Immune Defense Mechanism of Reticulitermes chinensis Snyder (Blattodea: Isoptera) against Serratia marcescens Bizio
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Insect Rearing and Experimental Treatment
2.2. RNA Isolation and Sequencing
2.3. Transcript Assembly and Sequence Annotation
2.4. Differential Gene Expression between R. chinensis Infected with SM1 and the Control R. chinensis
2.5. Quantitative Reverse-Transcription PCR (qRT-PCR)
2.6. Statistical Method
3. Results
3.1. Analysis of R. chinensis Transcriptome Data
3.1.1. R. chinensis Transcriptome Assembly
3.1.2. Functional Annotation of R. chinensis Transcripts
3.1.3. DEGs in the Response of R. chinensis to SM1 Infection
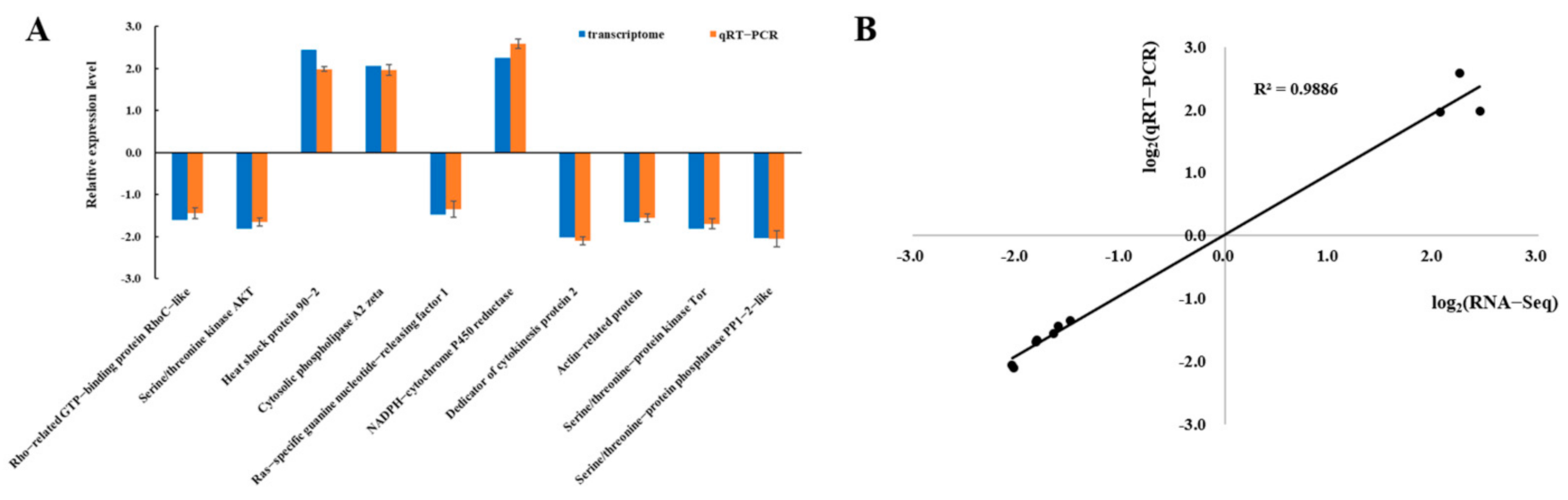
3.2. Verification of Transcriptomic Data by qRT-PCR
3.3. Immune-Related DEGs in R. chinensis Infected with SM1
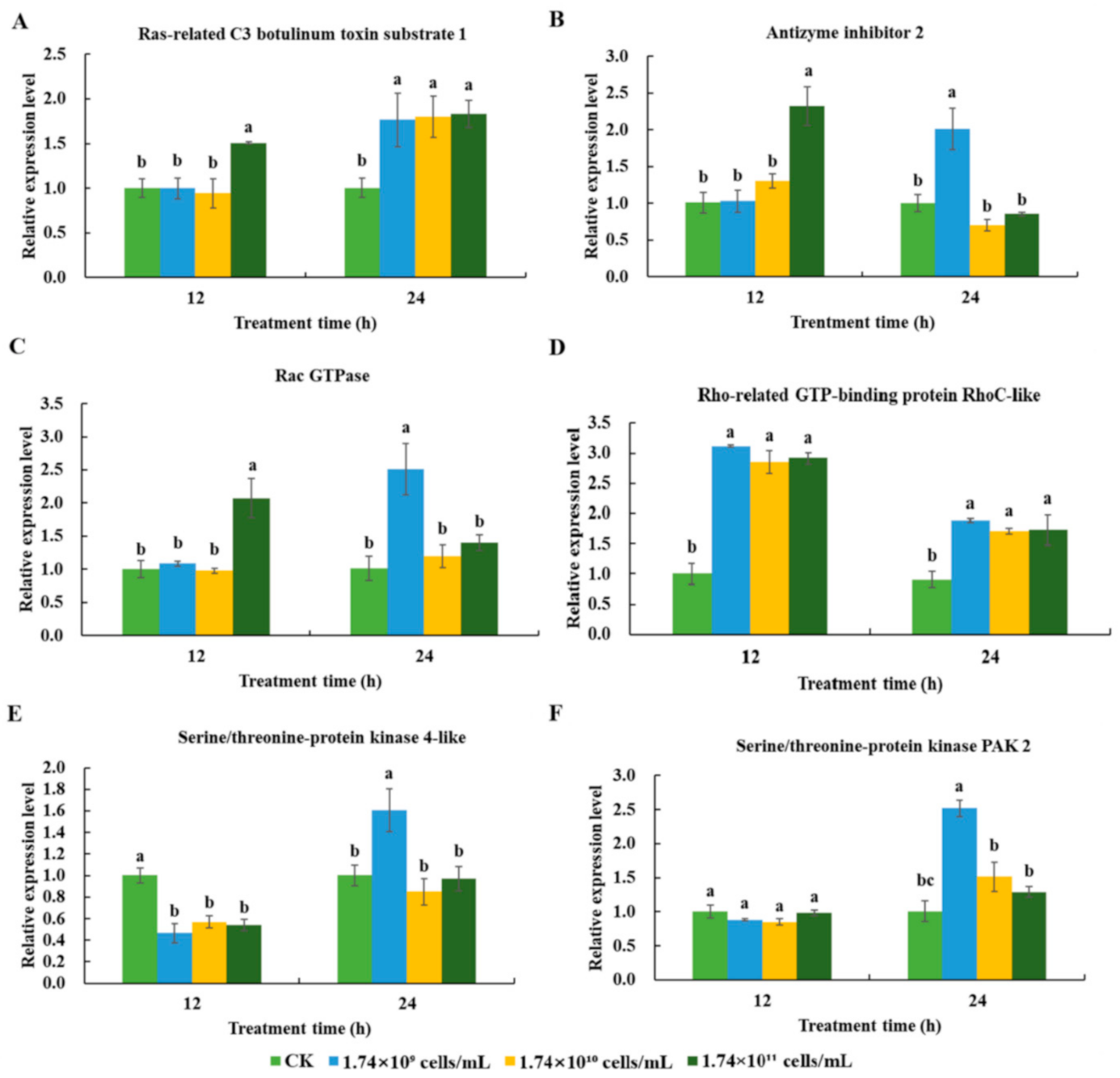
3.3.1. Cellular Immune Responses Induced by SM1 in R. chinensis
3.3.2. Expression Profiles of Cellular Immunity-related Genes in R. chinensis Infected with SM1
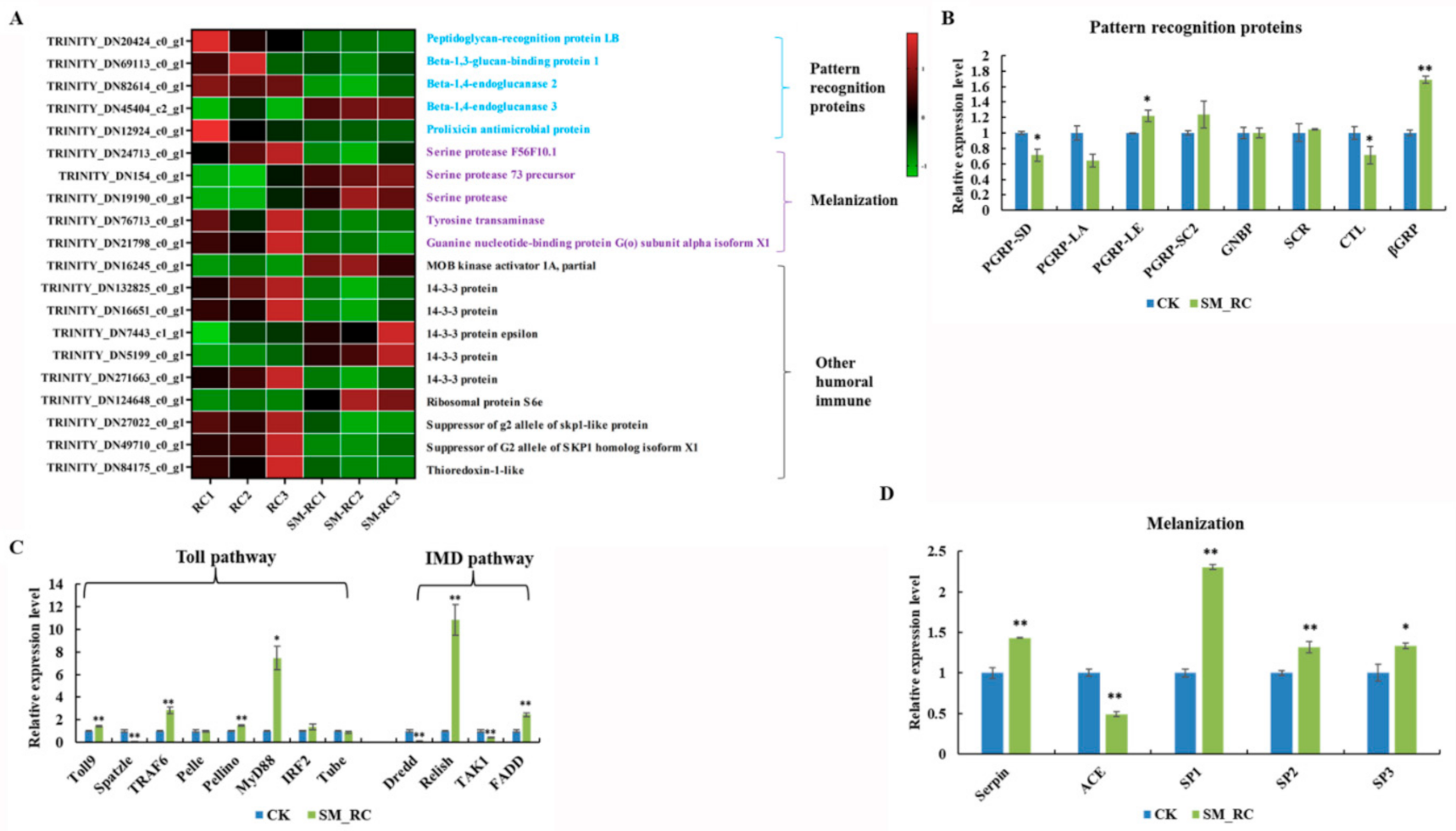
3.3.3. Humoral Immune Responses Induced by SM1 in R. chinensis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, X.; Li, X.; Wang, Y.; Peng, J.; Hong, H.; Yang, H. Phylogenetic diversity of nitrogen fixation genes in the intestinal tract of Reticulitermes chinensis Snyder. Curr. Microbiol. 2012, 65, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, G.; Husseneder, C.; Lei, C. Genetic analysis of population structure and reproductive mode of the termite Reticulitermes chinensis snyder. PLoS ONE 2013, 8, e69070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Tong, Y.; Xiong, Q.; Huang, Q. Efficacy of three kinds of baits against the subterranean termite Reticulitermes chinensis (Isoptera: Rhinotermitidae) in rural houses in China. Sociobiology 2010, 56, 209–222. [Google Scholar]
- Ahmad, F.; Fouad, H.; Liang, S.Y.; Hu, Y.; Mo, J.C. Termites and Chinese agricultural system: Applications and advances in integrated termite management and chemical control. Insect Sci. 2021, 28, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Atsbha, G.; Hintsa, M. Evaluation of chemical, botanical and cultural management options of termite in Tanqua Abergelle district, Ethiopia. Afr. J. Plant Sci. 2018, 12, 98–104. [Google Scholar] [CrossRef]
