Effect of Male Body Size on Female Reproduction in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
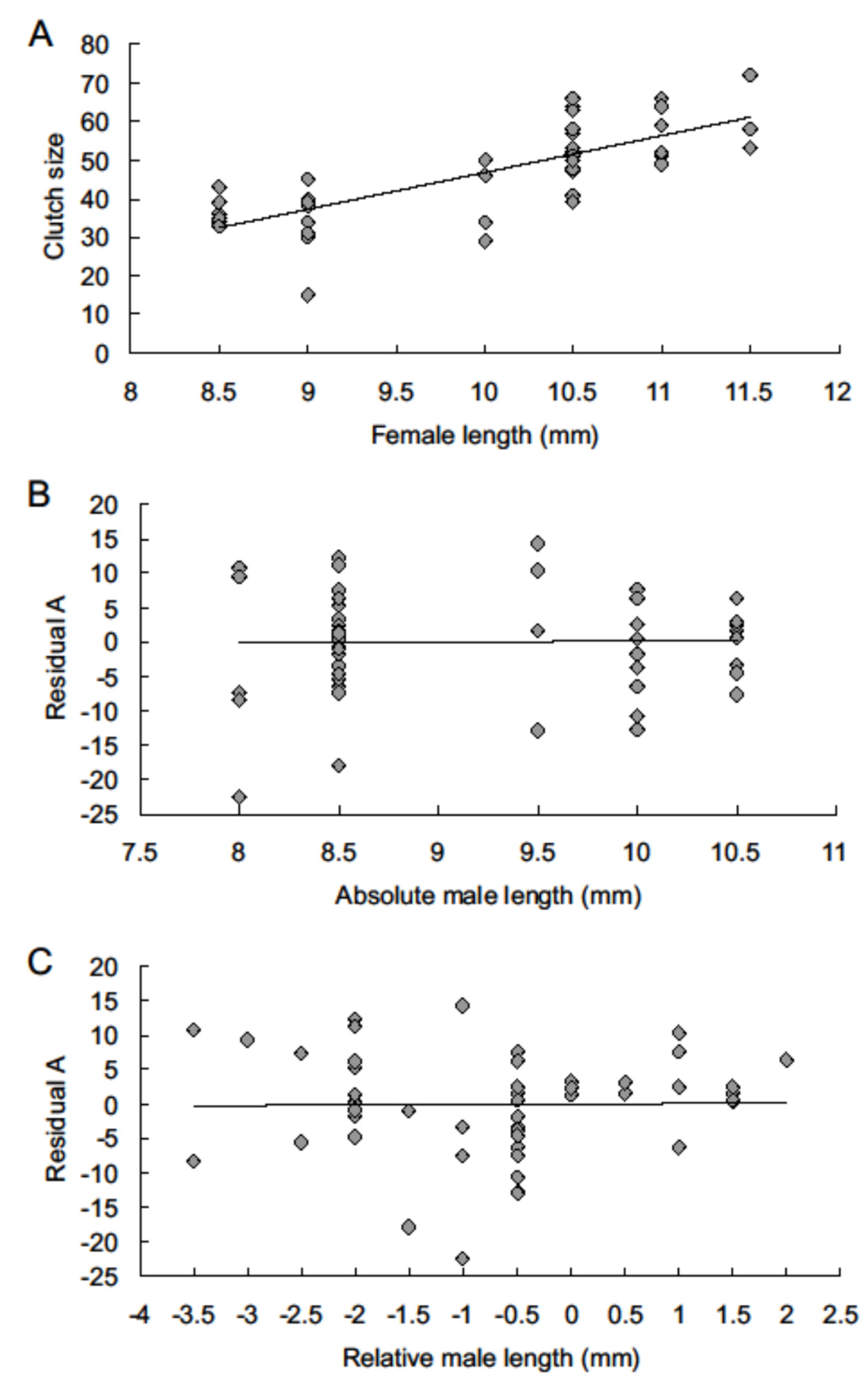
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Garcia-Barros, E. Body size, egg size, and their interspecific relationships with ecological and life history traits in butterflies (Lepidoptera : Papilionoidea, Hesperioidea). Biol. J. Linn. Soc. 2000, 70, 251–284. [Google Scholar] [CrossRef]
- Chown, S.L.; Gaston, K.J. Body size variation in insects: A macroecological perspective. Biol. Rev. 2010, 85, 139–169. [Google Scholar] [CrossRef] [PubMed]
- Quiring, D.T.; McNeill, J.N. Influence of intraspecific larval competition and mating on the longevity and reproductive performance of females of the leaf miner Agromyza frontella (Rondani) (Diptera, Agromyzidae). Can. J. Zool. 1984, 62, 2197–2200. [Google Scholar] [CrossRef]
- Nasci, R.S. The size of emerging and host seeking Aedes aegypti and the relation of size to blood feeding success in the field. J. Am. Mosq. Control Assoc. 1986, 2, 61–62. [Google Scholar]
- Lavadinho, A.M.P. Toxicological studies on adult Sitophilus granarius (L.)—Influence of indiidual body weight on susceptibility to DDT and Malathion. J. Stored Prod. Res. 1976, 12, 215–224. [Google Scholar] [CrossRef]
- Harvey, G.T. Egg weight as a factor in the overwintering survival of spruce budworm (Lepidoptera: Tortricidae) larvae. Can. Entomol. 1985, 117, 1451–1461. [Google Scholar] [CrossRef]
- Kidd, N.A.C.; Cleaver, A.M. The control of migratory urge in Aphis fabae Scopoli (Hemiptera, Aphididae). Bull. Entomol. Res. 1986, 76, 77–87. [Google Scholar] [CrossRef]
- Tsuji, J.S.; Kingsolver, J.G.; Watt, W.B. Thermal physiological ecology of Colias butterflies in flight. Oecologia 1986, 69, 161–170. [Google Scholar] [CrossRef]
- Bennettova, B.; Fraenkel, G. What determines the number of ovarioles in a fly ovary? J. Insect Physiol. 1981, 27, 403–410. [Google Scholar] [CrossRef]
- Injeyan, H.S.; Tobe, S.S. Phase polymorphism in Schistocerca gregaria: Reproductive parameters. J. Insect Physiol. 1981, 27, 97–102. [Google Scholar] [CrossRef]
- Leather, S.R.; Wellings, P.W. Ovariole number and fecundity in aphids. Entomol. Exp. Appl. 1981, 30, 128–133. [Google Scholar] [CrossRef]
- Schlein, Y. Ultraviolet light and puparial weight as factors in autogeny of fleshfly, Sarcophaga falculata. J. Insect Physiol. 1977, 23, 961–964. [Google Scholar] [CrossRef]
