Half Friend, Half Enemy? Comparative Phytophagy between Two Dicyphini Species (Hemiptera: Miridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of Mirid Predators
2.2. Phytophagy in Semi-Field Conditions
2.3. Fruit Damage
2.4. Location on Tomato Plant versus Fruit
2.5. Data Analysis
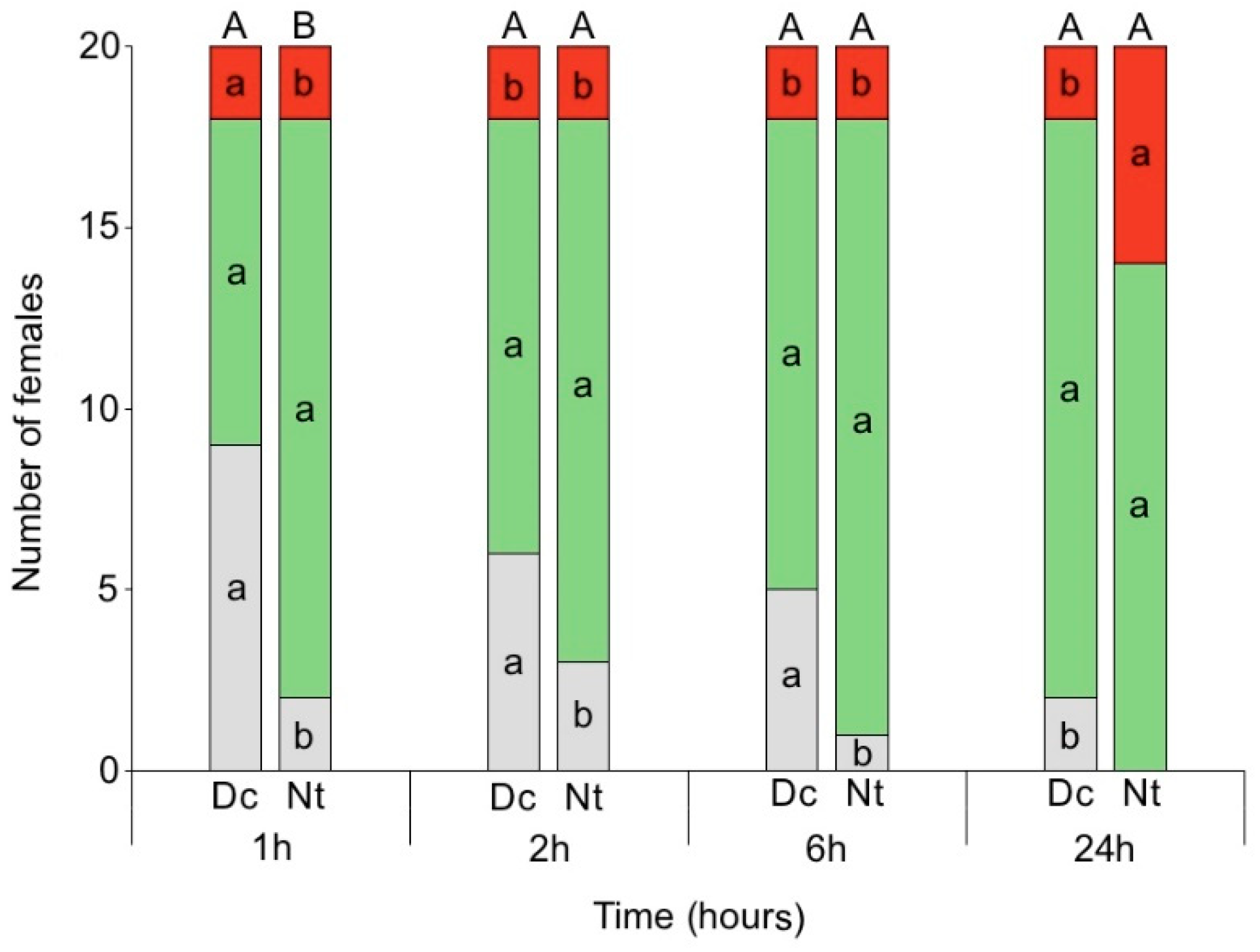
3. Results
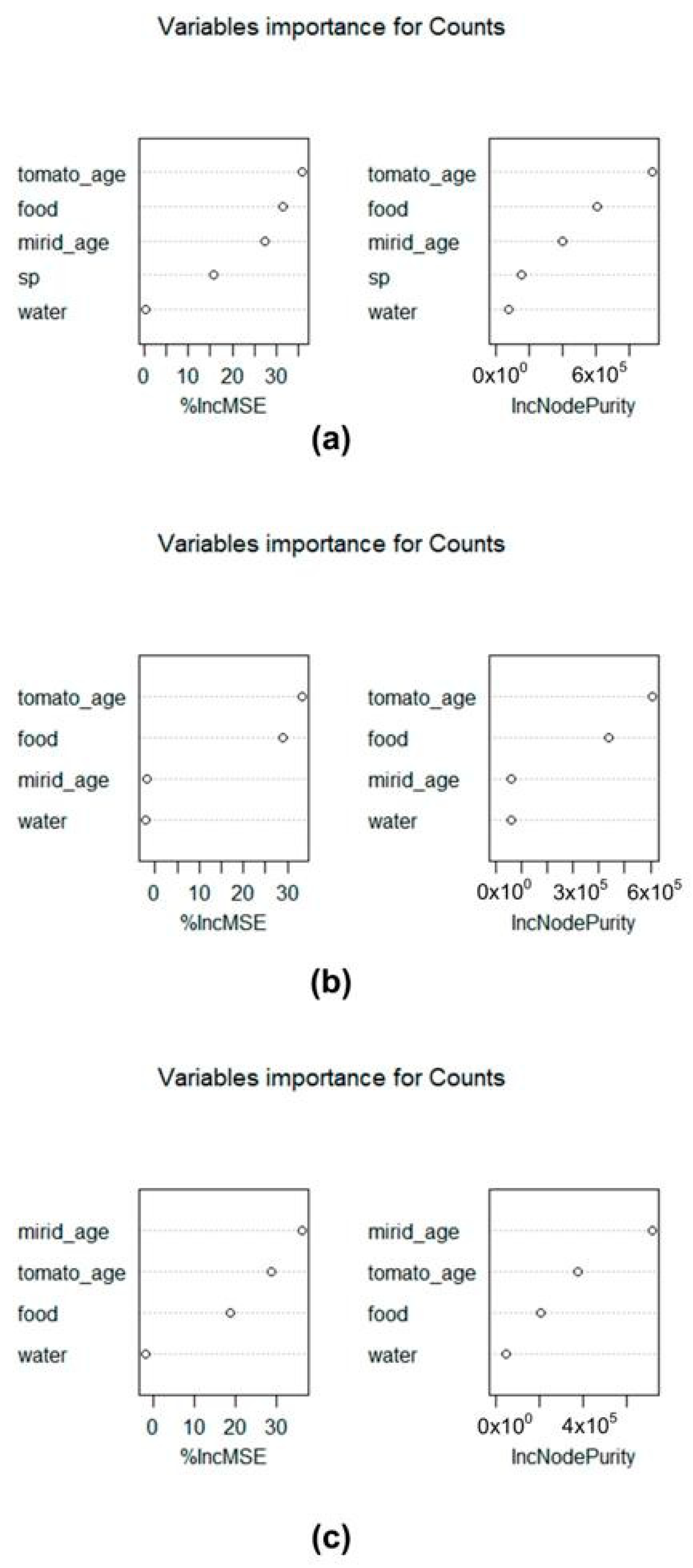
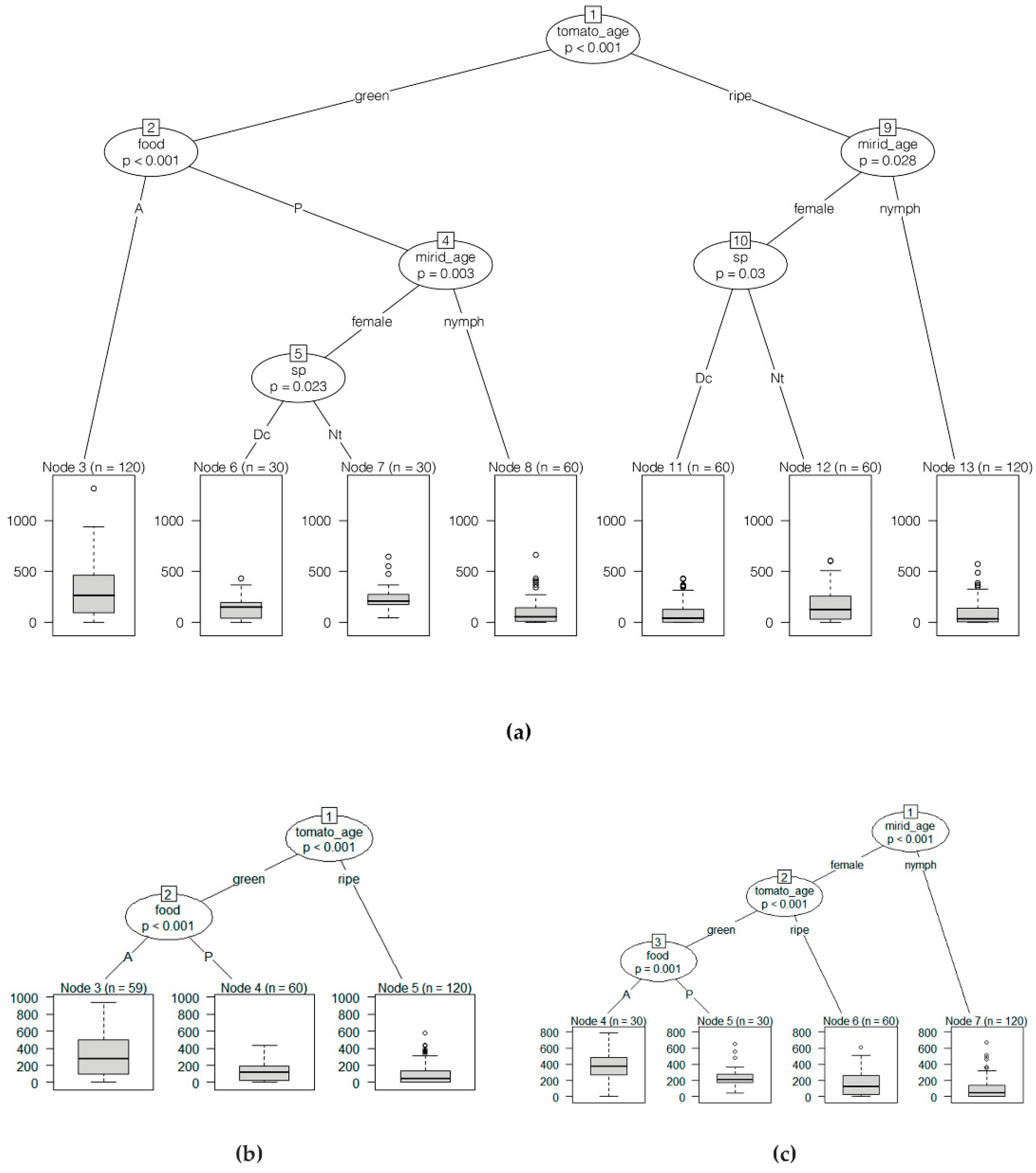
3.1. Phytophagy in Semi-Field Conditions
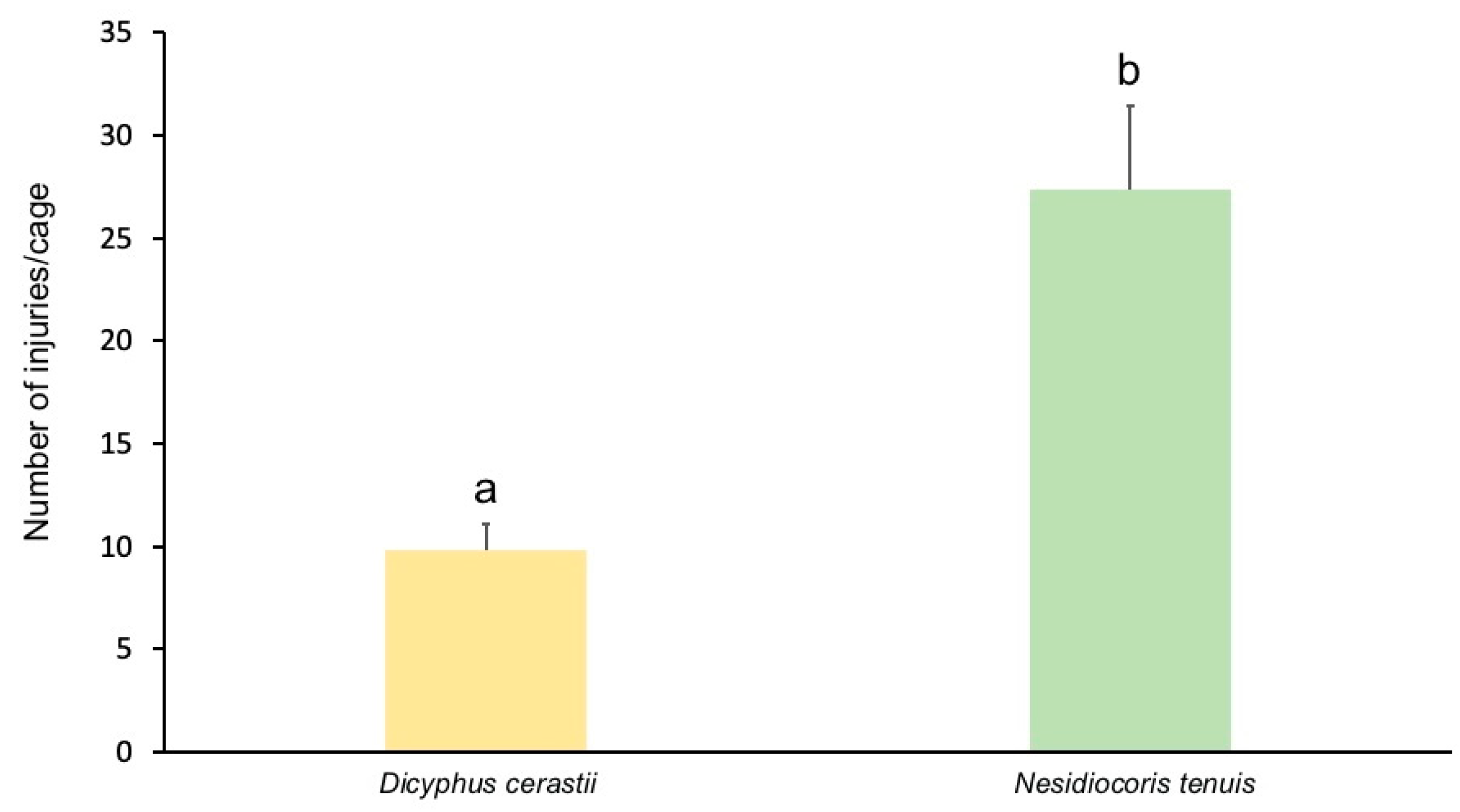
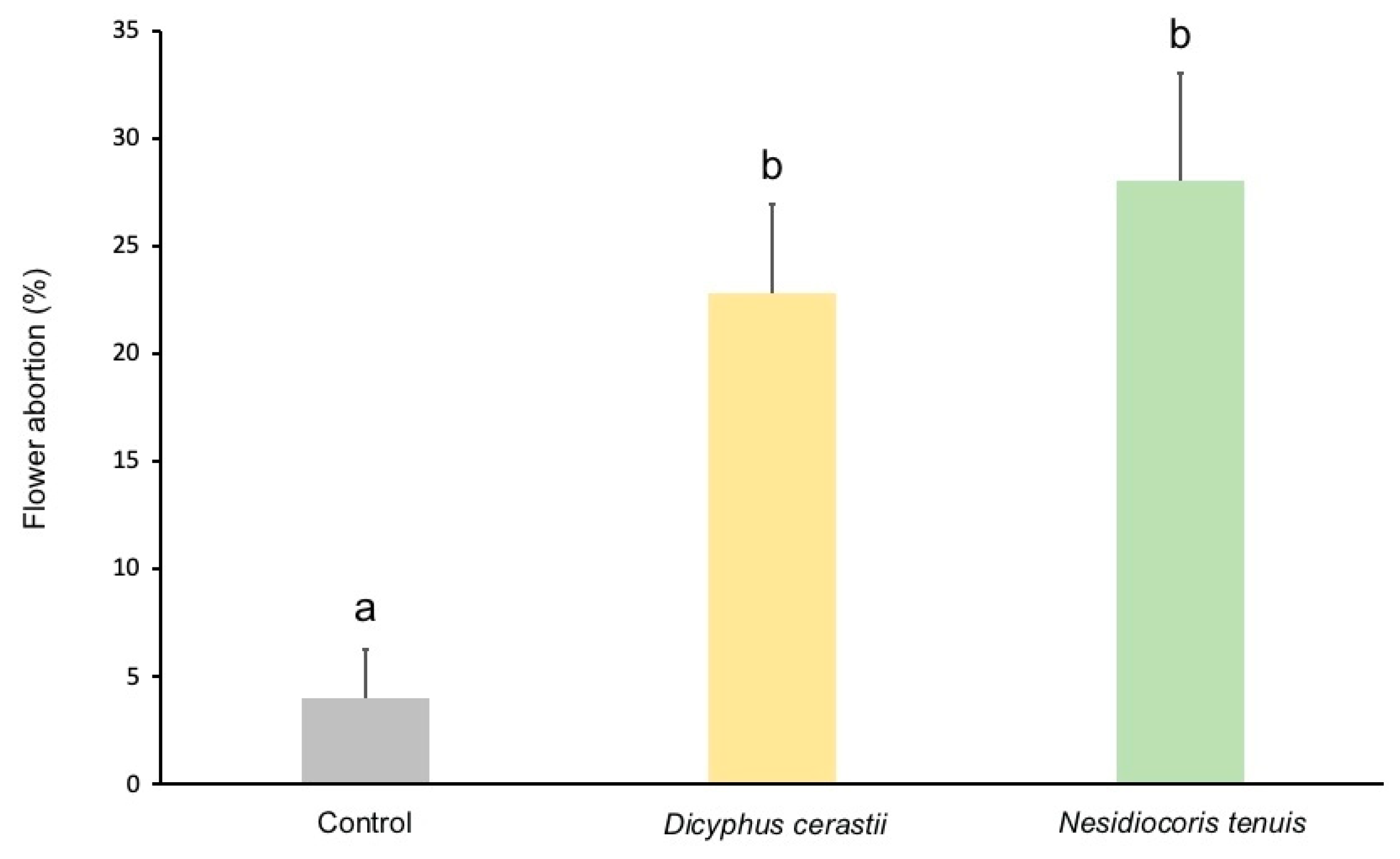
3.2. Fruit Damage
3.3. Location on Tomato Plant vs. Fruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, E.; Prieto, R.; Mexia, A.; Rodrigues, S.; Costa, C.A.; Godinho, M.C. Mirid bugs as biological control agents in protected tomato crops in the Oeste region. Acta Hortic. 2012, 927, 253–260. [Google Scholar] [CrossRef]
- Abraços-Duarte, G.; Ramos, S.; Valente, F.; Borges da Silva, E.; Figueiredo, E. Functional response and predation rate of Dicyphus cerastii Wagner (Hemiptera: Miridae). Insects 2021, 12, 530. [Google Scholar] [CrossRef]
- Figueiredo, E.; Martins, J.; Matos, T.; Duarte, G.; Silva, E.B.; Mexia, A. Mirid complex in Oeste region greenhouse—Dicyphus umbertae a promising biological control agent? IOBC/WPRS Bull. 2016, 119, 34–35. [Google Scholar]
