland Cells
2.3. Examination of Anatomical Parameters
2.4. Statistical Analysis
3. Results
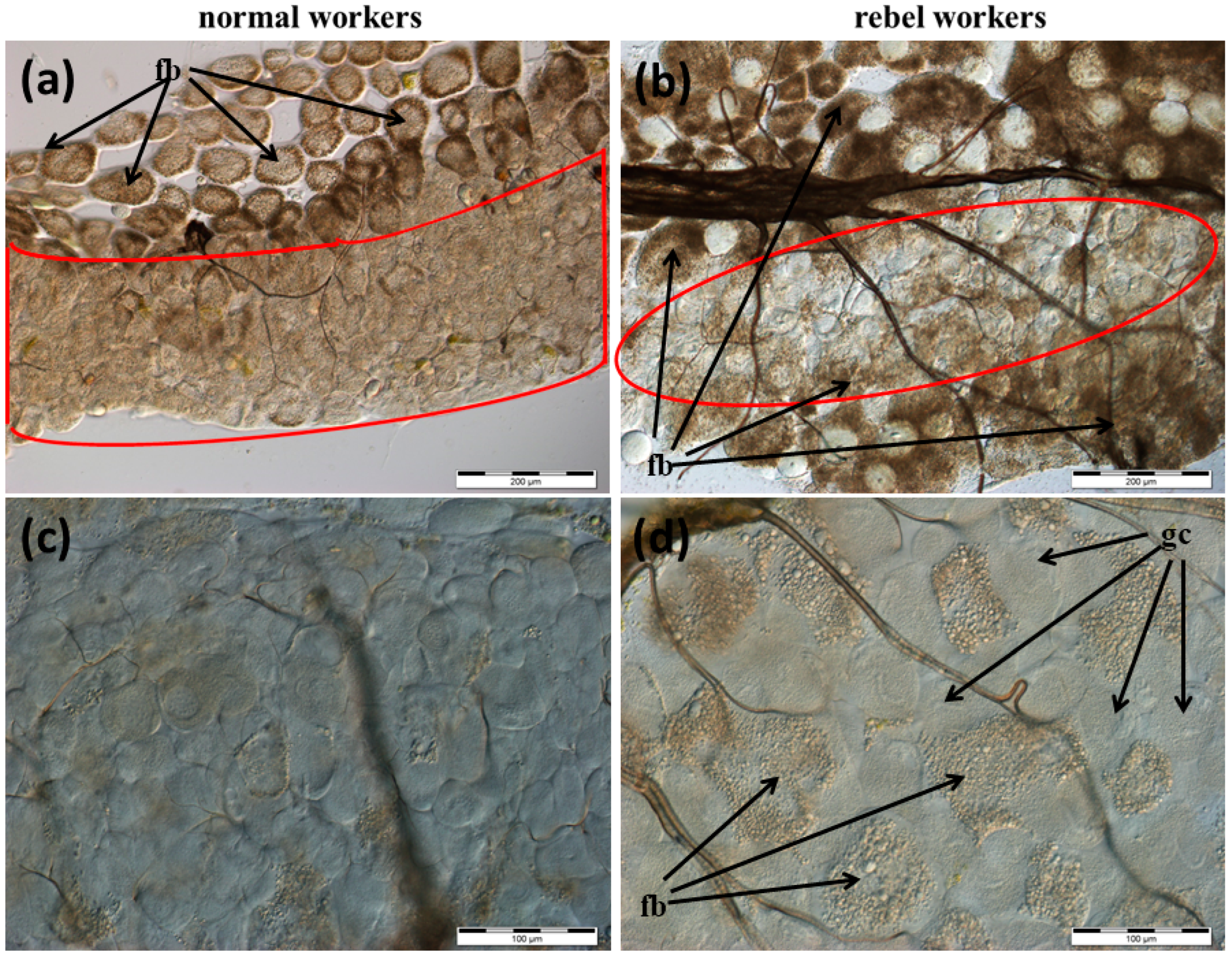
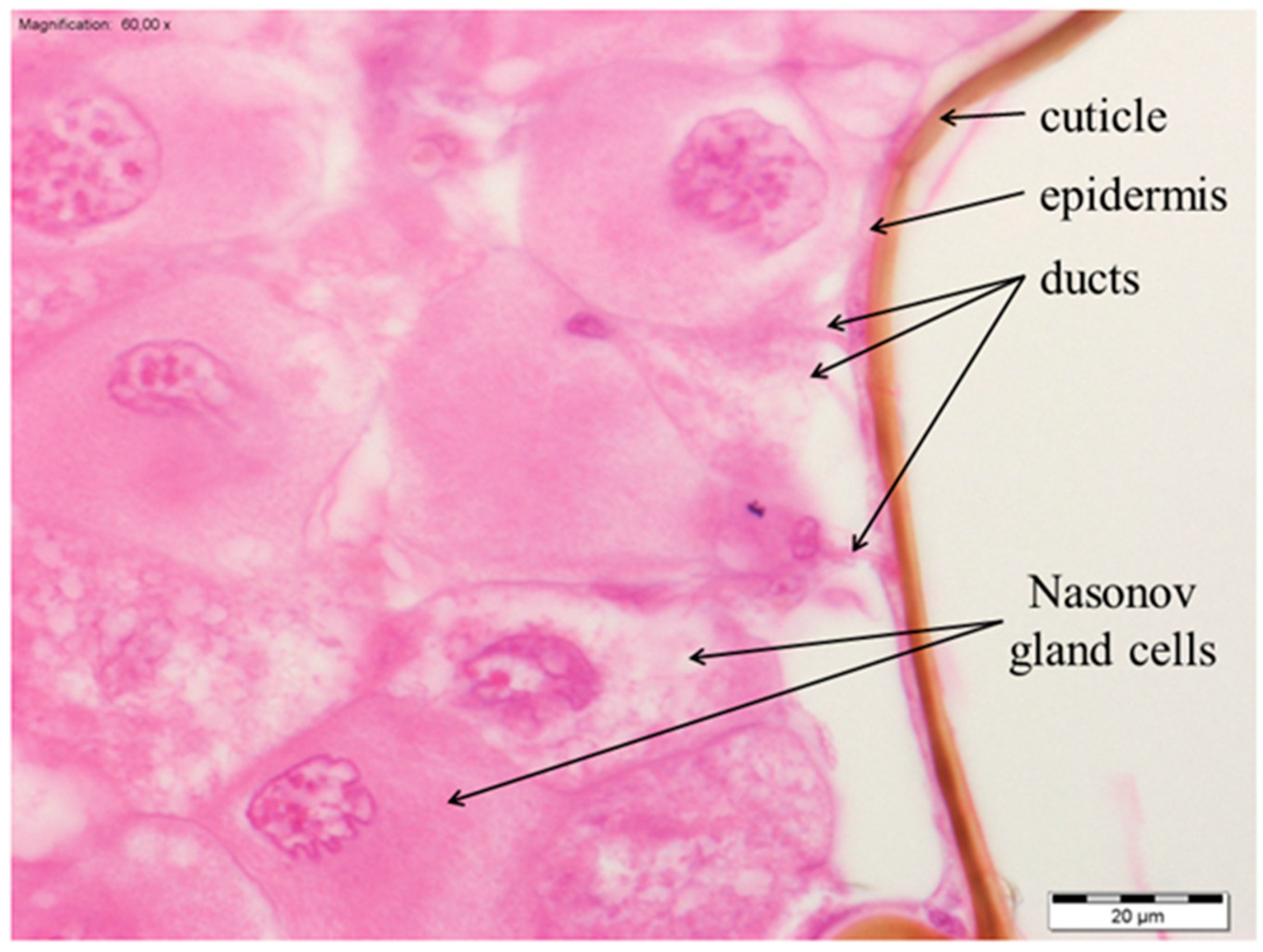
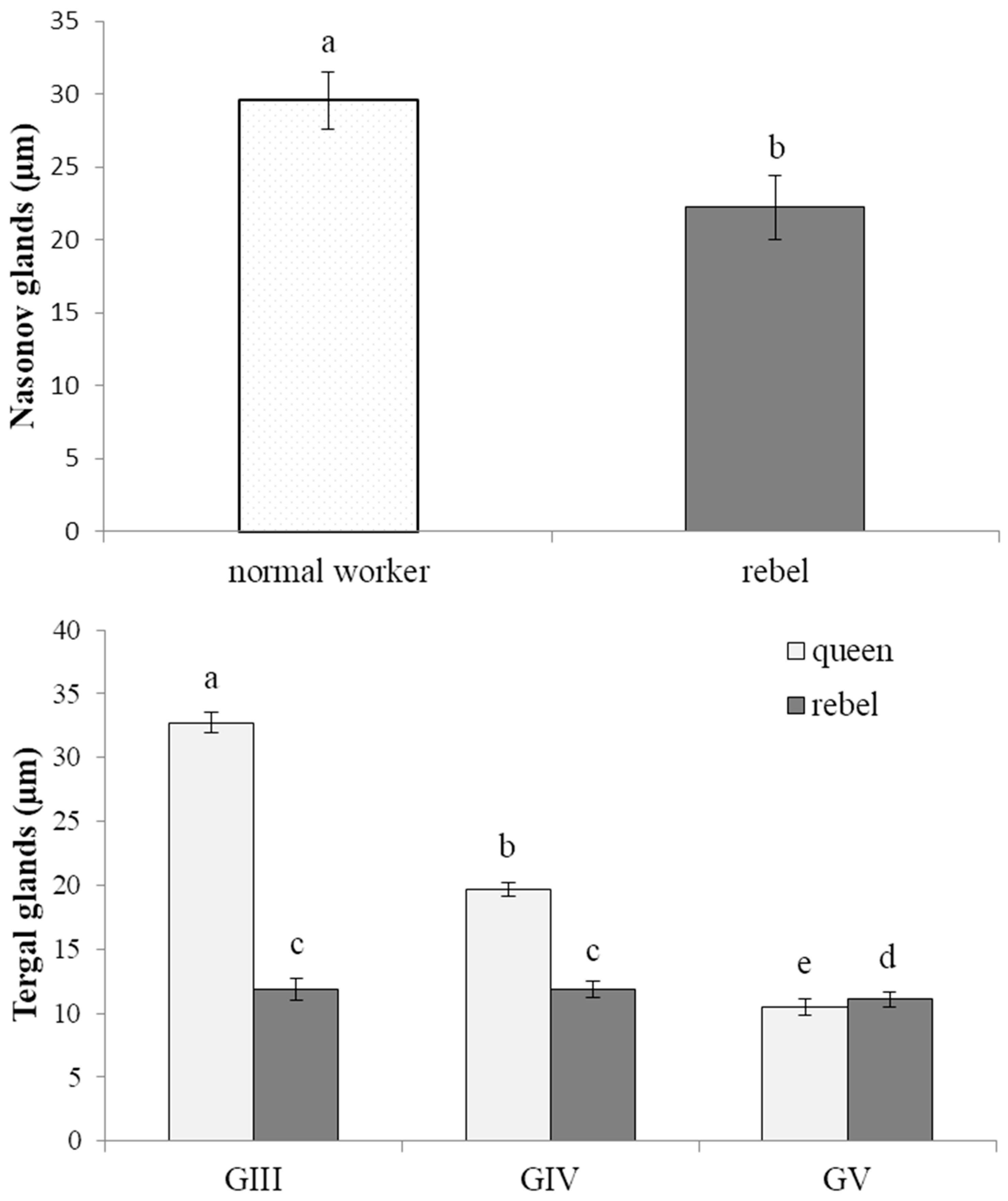
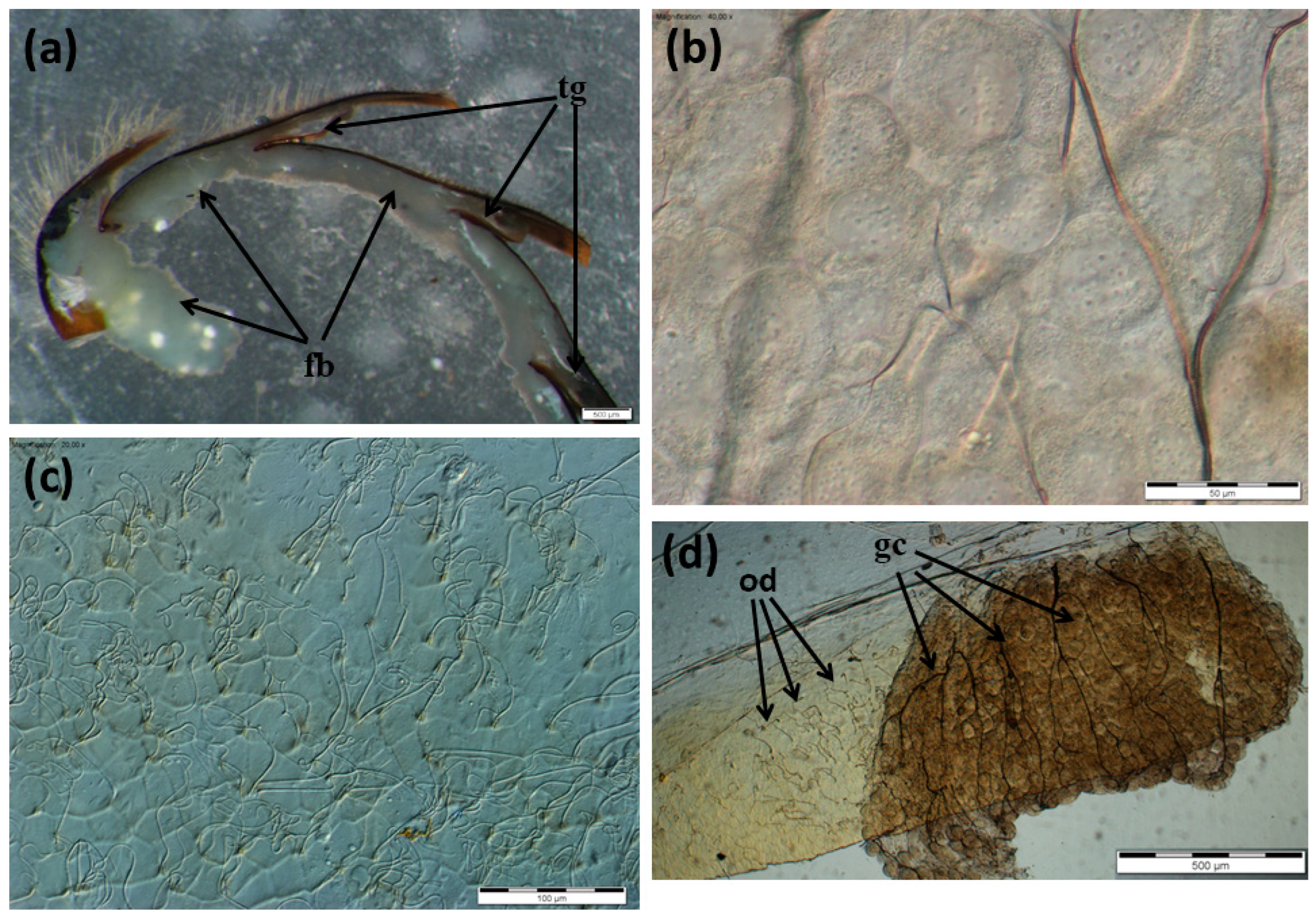
3.1. The Morphology of the Nasonov Gland
3.2. The Morphology of the Tergal Glands
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, D.; Iuzzolino, M.; Mankel, A.; Bozek, K.; Stephens, G.; Peleg, O. Flow-mediated olfactory communication in honeybee swarms. Proc. Natl. Acad. Sci. USA 2021, 118, e2011916118. [Google Scholar] [CrossRef] [PubMed]
- Mumoki, F.; Crewe, R. Pheromone communication in honey bees (Apis mellifera). In Insect Pheromone Biochemistry and Molecular Biology, 2nd ed.; Blomquist, G.J., Vogt, R.G., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 6, pp. 183–204. [Google Scholar]
- Slessor, K.; Winston, M.; Le Conte, Y. Pheromone communication in the honeybee (Apis mellifera L.). J. Chem. Ecol. 2005, 31, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.; Elgar, M. The evolution of pheromone diversity. Trends Ecol. Evol. 2008, 23, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E. Biosynthesis in Insects, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 28–56. [Google Scholar]
- Walsh, C.; Tang, Y. Natural Product Biosynthesis: Chemical Logic and Enzymatic Machinery, 1st ed.; Royal Society of Chemistry: London, UK, 2017; pp. 56–78. [Google Scholar]
- Alaux, C.; Maisonnasse, A.; Le Conte, Y. Pheromones in a superorganism: From gene to social regulation. Vitam. Horm. 2010, 83, 401–423. [Google Scholar] [PubMed]
- Brückner, A.; Parker, J. Molecular evolution of gland cell types and chemical interactions in animals. J. Exp. Biol. 2020, 223, jeb211938. [Google Scholar] [CrossRef] [PubMed]