- Francis, J.R. Biocontrol potential and genetic diversity of Metarhizium anisopliae lineage in agricultural habitats. J. Appl. Microbiol. 2019, 127, 556–564. [Google Scholar] [CrossRef]
- Wright, M.S.; Raina, A.K.; Lax, A.R. A strain of the fungus Metarhizium anisopliae for controlling subterranean termites. J. Econ. Entomol. 2005, 98, 1451–1458. [Google Scholar] [CrossRef]
- Chin, K.L.; H’ng, P.S.; Lee, C.L.; Wong, W.Z.; Go, W.Z.; Khoo, P.S.; Luqman, A.C.; Ashaari, Z. Application strategies by selective medium treated with entomopathogenic bacteria Serratia marcescens and Pseudomonas aeruginosa as potential biocontrol against Coptotermes curvignathus. R Soc. Open Sci. 2021, 8, 201311. [Google Scholar] [CrossRef]
- Devi, K.K.; Seth, N.; Kothamasi, S.; Kothamasi, D. Hydrogen cyanide-producing rhizobacteria kill subterranean termite Odontotermes obesus (Rambur) by cyanide poisoning under in vitro conditions. Curr. Microbiol. 2007, 54, 74–78. [Google Scholar] [CrossRef]
- Devi, K.K.; Kothamasi, D. Pseudomonas fluorescens CHA0 can kill subterranean termite Odontotermes obesus by inhibiting cytochrome c oxidase of the termite respiratory chain. FEMS Microbiol. Lett. 2009, 300, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.J.; Qi, X.L.; Feng, K.; Xia, X.R.; Tang, F. Identification and characters of a strain of Serratia marcescens isolated from the Odontotermes formosanus. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2019, 43, 76–82. [Google Scholar]
- Hejazi, A.; Falkiner, F.R. Serratia marcescens. J. Med. Microbiol. 1997, 46, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Montaner, B.; Perez-Tomas, R. The prodigiosins: A new family of anticancer drugs. Curr. Cancer Drug Targets 2003, 3, 57–65. [Google Scholar] [CrossRef]
- Khan, K.I.; FazaI, Q.; Jafri, R.H.; Ahmad, M. Susceptbility of various species of termites to a pathogen, Serratia marcescens. Pak. J. Sci. 1977, 29, 46–47. [Google Scholar]
- Ishii, K.; Adachi, T.; Hara, T.; Hamamoto, H.; Sekimizu, K. Identification of a Serratia marcescens virulence factor that promotes hemolymph bleeding in the silkworm, Bombyx mori. J. Invertebr. Pathol. 2014, 117, 61–67. [Google Scholar] [CrossRef]
- Aggarwal, C.; Paul, S.; Tripathi, V.; Paul, B.; Khan, M.A. Characterization of putative virulence factors of Serratia marcescens strain SEN for pathogenesis in Spodoptera litura. J. Invertebr. Pathol. 2017, 143, 115–123. [Google Scholar] [CrossRef]