- Urbaneja-Bernat, P.; Mollá, O.; Alonso, M.; Bolkcmans, K.; Urbaneja, A.; Tena, A. Sugars as complementary alternative food for the establishment of Nesidiocoris tenuis in greenhouse tomato. J. Appl. Entomol. 2015, 139, 161–167. [Google Scholar] [CrossRef]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
- Gillespie, D.R.; Vanlaerhoven, S.L.; McGregor, R.R.; Chan, S.; Roitberg, B.D. Plant feeding in an omnivorous mirid, Dicyphus hesperus: Why plant context matters. Psyche 2012, 2012, 495805. [Google Scholar] [CrossRef]
- Sinia, A.; Roitberg, B.; McGregor, R.R.; Gillespie, D.R. Prey feeding increases water stress in the omnivorous predator Dicyphus hesperus. Entomol. Exp. Appl. 2004, 110, 243–248. [Google Scholar] [CrossRef]
- Gillespie, D.R.; Mcgregor, R.R. The functions of plant feeding in the omnivorous predator Dicyphus hesperus: Water places limits on predation. Ecol. Entomol. 2000, 25, 380–386. [Google Scholar] [CrossRef]
- Han, P.; Bearez, P.; Adamowicz, S.; Lavoir, A.V.; Amiens-Desneux, E.; Desneux, N. Nitrogen and water limitations in tomato plants trigger negative bottom-up effects on the omnivorous predator Macrolophus pygmaeus. J. Pest Sci. 2015, 88, 685–691. [Google Scholar] [CrossRef]
- Sanchez, J.A. Density thresholds for Nesidiocoris tenuis (Heteroptera: Miridae) in tomato crops. Biol. Control 2009, 51, 493–498. [Google Scholar] [CrossRef]