- Griffiths, D. Phenology and larval-adult size relations in the ant-lion Macroleon quinquemaculatus. J. Anim. Ecol. 1985, 54, 573–581. [Google Scholar] [CrossRef]
- Kimura, T.; Tsubaki, Y. Female size and age-specific fecundity in the small white butterfly, Pieris rapae crucivora Boisduval (Lepidoptera, Pieridae). Res. Popul. Ecol. 1986, 28, 295–304. [Google Scholar] [CrossRef]
- Iga, M. Mating preferences of Dacus dorsalis Hendel (Diptera: Tephritidae) with reference to individual variations in size. Japanese J. Appl. Entomol. 1981, 25, 292–294. [Google Scholar] [CrossRef]
- Honek, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Murdie, G. The biological consequences of decreased size caused by crowding or rearing temperatures in apterae of the pea aphid, Acyrthosiphon pisum Harris. Trans. Ent. Soc. Lond. 1969, 121, 443–455. [Google Scholar] [CrossRef]
- Wedell, N. Ejaculate size in bushcrickets: The importance of being large. J. Evol. Biol. 1997, 10, 315–325. [Google Scholar] [CrossRef]
- Boivin, G.; Martel, V. Size-induced reproductive constraints in an egg parasitoid. J. Insect Physiol. 2012, 58, 1694–1700. [Google Scholar] [CrossRef]
- Hatala, A.J.; Harrington, L.C.; Degner, E.C. Age and body size influence sperm quantity in male Aedes albopictus (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2018, 55, 1051–1054. [Google Scholar] [CrossRef]
- Brown, W.D. Female remating and the intensity of female choice in black-horned tree crickets, Oecanthus nigricornis. Behav. Ecol. 1997, 8, 66–74. [Google Scholar] [CrossRef]
- South, A.; Lewis, S.M. The influence of male ejaculate quantity on female fitness: A meta-analysis. Biol. Rev. 2011, 86, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Savalli, U.M.; Czesak, M.E.; Fox, C.W. Paternal investment in the seed beetle Callosobruchus maculatus (Coleoptera: Bruchidae): Variation among populations. Ann. Entomol. Soc. Am. 2000, 93, 1173–1178. [Google Scholar] [CrossRef]
- Singh, P.; Mishra, G.; Omkar. Influence of body size and familiarity on mating and reproductive parameters in the zig-zag ladybird beetle, Menochilus sexmaculatus (Coleoptera: Coccinellidae). Can. J. Zool. 2019, 97, 453–463. [Google Scholar] [CrossRef]
- McLain, D.K. Behavioral and morphological correlates of male dominance and courtship persistence in the blister beetle Epicauta pennsylvanica (Coleoptera: Meloidae). Am. Midl. Nat. 1982, 107, 396–403. [Google Scholar] [CrossRef]
- O’Neill, K.M. The significance of body size in territorial interactions of male beewolves (Hymenoptera:Sphecidae, Philanthus). Anim. Behav. 1983, 31, 404–411. [Google Scholar] [CrossRef]