- Oi, C.; van Zweden, J.; Oliveira, R.; Van Oystaeyen, A.; Nascimento, F.; Wenseleers, T. The origin and evolution of social insect queen pheromones: Novel hypotheses and outstanding problems. BioEssays 2015, 37, 808–821. [Google Scholar] [CrossRef]
- Raguso, R.A.; Agrawal, A.; Douglas, A.; Jander, G.; Kessler, A.; Poveda, K.; Thaler, J. The raison d’être of chemical ecology. Ecology 2015, 96, 617–630. [Google Scholar] [CrossRef]
- Tittiger, C. Functional genomics and insect chemical ecology. J. Chem. Ecol. 2004, 30, 2335–2358. [Google Scholar] [CrossRef]
- Bortolotti, L.; Costa, C. Chemical Communication in the Honey Bee Society. In Neurobiology of Chemical Communication, 1st ed.; Mucignat-Caretta, C., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: New York, NY, USA, 2014; Volume 5, pp. 147–210. [Google Scholar]
- Klett, K.; Zhang, J.J.; Zhang, Y.Y.; Wang, Z.; Dong, S.; Tan, K. The Nasonov gland pheromone as a potential source of death cue in Apis cerana. J. Insect Physiol. 2021, 8, 104238. [Google Scholar] [CrossRef]
- Renner, M.; Baumann, M. Über Komplexe von subepidermalen Drüsenzellen (Duftdrüsen?) der Bienenkönigin. Naturwissenschaften 1964, 3, 68–69. [Google Scholar] [CrossRef]
- Billen, J.; Dumortier, K.; Velthuis, H. Plasticity of honey bee castes: Occurrence of tergal glands in workers. Naturwissenschaften 1986, 73, 332–333. [Google Scholar] [CrossRef]
- Wossler, T.; Veale, R.; Crewe, R. How queen-like are the tergal glands in workers of Apis mellifera capensis and Apis mellifera scutellata? Apidologie 2000, 31, 55–66. [Google Scholar] [CrossRef]
- Moritz, R.; Crewe, R. Chemical signals of queens in kin recognition of honey bees, Apis mellifera L. J. Comp. Physiol. A 1988, 164, 83–89. [Google Scholar] [CrossRef]