- Moerkens, R.; Berckmoes, E.; van Damme, V.; Ortega-Parra, N.; Hanssen, I.; Wuytack, M.; Wittemans, L.; Casteels, H.; Tirry, L.; De Clercq, P.; et al. High population densities of Macrolophus pygmaeus on tomato plants can cause economic fruit damage: Interaction with Pepino mosaic virus? Pest Manag. Sci. 2016, 72, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Puentes, A.; Stephan, J.G.; Björkman, C. A systematic review on the effects of plant-feeding by omnivorous arthropods: Time to catch-up with the mirid-tomato bias? Front. Ecol. Evol. 2018, 6, 218. [Google Scholar] [CrossRef] [Green Version]
- Moerkens, R.; Pekas, A.; Bellinkx, S.; Hanssen, I.; Huysmans, M.; Bosmans, L.; Wäckers, F. Nesidiocoris tenuis as a pest in Northwest Europe: Intervention threshold and influence of Pepino mosaic virus. J. Appl. Entomol. 2020, 144, 566–577. [Google Scholar] [CrossRef]
- Moerkens, R.; Berckmoes, E.; van Damme, V.; Wittemans, L.; Tirry, L.; Casteels, H.; De Clercq, P.; De Vis, R. Inoculative release strategies of Macrolophus pygmaeus Rambur (Hemiptera: Miridae) in tomato crops: Population dynamics and dispersal. J. Plant Dis. Prot. 2017, 124, 295–303. [Google Scholar] [CrossRef]