- Burk, T. Male-male interactionsin Caribbean fruit flies, Anasterpha suspensa (Loew) (Diptera, Tephritidae)—Territorial fights and signaling stimulation. Fla. Entomol. 1984, 67, 542–547. [Google Scholar] [CrossRef]
- McLain, D.K.; Boromisa, R.D. Male choice, fighting ability, assortative mating and the intensity of sexual selection in the milkweed longhorn beetle, Tetraopes tetraophthalmus (Coleoptera, Cerambycidae). Behav. Ecol. Sociobiol. 1987, 20, 239–246. [Google Scholar] [CrossRef]
- Frey, D. Resistance to mating by female monarch butterflies. In The 1997 North American Conference on the Monarch Butterfly; Hoth, J., Merino, L., Oberhauser, K., Pisanty, I., Price, S., Wilkinson, T.P., Eds.; Intercept Commision for Environmental Cooperation: Montreal, QC, Canada, 1999; pp. 79–87. [Google Scholar]
- Jorge, A.S.; Lomonaco, C. Body size, symmetry and courtship behavior of Dysdercus maurus Distant (Hemiptera: Pyrrhocoridae). Neotrop. Entomol. 2011, 40, 305–311. [Google Scholar] [CrossRef][Green Version]
- Conroy, L.P.; Gray, D.A. Male armaments and reproductive behavior in "Nutcracker" Camel Crickets (Rhaphidophoridae, Pristoceuthophilus). Insects 2015, 6, 85–99. [Google Scholar] [CrossRef]
- Hayashi, K. Alternative mating strategies in the water strider Gerris elongatus (Heteroptera, Gerridae). Behav. Ecol. Sociobiol. 1985, 16, 301–306. [Google Scholar] [CrossRef]
- Montiglio, P.O.; Wey, T.W.; Chang, A.T.; Fogarty, S.; Sih, A. Multiple mating reveals complex patterns of assortative mating by personality and body size. J. Anim. Ecol. 2016, 85, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Forrest, T.G.; Sylvester, J.L.; Testa, S.; Smith, S.W.; Dinep, A.; Cupit, T.L.; Huggins, J.M.; Atkins, K.L.; Eubanks, M. Mate choice in ground crickets (Gryllidae, Nemoniidae). Fla. Entomol. 1991, 74, 74–80. [Google Scholar] [CrossRef]
- Hingle, A.; Fowler, K.; Pomiankowski, A. Size-dependent mate preference in the stalk-eyed fly Cyrtodiopsis dalmanni. Anim. Behav. 2001, 61, 589–595. [Google Scholar] [CrossRef]
- Beeler, A.E.; Rauter, C.M.; Moore, A.J. Mate discrimination by females in the burying beetle Nicrophorus orbicollis: The influence of male size on attractiveness to females. Ecol. Entomol. 2002, 27, 1–6. [Google Scholar] [CrossRef]
- Watson, N.L.; Simmons, L.W. Mate choice in the dung beetle Onthophagus sagittarius: Are female horns ornaments? Behav. Ecol. 2010, 2, 424–430. [Google Scholar] [CrossRef]