- Wossler, T.; Crewe, R. The releaser effects of the tergal gland secretion of queens honey bees (Apis mellifera L.). J. Insect Behav. 1999, 12, 343–351. [Google Scholar] [CrossRef]
- Grozinger, C.; Fischer, P.; Hampton, J. Uncoupling primer and releaser responses to pheromone in honey bees. Naturwissenschaften 2007, 94, 375–379. [Google Scholar] [CrossRef]
- Ferguson, A.; Free, J. Factors determining the release of Nasonov pheromone by honey bees at the hive entrance. Physiolol. Entomol. 1981, 6, 15–19. [Google Scholar] [CrossRef]
- Al-Kahtani, S.; Bienefeld, K. The Nasonov gland pheromone is involved in recruiting honey bee workers for individual larvae to be reared as queens. J. Insect Behav. 2012, 25, 392–400. [Google Scholar] [CrossRef]
- Winston, M. The Biology of Honeybee, 1st ed.; Harvard University Press: Cambridge, MA, USA, 1987; pp. 129–168. [Google Scholar]
- Woyciechowski, M.; Kuszewska, K.; Pitorak, J.; Kierat, J. Honeybee worker larvae perceive queen pheromones in their food. Apidologie 2017, 48, 144–149. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Kuszewska, K. Swarming generates rebel workers in honeybees. Curr. Biol. 2012, 22, 707–711. [Google Scholar] [CrossRef][Green Version]
- Kuszewska, K.; Woyciechowski, M. Age at which larvae are orphaned determines their development into typical or rebel workers in the honeybee (Apis mellifera L.). PLoS ONE 2015, 10, e0123404. [Google Scholar] [CrossRef]
- Kuszewska, K.; Miler, K.; Rojek, W.; Woyciechowski, M. Honeybee workers with higher reproductive potential live longer lives. Exp. Gerontol. 2017, 98, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kuszewska, K.; Miler, K.; Rojek, W.; Ostap-Chęć, M.; Woyciechowski, M. Rebel honeybee workers have a tendency to become intraspecific reproductive parasites. Ecol. Evol. 2018, 8, 11914–11920. [Google Scholar] [CrossRef] [PubMed]