- Niu, H.T.; Wang, N.; Liu, B.S.; Xiao, L.J.J.; Wang, L.H.; Guo, H.F. Synergistic and additive interactions of Serratia marcescens S-JS1 to the chemical insecticides for controlling Nilaparvata lugens (Hemiptera: Delphacidae). J. Econ. Entomol. 2018, 111, 823–828. [Google Scholar] [CrossRef]
- Shokal, U.; Yadav, S.; Atri, J.; Accetta, J.; Kenney, E.; Banks, K.; Katakam, A.; Jaenike, J.; Eleftherianos, I. Effects of co-occurring Wolbachia and Spiroplasma endosymbionts on the Drosophila immune response against insect pathogenic and non-pathogenic bacteria. BMC Microbiol. 2016, 16, 16. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; De Mandal, S.; Xu, X.; Jin, F. The tripartite interaction of host immunity-Bacillus thuringiensis infection-gut microbiota. Toxins 2020, 12, 514. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The gene ontology consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M. The KEGG database. Novart Fdn Symp. 2002, 247, 91–101; discussion 101–103, 119–128, 244–252. [Google Scholar]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Giulietti, A.; Oververgh, L.; Valckx, D.; Decallonne, B.; Bouillon, R.; Mathieu, C. An overview of real-time quantitative PCR: Applications to quantify cytokine gene expression. Methods 2001, 25, 386–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishilevich, E.; Vélez, A.M.; Storer, N.P.; Li, H.; Bowling, A.J.; Rangasamy, M.; Worden, S.E.; Narva, K.E.; Siegfried, B.D. RNAi as a management tool for the western corn rootworm, Diabrotica virgifera virgifera. Pest Manag. Sci. 2016, 72, 1652–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitsikas, V.; Corrêa, I.R.J.; Nichols, B.J. Clathrin-independent pathways do not contribute significantly to endocytic flux. Elife 2014, 3, e03970. [Google Scholar] [CrossRef]
- Jo, Y.H.; Lee, J.H.; Patnaik, B.B.; Keshavarz, M.; Lee, Y.S.; Han, Y.S. Autophagy in Tenebrio molitor immunity: Conserved antimicrobial functions in insect defenses. Front. Immunol. 2021, 12, 667664. [Google Scholar] [CrossRef]
- Sandiford, S.L.; Dong, Y.; Pike, A.; Blumberg, B.J.; Bahia, A.C.; Dimopoulos, G. Cytoplasmic actin is an extracellular insect immune factor which is secreted upon immune challenge and mediates phagocytosis and direct killing of bacteria, and is a Plasmodium antagonist. PLoS Pathog 2015, 11, e1004631. [Google Scholar] [CrossRef] [Green Version]