- Kosal, E.F.; Niedzlek-Feave, M. Female preferences for large, heavy mates in Schistocerca americana (Orthoptera: Acrididae). J. Insect Behav. 1997, 10, 711–725. [Google Scholar] [CrossRef]
- Fukaya, M.; Yasuda, T.; Akino, T.; Yasui, H.; Wakamura, S.; Fukuda, T.; Ogawa, Y. Effects of male body size on mating behavior and female mate refusal in the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2004, 39, 731–737. [Google Scholar] [CrossRef][Green Version]
- Cordeir, E.M.C.; Correa, A.S.; Rosi-Denadai, C.A.; Tome, H.V.V.; Guedes, R.N.C. Insecticide resistance and size assortative mating in females of the maize weevil (Sitophilus zeamais). Pest Manag. Sci. 2017, 73, 823–829. [Google Scholar] [CrossRef]
- Capone, T.A. Mutual preference for large mates in green stink bugs, Acrosternum hilare (Hemiptera, Pentatomidae). Anim. Behav. 1995, 49, 1335–1344. [Google Scholar] [CrossRef][Green Version]
- Kristenova, M.; Exnerova, A.; Stys, P. Seed preferences of Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae): A re there specialized trophic populations? Eur. J. Entomol. 2011, 108, 581–586. [Google Scholar] [CrossRef]
- Honek, A. Body size and mating success in Pyrrhocoris apterus (Heteroptera). Eur. J. Entomol. 2003, 100, 55–60. [Google Scholar] [CrossRef]
- Socha, R. Wing morph- and age-related differences in fertilization success of adult males of a flightless bug, Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). Eur. J. Entomol. 2008, 108, 93–98. [Google Scholar] [CrossRef]
- Bangham, J.; Chapman, T.; Partridge, L. Effects of body size, accessory gland and testis size on pre- and postcopulatory success in Drosophila melanogaster. Anim. Behav. 2002, 64, 915–921. [Google Scholar] [CrossRef]
- Fairn, E.R.; Schulte-Hostedde, A.I.; Alarie, Y. Sexual selection on accessory glands, genitalia and protarsal pads in the whirligig beetle Dineutus nigrior Roberts (Coleoptera: Gyrinidae). Ethology 2007, 113, 257–266. [Google Scholar] [CrossRef]
- Lemmen, J.; Keddie, B.A.; Evenden, M.L. Size and protein content of accessory glands in adult male Caloptilia fraxinella in different physiological states. Physiol. Entomol. 2016, 41, 74–82. [Google Scholar] [CrossRef]
- Socha, R. Endocrine control of wing morph-related differences in mating success and accessory gland size in male firebugs. Anim. Behav. 2006, 71, 1273–1281. [Google Scholar] [CrossRef]
- Systat Software Inc. SigmaStat 3.5; Systat Software, Inc.: Point Richmond, CA, USA, 2006. [Google Scholar]