- Moritz, R.; Crewe, R.; Hepburn, H. Queen avoidance and mandibular gland secretion of honeybee workers (Apis mellifera L.). Insect Sociaux 2002, 49, 86–91. [Google Scholar] [CrossRef]
- Malka, O.; Shnieor, S.; Hefetz, A.; Katzav-Gozansky, T. Reversible royalty in worker honeybees (Apis mellifera) under the queen influence. Behav. Ecol. Sociobiol. 2007, 61, 465–473. [Google Scholar] [CrossRef]
- Brockmann, A.; Dietz, D.; Spaethe, J.; Tautz, J. Beyond 9-ODA: Sex pheromone communication in the European honey bee Apis mellifera L. J. Chem. Ecol. 2006, 32, 657–667. [Google Scholar] [CrossRef]
- Büchler, R.; Andonov, S.; Bienefeld, K.; Costa, C.; Hatjina, F.; Kezic, N.; Kryger, P.; Spivak, M.; Uzunov, A.; Wilde, J. Standard methods for rearing and selection of Apis mellifera queens. J. Apic. Res. 2013, 52, 1–30. [Google Scholar] [CrossRef]
- Strachecka, A.; Chobotow, J.; Paleolog, J.; Łoś, A.; Schulz, M.; Teper, D.; Kucharczyk, H.; Grzybek, M. Insights into the biochemical defence and methylation of the solitary bee Osmia rufa L.: A foundation for examining eusociality development. PLoS ONE 2017, 12, e0176539. [Google Scholar] [CrossRef]
- Kuszewska, K.; Miler, K.; Woyciechowski, M. Honeybee rebel workers invest less in risky foraging than normal workers. Sci. Rep. 2018, 8, 9459. [Google Scholar] [CrossRef]
- Kuszewska, K.; Wącławska, A.; Woyciechowski, M. Reproduction of rebel workers in honeybee (Apis mellifera) colonies. Apidologie 2018, 49, 162–171. [Google Scholar] [CrossRef]
- Kuszewska, K.; Miler, K.; Woyciechowski, M. Honeybee rebel workers preferentially respond to high concentrations of sucrose. Apidologie 2019, 50, 253–261. [Google Scholar] [CrossRef]