- Urbaneja, A.; González-Cabrera, J.; Arnó, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 2012, 68, 1215–1222. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Gallego, C.; Roda, A.; Kostyk, B.; Stansly, P.A.; Qureshi, J.; Triana, M.; Alférez, F.; Urbaneja, A. Biological traits of the predatory mirid Macrolophus praeclarus, a candidate biocontrol agent for the Neotropical region. Bull. Entomol. Res. 2021, 111, 429–437. [Google Scholar] [CrossRef]
- Roda, A.; Castillo, J.; Allen, C.; Urbaneja, A.; Pérez-Hedo, M.; Weihman, S.; Stansly, P.A. Biological control potential and drawbacks of three zoophytophagous mirid predators against Bemisia tabaci in the United States. Insects 2020, 11, 670. [Google Scholar] [CrossRef]
- Dumont, F.; Lucas, E.; Réale, D. Coexistence of zoophytophagous and phytozoophagous strategies linked to genotypic diet specialization in plant bug. PLoS ONE 2017, 12, e0176369. [Google Scholar] [CrossRef]
- Chinchilla-Ramírez, M.; Pérez-Hedo, M.; Pannebakker, B.A.; Urbaneja, A. Genetic variation in the feeding behavior of isofemale lines of Nesidiocoris tenuis. Insects 2020, 11, 513. [Google Scholar] [CrossRef]
- Calvo, J.; Bolckmans, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 2009, 54, 237–246. [Google Scholar] [CrossRef]
- Fantinou, A.A.; Perdikis, D.C.; Labropoulos, P.D.; Maselou, D.A. Preference and consumption of Macrolophus pygmaeus preying on mixed instar assemblages of Myzus persicae. Biol. Control 2009, 51, 76–80. [Google Scholar] [CrossRef]