- Pieters, J. Coronin 1 in innate immunity. Subcell Biochem. 2008, 48, 116–123. [Google Scholar]
- Hinton, A.; Bond, S.; Forgac, M. V-ATPase functions in normal and disease processes. Pflug. Arch. 2009, 457, 589–598. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, N.; Xie, X.; Bi, G.; Ba, H.; Li, L.; Zhang, J.; Deng, X.; Yao, Y.; Tang, Z.; et al. The macrophage-specific V-ATPase subunit ATP6V0D2 restricts inflammasome activation and bacterial infection by facilitating autophagosome-lysosome fusion. Autophagy 2019, 15, 960–975. [Google Scholar] [CrossRef]
- Malagoli, D.; Abdalla, F.C.; Cao, Y.; Feng, Q.; Fujisaki, K.; Gregorc, A.; Matsuo, T.; Nezis, I.P.; Papassideri, I.S.; Sass, M.; et al. Autophagy and its physiological relevance in arthropods: Current knowledge and perspectives. Autophagy 2010, 6, 575–588. [Google Scholar] [CrossRef] [Green Version]
- Romanelli, D.; Casati, B.; Franzetti, E.; Tettamanti, G. A molecular view of autophagy in Lepidoptera. BioMed. Res. Int. 2014, 2014, 902315. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Ma, L.; Guo, E.E.; Deng, X.J.; Ma, S.Y.; Xia, Q.Y.; Cao, Y.; Li, S. 20-hydroxyecdysone upregulates Atg genes to induce autophagy in the Bombyx fat body. Autophagy 2013, 9, 1172–1187. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.J.; Hansen, M.; Troemel, E. Autophagy and innate immunity: Insights from invertebrate model organisms. Autophagy 2018, 14, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.P.A.; Boucrot, E. Mechanisms of carrier formation during clathrin-independent endocytosis. Trends Cell Biol. 2018, 28, 188–200. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012, 7, 61–98. [Google Scholar] [CrossRef]
- Freeman, S.A.; Grinstein, S. Phagocytosis: Receptors, signal integration, and the cytoskeleton. Immunol. Rev. 2014, 262, 193–215. [Google Scholar] [CrossRef]
- Jezegou, A.; Llinares, E.; Anne, C.; Kieffer-Jaquinod, S.; O’Regan, S.; Aupetit, J.; Chabli, A.; Sagne, C.; Debacker, C.; Chadefaux-Vekemans, B. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc. Natl. Acad. Sci. USA 2012, 109, E3434–E3443. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Du, H.; Rutkowski, R.; Gartner, A.; Wang, X. LAAT-1 is the lysosomal lysine/arginine transporter that maintains amino acid homeostasis. Science 2012, 337, 351–354. [Google Scholar] [CrossRef] [Green Version]