- Zdarek, J. Le comportement d’accouplement á la fin de la diapause imaginale et son controle hormonal dans le cas de la punaise Pyrrhocoris apterus L. (Pyrrhocoridae, Heteroptera). Ann. Endocrin. 1968, 29, 703–707. [Google Scholar]
- Socha, R. Decreased mating propensity of macropterous morph in a flightless wing-polymorphic insect, Pyrrhocoris apterus (Heteroptera). Eur. J. Entomol. 2004, 101, 539–545. [Google Scholar] [CrossRef]
- Socha, R.; Zemek, R. Mating behaviour and wing morp-related differences in the sexual activity of a flightless bug, Pyrrhocoris apterus (L.) (Heteroptera). Ethol. Ecol. Evol. 2004, 16, 217–229. [Google Scholar] [CrossRef]
- Honek, A.; Martinkova, Z.; Brabec, M. Mating activity of Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae) in nature. Eur. J. Entomol. 2019, 116, 187–193. [Google Scholar] [CrossRef]
- Honek, A.; Martinkova, Z. Behavioural thermoregulation hastens spring mating activity in Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). J. Therm. Biol. 2019, 84, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Honek, A. Body size and fecundity in natural populations of Pyrrhocoris apterus L. (Heteroptera, Pyrrhocoridae). Zool. Jahrb., Abt. Syst. Geogr. Biol. Tiere. 1986, 113, 125–140. [Google Scholar]
- Honek, A.; Hodek, I. Diapause of Chrysopa carnea (Chrysopidae: Neuroptera) females in the field. Věstník Československé Společnosti Zoologické 1973, 37, 95–100. [Google Scholar]
- Honek, A. Regulation of body size in a heteropteran bug, Pyrrhocoris apterus. Entomol. Exp. Appl. 1987, 44, 257–262. [Google Scholar] [CrossRef]
| 2004 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Male Size (mm) | |||||||||
| 8 | 8.5 | 9 | 9.5 | 10 | 10.5 | 11 | Sum | ||
| Female size (mm) | 8.5 | 3 | 1 | 1 | 1 | 6 | |||
| 9 | 2 | 8 | 1 | 4 | 3 | 18 | |||
| 9.5 | 0 | ||||||||
| 10 | 2 | 1 | 1 | 4 | |||||
| 10.5 | 12 | 1 | 7 | 20 | |||||
| 11 | 1 | 2 | 3 | 6 | |||||
| 11.5 | 2 | 1 | 3 | ||||||
| Sum | 5 | 27 | 0 | 4 | 12 | 9 | 0 | 57 | |
| 2022 | |||||||||
| Male Size (mm) | |||||||||
| 8 | 8.5 | 9 | 9.5 | 10 | 10.5 | 11 | Sum | ||
| Female size (mm) | 8.5 | 2 | 1 | 2 | 1 | 6 | |||
| 9 | 9 | 9 | |||||||
| 9.5 | 1 | 1 | 3 | 3 | 8 | ||||
| 10 | 1 | 2 | 3 | ||||||
| 10.5 | 6 | 1 | 4 | 1 | 12 | ||||
| 11 | 1 | 2 | 1 | 1 | 5 | ||||
| 11.5 | 1 | 1 | 2 | ||||||
| Sum | 3 | 11 | 3 | 7 | 12 | 7 | 2 | 45 | |
| Regression | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clutch | n Eggs | (a) Female Size | (b) Male Size (abs) | (c) Male Size (rel) | |||||||||||