- Silva, D.B.; Bueno, V.H.P.; Calvo, F.J.; van Lenteren, J.C. Do nymphs and adults of three neotropical zoophytophagous mirids damage leaves and fruits of tomato? Bull. Entomol. Res. 2017, 107, 200–207. [Google Scholar] [CrossRef]
- McGregor, R.R.; Gillespie, D.R.; Park, C.G.; Quiring, D.M.J.; Foisy, M.R.J. Leaves or fruit? The potential for damage to tomato fruits by the omnivorous predator, Dicyphus hesperus. Entomol. Exp. Appl. 2000, 95, 325–328. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased recursive partitioning: A conditional inference framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Text Mining with Machine Learning, 1st ed.; Random Forests; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–122. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Kyle, T.; Gibson, J.; Lawler, J.J.; Beard, H.; Hess, T. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Arnó, J.; Castañé, C.; Riudavets, J.; Roig, J.; Gabarra, R. Characterization of damage to tomato plants produced by the zoophytophagous predator Nesidiocoris tenuis. IOBC Wprs Bull. 2006, 29, 249–254. [Google Scholar]
- Raman, K.; Sanjayan, K.P. Histology and histopathology of the feeding lesions by Cyrtopeltis tenuis Reut. (Hemiptera: Miridae) on Lycopersicon esculentum Mill. (Solanaceae). Proc. Indian Acad. Sci. 1984, 93, 543–547. [Google Scholar] [CrossRef]