- Rong, Y.G.; McPhee, C.K.; Deng, S.; Huang, L.; Chen, L.; Liu, M.; Tracy, K.; Baehrecke, E.H.; Yu, L.; Lenardo, M.J. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc. Natl. Acad. Sci. USA 2011, 108, 7826–7831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagne, C.; Agulhon, C.; Ravassard, P.; Darmon, M.; Hamon, M.; El Mestikawy, S.; Gasnier, B.; Giros, B. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc. Natl. Acad. Sci. USA 2001, 98, 7206–7211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Gökçen, F.; Felföldi, G.; Millichap, P.J.; Trenczek, T.E.; Ffrench-Constant, R.H.; Reynolds, S.E. The immunoglobulin family protein hemolin mediates cellular immune responses to bacteria in the insect Manduca sexta. Cell Microbiol. 2007, 9, 1137–1147. [Google Scholar] [CrossRef]
- Arteaga, B.L.A.; Crispim, J.S.; Fernandes, K.M.; De Oliveira, L.L.; Pereira, M.F.; Bazzolli, D.M.S.; Bazzolli, G.F. Differential cellular immune response of Galleria mellonella to Actinobacillus pleuropneumoniae. Cell Tissue Res. 2017, 370, 153–168. [Google Scholar] [CrossRef]
- Gul, I.; Kausar, S.; You, Q.; Sun, W.; Li, Z.; Abbas, M.N.; Cui, H. Identification and the immunological role of two nimrod family genes in the silkworm, Bombyx mori. Int J. Biol. Macromol. 2021, 193, 154–165. [Google Scholar] [CrossRef]
- Sheehan, G.; Garvey, A.; Croke, M.; Kavanagh, K. Innate humoral immune defences in mammals and insects: The same, with differences? Virulence 2018, 9, 1625–1639. [Google Scholar] [CrossRef] [Green Version]
- Silverman, N.; Paquette, N.; Aggarwal, K. Specificity and signaling in the Drosophila immune response. ISJ-Invert. Surviv. J. 2009, 6, 163–174. [Google Scholar]
- Tanji, T.; Ip, Y.T. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol. 2005, 26, 193–198. [Google Scholar] [CrossRef]
- Grizanova, E.V.; Dubovskiy, I.M.; Whitten, M.M.; Glupov, V.V. Contributions of cellular and humoral immunity of Galleria mellonella larvae in defence against oral infection by Bacillus thuringiensis. J. Invertebr. Pathol. 2014, 119, 40–46. [Google Scholar] [CrossRef]