- Kuszewska, K.; Rojek, W. Honeybee workers with higher reproductive potential have a greater learning ability. Apidologie 2021, 52, 608–619. [Google Scholar] [CrossRef]
- Marshall, H.; Lonsdale, Z.; Mallon, E. Methylation and gene expression differences between reproductive and sterile bumblebee workers. Evol. Lett. 2019, 3, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Herman, J.; Rueppell, O. Reproductive activation in honeybee (Apis mellifera) workers protects against abiotic and biotic stress. Philos. Trans. R. Soc. B 2021, 376, 20190737. [Google Scholar] [CrossRef] [PubMed]
- Ratnieks, F.; Visscher, P. Worker policing in the honeybee. Nature 1989, 342, 796–797. [Google Scholar] [CrossRef]
- Okosun, O.; Yusuf, A.; Crewe, R.; Pirk, C. Effects of age and reproductive status on tergal gland secretions in queenless honey bee workers, Apis mellifera scutellata and A. m. capensis. J. Chem. Ecol. 2015, 41, 896–903. [Google Scholar] [CrossRef]
- Okosun, O.; Yusuf, A.; Crewe, R.; Pirk, C.W. Tergal gland components of reproductively dominant honey bee workers have both primer and releaser effects on subordinate workers. Apidologie 2019, 50, 173–182. [Google Scholar] [CrossRef]
- De Hazan, M.; Lensky, Y.; Cassier, P. Effects of queen honeybee (Apis mellifera L.) ageing on her attractiveness to workers. Comp. Biochem. Physiol. Part A 1989, 93, 777–783. [Google Scholar] [CrossRef]
- Smith, R.; Spivak, M.; Taylor, J.; Bennett, C.; Smith, M. Maturation of tergal gland alkene profiles in European honey bee queens, Apis mellifera L. J. Chem. Ecol. 1993, 19, 133–142. [Google Scholar] [CrossRef]
- Malka, O.; Shnieor, S.; Katzav-Gozansky, T.; Hefetz, A. Aggressive reproductive competition among hopelessly queenless honey bee workers triggered by pheromone signaling. Naturwissenschaften 2008, 95, 553–559. [Google Scholar] [CrossRef]