- Calvo, F.J.; Bolckmans, K.; Belda, J.E. Release rate for a pre-plant application of Nesidiocoris tenuis for Bemisia tabaci control in tomato. BioControl 2012, 57, 809–817. [Google Scholar] [CrossRef]
- Sanchez, J.A.; del Pino-Pérez, M.; del Mar Davó, M.; Cascales, J.I.M.; Lacasa, A. Zoophytophagy of the plantbug Nesidiocoris tenuis in tomato crops in southeast Spain. IOBC/Wprs Bull. 2006, 29, 243–248. [Google Scholar]
- Sanchez, J.A.; López-Gallego, E.; Pérez-Marcos, M.; Perera-Fernández, L.G.; Ramírez-Soria, M.J. How safe is it to rely on Macrolophus pygmaeus (Hemiptera: Miridae) as a biocontrol agent in tomato crops? Front. Ecol. Evol. 2018, 6, 132. [Google Scholar] [CrossRef] [Green Version]
- Urbaneja-Bernat, P.; Alonso, M.; Tena, A.; Bolckmans, K.; Urbaneja, A. Sugar as nutritional supplement for the zoophytophagous predator Nesidiocoris tenuis. BioControl 2013, 58, 57–64. [Google Scholar] [CrossRef]
- Urbaneja-Bernat, P.; Bru, P.; González-Cabrera, J.; Urbaneja, A.; Tena, A. Reduced phytophagy in sugar-provisioned mirids. J. Pest Sci. 2019, 92, 1139–1148. [Google Scholar] [CrossRef]