- Shao, Q.M.; Yang, B.; Xu, Q.Y.; Li, X.Q.; Lu, Z.Q.; Wang, C.S.; Huang, Y.P.; Soderhall, K.; Ling, E.J. Hindgut innate immunity and regulation of fecal microbiota through melanization in insects. J. Biol. Chem. 2012, 287, 14270–14279. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Yu, X.Q.; Wang, Q.; Tao, X.; Li, J.; Zhang, S.; Xia, X.; You, M. Immune responses to Bacillus thuringiensis in the midgut of the diamondback moth, Plutella xylostella. Dev. Comp. Immunol. 2020, 107, 103661. [Google Scholar] [CrossRef]
- Valanne, S.; Wang, J.H.; Ramet, M. The Drosophila Toll signaling pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Silverman, N.; Maniatis, T. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001, 15, 2321–2342. [Google Scholar] [CrossRef] [Green Version]
- Baeg, G.H.; Zhou, R.; Perrimon, N. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 2005, 19, 1861–1870. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biot. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [Green Version]
- Choe, K.M.; Lee, H.; Anderson, K.V. Drosophila peptidoglycan recognition protein LC (PGRP-LC) acts as a signal-transducing innate immune receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 1122–1126. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, T.; Yano, T.; Aggarwal, K.; Lim, J.H.; Ueda, K.; Oshima, Y.; Peach, C.; Erturk-Hasdemir, D.; Goldman, W.E.; Oh, B.H.; et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the Drosophila immune response to monomeric DAP-type peptidoglycan. Nat. Immunol. 2006, 7, 715–723. [Google Scholar] [CrossRef]
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426, 33–38. [Google Scholar] [CrossRef]
- Stoven, S.; Ando, I.; Kadalayil, L.; Engstrom, Y.; Hultmark, D. Activation of the Drosophila NF-kB factor relish by rapid endoproteolytic cleavage. EMBO Rep. 2000, 1, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Stoven, S.; Silverman, N.; Junell, A.; Hedengren-Olcott, M.; Erturk, D.; Engstrom, Y.; Maniatis, T.; Hultmark, D. Caspase-mediated processing of the Drosophila NF-kB factor relish. Proc. Natl. Acad. Sci. USA. 2003, 100, 5991–5996. [Google Scholar] [CrossRef] [Green Version]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef]
- Xia, X.; You, M.; Rao, X.J.; Yu, X.Q. Insect C-type lectins in innate immunity. Dev. Comp. Immunol. 2018, 83, 70–79. [Google Scholar] [CrossRef]