| N | Mean ± SE | a | Rsqr | F | P | a | Rsqr | F | P | a | Rsqr | F | P | ||
| 2004 | |||||||||||||||
| 1 | 57 | 45.9 ± 1.52 | 9.549 | 0.5970 | 81.44 | <0.001 | 0.174 | 0.0004 | 0.02 | 0.877 | 0.079 | 0.0002 | 0.01 | 0.917 | |
| 2 | 57 | 53.1 ± 1.38 | 7.717 | 0.4750 | 49.84 | <0.001 | −0.222 | 0.0007 | 0.04 | 0.848 | −0.101 | 0.0003 | 0.02 | 0.897 | |
| 3 | 57 | 46.8 ± 1.63 | 6.522 | 0.2420 | 17.56 | <0.001 | 0.799 | 0.0043 | 0.24 | 0.628 | 0.364 | 0.0020 | 0.11 | 0.743 | |
| 4 | 42 | 37.8 ± 2.24 | 7.308 | 0.2110 | 10.69 | 0.002 | 2.273 | 0.0238 | 0.98 | 0.329 | 1.017 | 0.0107 | 0.43 | 0.515 | |
| 5 | 21 | 31.2 ± 2.69 | 3.072 | 0.0479 | 0.96 | 0.341 | 1.049 | 0.0062 | 0.12 | 0.735 | 0.531 | 0.0031 | 0.06 | 0.810 | |
| 1 + 2 | 57 | 98.9 ± 2.70 | 17.266 | 0.6220 | 90.43 | <0.001 | −0.048 | 0.0000 | 0.00 | 0.980 | −0.022 | 0.0000 | 0.00 | 0.987 | |
| 1 + 2 + 3 | 57 | 145.7 ± 3.90 | 23.788 | 0.5650 | 71.54 | <0.001 | 0.751 | 0.0012 | 0.06 | 0.801 | 0.343 | 0.0005 | 0.03 | 0.865 | |
| 1 + 2 + 3 + 4 | 42 | 184.9 ± 5.37 | 30.557 | 0.5770 | 54.56 | <0.001 | 3.730 | 0.0187 | 0.76 | 0.387 | 1.668 | 0.0084 | 0.34 | 0.564 | |
| 1 + 2 + 3 + 4 + 5 | 21 | 217.5 ± 8.96 | 32.143 | 0.4740 | 17.11 | <0.001 | 7.027 | 0.0454 | 0.90 | 0.354 | 3.555 | 0.0230 | 0.45 | 0.512 | |
| 2022 | |||||||||||||||
| 1 | 45 | 44.1 ± 1.30 | 4.269 | 0.1800 | 10.32 | 0.002 | 1.761 | 0.0339 | 1.65 | 0.205 | 0.648 | 0.0125 | 0.60 | 0.444 | |
| 2 | 44 | 48.8 ± 1.73 | 5.005 | 0.1420 | 6.98 | 0.012 | 1.963 | 0.0255 | 1.10 | 0.301 | 1.126 | 0.6960 | 0.38 | 0.540 | |
| 3 | 43 | 52.3 ± 1.60 | 2.683 | 0.0489 | 2.11 | 0.154 | 3.381 | 0.0830 | 3.71 | 0.061 | 1.222 | 0.0300 | 1.27 | 0.267 | |
| 4 | 40 | 54.7 ± 1.50 | 4.916 | 0.2050 | 9.79 | 0.003 | −1.848 | 0.0340 | 1.34 | 0.255 | −0.662 | 0.0122 | 0.47 | 0.498 | |
| 5 | 30 | 51.5 ± 2.18 | 6.098 | 0.1680 | 6.88 | 0.013 | −0.730 | 0.0025 | 0.09 | 0.771 | −0.229 | 0.0008 | 0.03 | 0.871 | |
| 1 + 2 | 44 | 93.6 ± 2.62 | 9.189 | 0.2090 | 11.11 | 0.002 | 4.195 | 0.0549 | 2.44 | 0.126 | 1.490 | 0.0195 | 0.84 | 0.366 | |
| 1 + 2 + 3 | 43 | 147.1 ± 3.65 | 10.56 | 0.1450 | 6.96 | 0.012 | 7.830 | 0.0949 | 4.30 | 0.044 | 2.829 | 0.0343 | 1.46 | 0.234 | |
| 1 + 2 + 3 + 4 | 40 | 204.0 ± 4.57 | 15.836 | 0.2060 | 9.59 | 0.004 | 3.900 | 0.0145 | 0.55 | 0.465 | 1.280 | 0.0048 | 0.18 | 0.676 | |
| 1 + 2 + 3 + 4 + 5 | 30 | 251.6 ± 6.24 | 21.15 | 0.2580 | 11.46 | 0.002 | 2.090 | 0.0028 | 0.09 | 0.762 | 0.644 | 0.0009 | 0.03 | 0.866 | |
| Regression | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clutch | Egg Mass | (a) Female Size | (b) Male Size (abs) | (c) Male Size (rel) | ||||||||||