- Deseyn, J.; Billen, J. Age-dependent morphology and ultrastructure of the hypopharyngeal gland of Apis mellifera workers (Hymenoptera, Apidae). Apidologie 2005, 36, 49–57. [Google Scholar] [CrossRef]
- Amdam, G.; Aase, A.; Seehuus, S.; Kim Fondrk, M.; Norberg, K.; Hartfelder, K. Social reversal of immunosenescence in honey bee workers. Exp. Gerontol. 2005, 40, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Woyciechowski, M.; Kozłowski, J. Division of labor by division of risk according to worker life expectancy in the honey bee (Apis mellifera L.). Apidologie 1998, 29, 191–205. [Google Scholar] [CrossRef]
- Oldroyd, B.; Beekman, M. Effects of selection for honey bee worker reproduction on foraging traits. PLoS Biol. 2008, 6, e56. [Google Scholar] [CrossRef] [PubMed]
- Woyciechowski, M.; Moroń, D. Life expectancy and onset of foraging in the honeybee (Apis mellifera). Insect Sociaux 2009, 56, 193–201. [Google Scholar] [CrossRef]
- Favaro, R.; Roved, J.; Haase, A.; Angeli, S. Impact of chronic exposure to two neonicotinoids on honey bee antennal responses to flower volatiles and pheromonal compounds. Front. Insect Sci. 2022, 2, 821145. [Google Scholar] [CrossRef]
- Strachecka, A.; Migdał, P.; Kuszewska, K.; Skowronek, P.; Grabowski, M.; Paleolog, J.; Woyciechowski, M. Humoral and cellular defense mechanisms in rebel workers of Apis mellifera. Biology 2021, 10, 1146. [Google Scholar] [CrossRef]
- Hamilton, W. The genetical evolution of social behaviour I-II. J. Theor. Biol. 1964, 7, 17–52. [Google Scholar] [CrossRef]
- Smith, M. Kin selection and group selection. Nature 1964, 201, 1145–1147. [Google Scholar] [CrossRef]
- Gadagkar, R. The evolution of eusociality, including a review of the social status of Ropalidia marginata. In Natural History and Evolution of Paper Wasps, 1st ed.; Turillazzi, S., West-Eberhard, M.J., Eds.; Oxford University Press: Oxford, UK, 1996; Volume 1, pp. 248–271. [Google Scholar]