- Chu, Y.; Hong, F.; Liu, Q.; An, C. Serine protease SP105 activates prophenoloxidase in Asian corn borer melanization, and is regulated by serpin-3. Sci. Rep. 2017, 7, 45256. [Google Scholar] [CrossRef] [Green Version]
- Huybrechts, R.; Coltura, L. Immune-induced angiotensin-converting enzyme assures the appearance of complementary peptides in Locusta migratoria for fine-tuning the innate immune response by inhibiting immune-activated phenoloxidase. Trends Entomol. 2018, 14, 11–16. [Google Scholar] [CrossRef]
- Christensen, B.M.; Li, J.; Chen, C.C.; Nappi, A.J. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhou, F.; Liu, Y.; Hong, F.; Wang, G.R.; An, C.J. Ostrinia furnacalis serpin-3 regulates melanization cascade by inhibiting a prophenoloxidase-activating protease. Insect Biochem. Mol. Biol. 2015, 61, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.R.; Jiang, H.B.; Kanost, M.R. Identification of plasma proteases inhibited by Manduca sexta serpin-4 and serpin-5 and their association with components of the prophenol oxidase activation pathway. J. Biol. Chem. 2005, 280, 14932–14942. [Google Scholar] [CrossRef] [Green Version]



| Transcript Length Interval | Number of Transcripts | Percentage (%) |
|---|---|---|
| <500 bp | 242,703 | 61.55 |
| 500–1 k | 86,025 | 21.82 |
| 1–2 k | 40,128 | 10.18 |
| >2 k | 25,434 | 6.45 |
| Total | 394,290 | |
| Length of all transcripts | 285,969,777 | |
| N50 (bp) | 1088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Wang, Z.; Tang, F.; Feng, K. Immune Defense Mechanism of Reticulitermes chinensis Snyder (Blattodea: Isoptera) against Serratia marcescens Bizio. Insects 2022, 13, 226. https://doi.org/10.3390/insects13030226
Luo J, Wang Z, Tang F, Feng K. Immune Defense Mechanism of Reticulitermes chinensis Snyder (Blattodea: Isoptera) against Serratia marcescens Bizio. Insects. 2022; 13(3):226. https://doi.org/10.3390/insects13030226
Chicago/Turabian StyleLuo, Jian, Zhiqiang Wang, Fang Tang, and Kai Feng. 2022. "Immune Defense Mechanism of Reticulitermes chinensis Snyder (Blattodea: Isoptera) against Serratia marcescens Bizio" Insects 13, no. 3: 226. https://doi.org/10.3390/insects13030226
APA StyleLuo, J., Wang, Z., Tang, F., & Feng, K. (2022). Immune Defense Mechanism of Reticulitermes chinensis Snyder (Blattodea: Isoptera) against Serratia marcescens Bizio. Insects, 13(3), 226. https://doi.org/10.3390/insects13030226