| N | mean ± SE | a | Rsqr | F | P | a | Rsqr | F | P | a | Rsqr | F | P | |
| 1 | 45 | 3.80 ± 0.070 | 0.296 | 0.3180 | 20.02 | <0.001 | 0.037 | 0.0068 | 0.30 | 0.590 | 0.014 | 0.0026 | 0.11 | 0.738 |
| 2 | 44 | 3.61 ± 0.059 | 0.074 | 0.0269 | 1.13 | 0.293 | −0.112 | 0.0615 | 2.69 | 0.109 | −0.040 | 0.0218 | 0.91 | 0.345 |
| 3 | 43 | 3.61 ± 0.052 | 0.053 | 0.0193 | 0.71 | 0.405 | −0.070 | 0.0383 | 1.44 | 0.239 | −0.025 | 0.0129 | 0.49 | 0.491 |
| 4 | 40 | 3.55 ± 0.048 | 0.081 | 0.0659 | 2.40 | 0.131 | 0.037 | 0.0120 | 0.41 | 0.525 | 0.012 | 0.0039 | 0.13 | 0.718 |
| 5 | 30 | 3.38 ± 0.082 | 0.021 | 0.0017 | 0.05 | 0.825 | 0.093 | 0.0251 | 0.75 | 0.395 | 0.028 | 0.0075 | 0.22 | 0.644 |
| Regression | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clutch | To (Days) | (a) Female Size | (b) Male Size (abs) | (c) Male Size (rel) | ||||||||||
| N | mean ± SE | a | Rsqr | F | P | a | Rsqr | F | P | a | Rsqr | F | P | |
| 1 | 45 | 7.4 ± 0.15 | 0.188 | 0.0273 | 1.29 | 0.262 | 0.053 | 0.0021 | 0.10 | 0.758 | 0.020 | 0.0008 | 0.04 | 0.850 |
| 2 | 44 | 12.2 ± 0.23 | 0.406 | 0.0542 | 2.35 | 0.133 | 0.088 | 0.0027 | 0.11 | 0.739 | 0.032 | 0.0010 | 0.04 | 0.841 |
| 3 | 43 | 18.2 ± 0.41 | 0.445 | 0.0205 | 0.86 | 0.360 | 0.295 | 0.0094 | 0.39 | 0.537 | 0.107 | 0.0034 | 0.14 | 0.711 |
| 4 | 40 | 23.2 ± 0.59 | 0.893 | 0.0423 | 1.72 | 0.197 | 0.735 | 0.0287 | 1.15 | 0.290 | 0.265 | 0.0103 | 0.41 | 0.527 |
| 5 | 30 | 28.0 ± 0.77 | 0.257 | 0.0025 | 0.08 | 0.776 | 1.113 | 0.0390 | 1.34 | 0.256 | 0.343 | 0.0120 | 0.40 | 0.531 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honek, A.; Martinkova, Z. Effect of Male Body Size on Female Reproduction in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae). Insects 2022, 13, 902. https://doi.org/10.3390/insects13100902
Honek A, Martinkova Z. Effect of Male Body Size on Female Reproduction in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae). Insects. 2022; 13(10):902. https://doi.org/10.3390/insects13100902
Chicago/Turabian StyleHonek, Alois, and Zdenka Martinkova. 2022. "Effect of Male Body Size on Female Reproduction in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae)" Insects 13, no. 10: 902. https://doi.org/10.3390/insects13100902
APA StyleHonek, A., & Martinkova, Z. (2022). Effect of Male Body Size on Female Reproduction in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae). Insects, 13(10), 902. https://doi.org/10.3390/insects13100902