- Nonacs, P. Kinship, greenbeards, and runaway social selection in the evolution of social insect cooperation. Proc. Natl. Acad. Sci. USA 2011, 108, 10808–10815. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Lomnicki, A. Multiple mating of queens and the sterility of workers among eusocial Hymenoptera. J. Theor. Biol. 1987, 128, 317–327. [Google Scholar] [CrossRef]
- Ptaszyńska, A.; Borsuk, G.; Mułenko, W.; Demetraki-Paleolog, J. Differentiation of Nosema apis and Nosema ceranae spores under Scanning Electron Microscopy (SEM). J. Apicul. Res. 2014, 53, 537–544. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strachecka, A.; Chobotow, J.; Kuszewska, K.; Olszewski, K.; Skowronek, P.; Bryś, M.; Paleolog, J.; Woyciechowski, M. Morphology of Nasonov and Tergal Glands in Apis mellifera Rebels. Insects 2022, 13, 401. https://doi.org/10.3390/insects13050401
Strachecka A, Chobotow J, Kuszewska K, Olszewski K, Skowronek P, Bryś M, Paleolog J, Woyciechowski M. Morphology of Nasonov and Tergal Glands in Apis mellifera Rebels. Insects. 2022; 13(5):401. https://doi.org/10.3390/insects13050401
Chicago/Turabian StyleStrachecka, Aneta, Jacek Chobotow, Karolina Kuszewska, Krzysztof Olszewski, Patrycja Skowronek, Maciej Bryś, Jerzy Paleolog, and Michał Woyciechowski. 2022. "Morphology of Nasonov and Tergal Glands in Apis mellifera Rebels" Insects 13, no. 5: 401. https://doi.org/10.3390/insects13050401
APA StyleStrachecka, A., Chobotow, J., Kuszewska, K., Olszewski, K., Skowronek, P., Bryś, M., Paleolog, J., & Woyciechowski, M. (2022). Morphology of Nasonov and Tergal Glands in Apis mellifera Rebels. Insects, 13(5), 401. https://doi.org/10.3390/insects13050401