- Opara, U.L.; Al-Ani, M.R.; Al-Rahbi, N.M. Effect of fruit ripening stage on physico-chemical properties, nutritional composition and antioxidant components of tomato (Lycopersicum esculentum) cultivars. Food Bioprocess Technol. 2012, 5, 3236–3243. [Google Scholar] [CrossRef]
- Duma, M.; Alsina, I.; Dubova, L.; Erdberga, I. Chemical composition of tomatoes depending on the stage of ripening. Chem. Technol. 2015, 66, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Lucas, R.; Alomar, O. Impact of Macrolophus caliginosus presence on damage production by Dicyphus tamaninii (Heteroptera: Miridae) on tomato fruits. J. Econ. Entomol. 2002, 95, 1123–1129. [Google Scholar] [CrossRef]
- Siscaro, G.; Lo Pumo, C.; Tropea Garzia, G.; Tortorici, S.; Gugliuzzo, A.; Ricupero, M.; Biondi, A.; Zappalà, L. Temperature and tomato variety influence the development and the plant damage induced by the zoophytophagous mirid bug Nesidiocoris tenuis. J. Pest Sci. 2019, 92, 1049–1056. [Google Scholar] [CrossRef]
- Garantonakis, N.; Pappas, M.L.; Varikou, K.; Skiada, V.; Broufas, G.D.; Kavroulakis, N.; Papadopoulou, K.K. Tomato inoculation with the endophytic strain Fusarium solani K results in reduced feeding damage by the zoophytophagous predator Nesidiocoris tenuis. Front. Ecol. Evol. 2018, 6, 126. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Hedo, M.; Bouagga, S.; Jaques, J.A.; Flors, V.; Urbaneja, A. Tomato plant responses to feeding behavior of three zoophytophagous predators (Hemiptera: Miridae). Biol. Control 2015, 86, 46–51. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Riahi, C.; Urbaneja, A. Use of zoophytophagous mirid bugs in horticultural crops: Current challenges and future perspectives. Pest Manag. Sci. 2021, 77, 33–42. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souto, P.; Abraços-Duarte, G.; da Silva, E.B.; Figueiredo, E. Half Friend, Half Enemy? Comparative Phytophagy between Two Dicyphini Species (Hemiptera: Miridae). Insects 2022, 13, 175. https://doi.org/10.3390/insects13020175
Souto P, Abraços-Duarte G, da Silva EB, Figueiredo E. Half Friend, Half Enemy? Comparative Phytophagy between Two Dicyphini Species (Hemiptera: Miridae). Insects. 2022; 13(2):175. https://doi.org/10.3390/insects13020175
Chicago/Turabian StyleSouto, Paula, Gonçalo Abraços-Duarte, Elsa Borges da Silva, and Elisabete Figueiredo. 2022. "Half Friend, Half Enemy? Comparative Phytophagy between Two Dicyphini Species (Hemiptera: Miridae)" Insects 13, no. 2: 175. https://doi.org/10.3390/insects13020175
APA StyleSouto, P., Abraços-Duarte, G., da Silva, E. B., & Figueiredo, E. (2022). Half Friend, Half Enemy? Comparative Phytophagy between Two Dicyphini Species (Hemiptera: Miridae). Insects, 13(2), 175. https://doi.org/10.3390/insects13020175






