Females of Halyomorpha halys (Hemiptera: Pentatomidae) Experience a Facultative Reproductive Diapause in Northern Greece
Abstract
Simple Summary
Abstract
1. Introduction
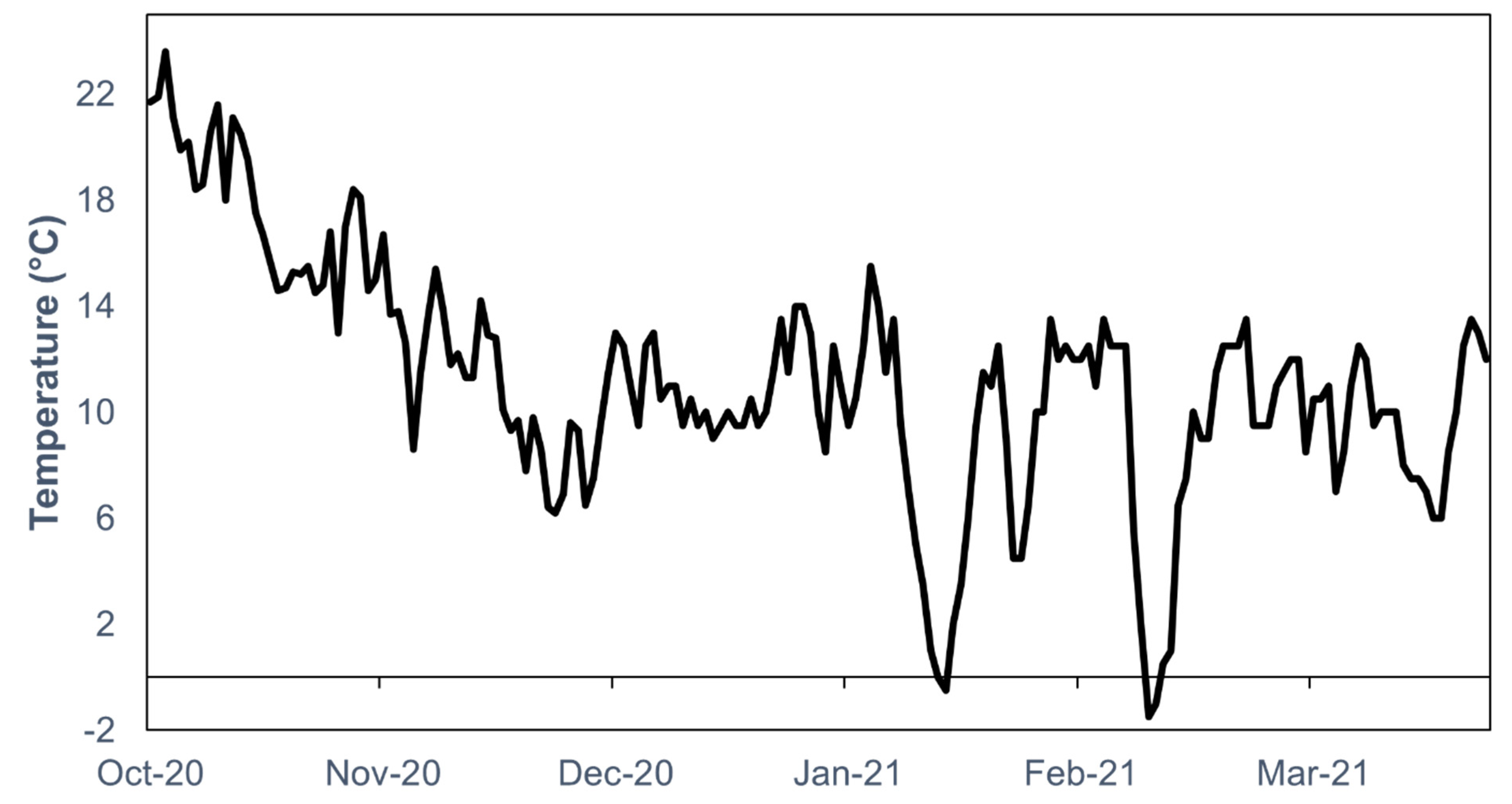
2. Materials and Methods
2.1. Specimen Collection
2.2. Colony Maintenance
2.3. Bioassay
2.4. Female Reproductive Status
2.5. Statistical Analysis
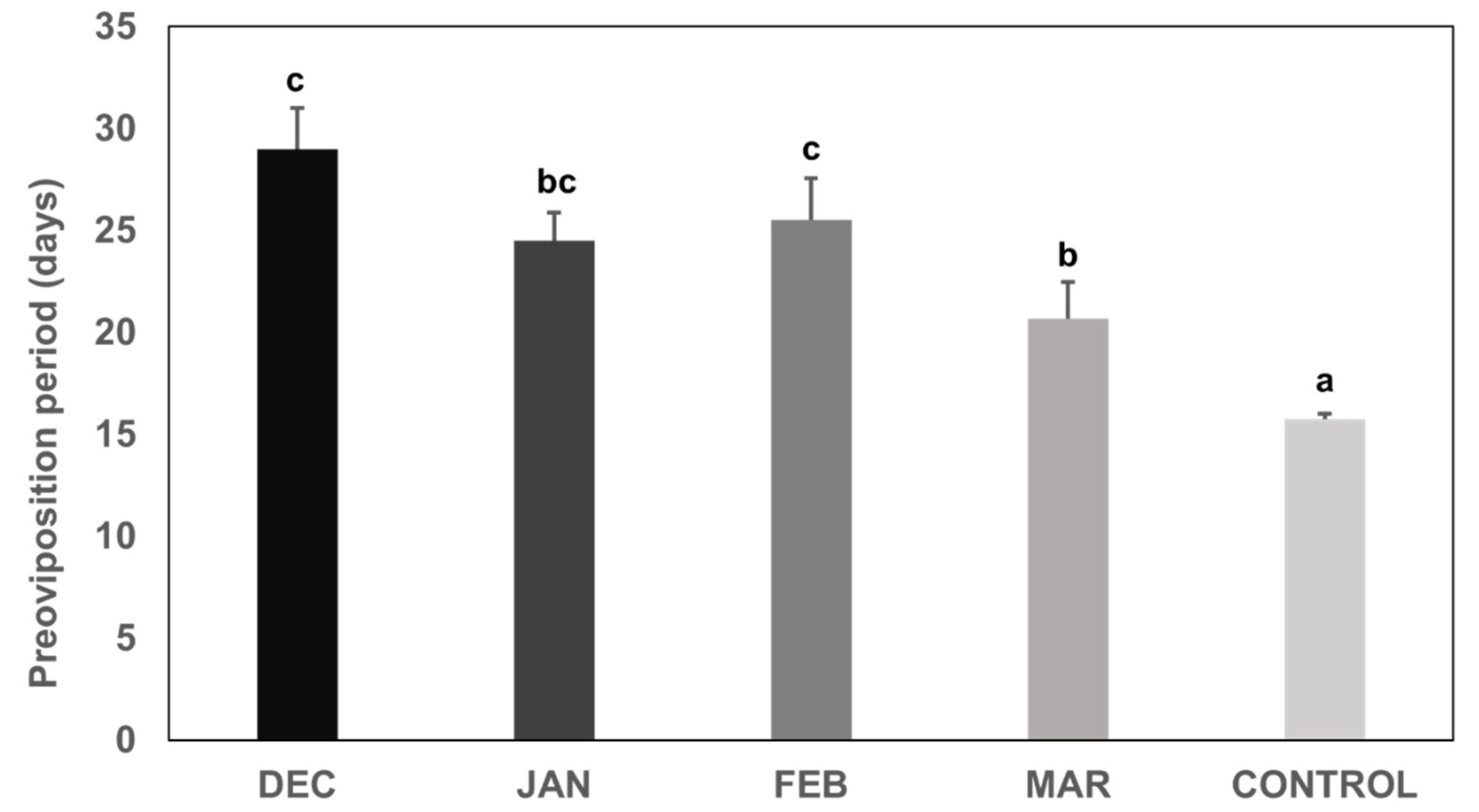
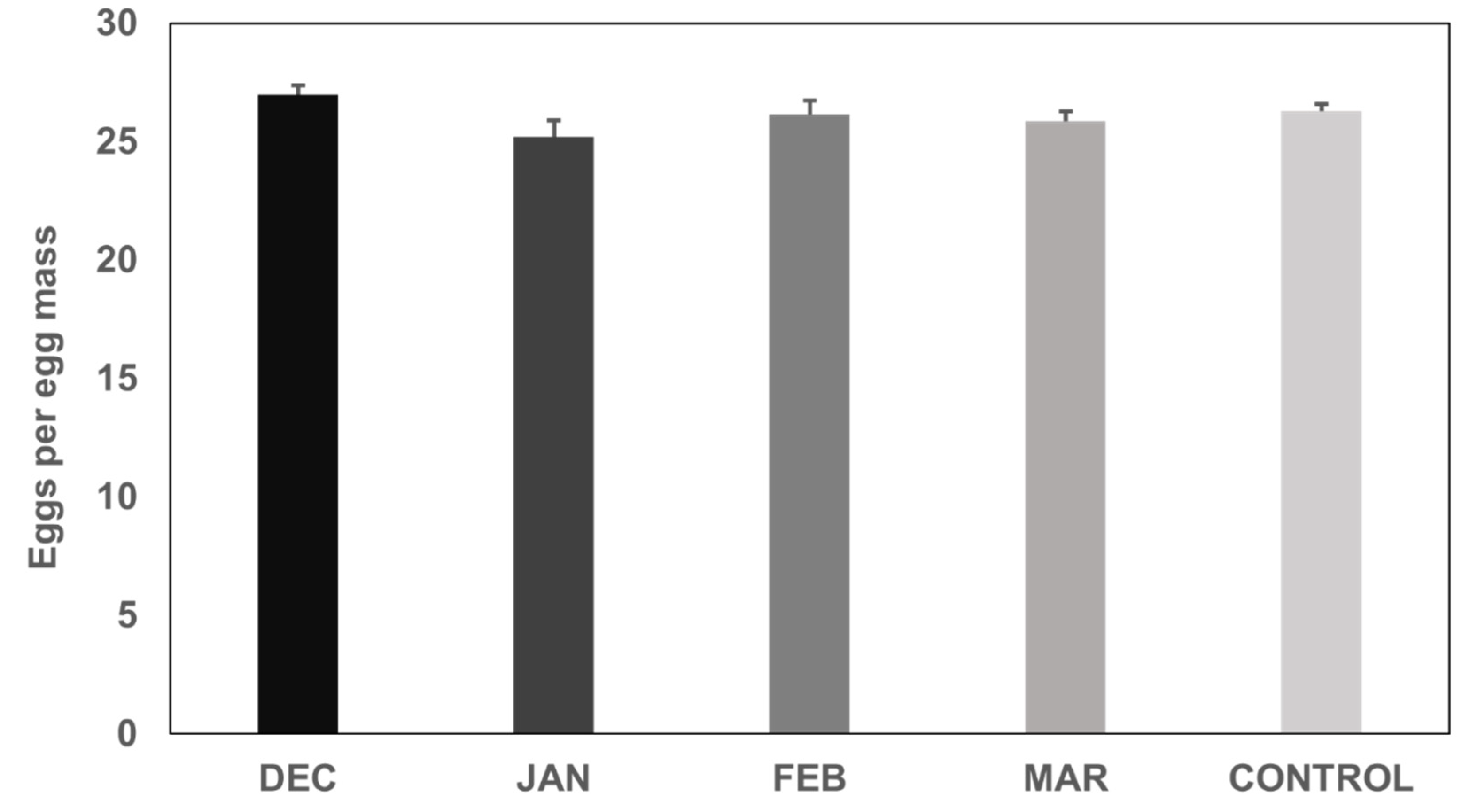
3. Results
3.1. Preoviposition Period
3.2. Fecundity of H. halys Females
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoebeke, E.R.; Carter, M.E. Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Wash. 2003, 105, 225–237. [Google Scholar]
- Fogain, R.; Graff, S. First records of the invasive pest, Halyomorpha halys (Hemiptera: Pentatomidae), in Ontario and Quebec. J. Entomol. Soc. Ont. 2011, 142, 45–48. [Google Scholar]
- Arnold, K. Halyomorpha halys (Stål, 1855), eine für die europäische Fauna neu nachgewiesene Wanzenart (Insecta: Heterop-tera: Pentatomidae: Cappaeini). Mitt. Des. Thüring. Entomol. 2009, 16, 19. [Google Scholar]
- Wermelinger, B.; Wyniger, D.; Forster, B. First records of an invasive bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Bull. Société Entomol. Suisse 2008, 81, 1–8. [Google Scholar]
- Bergmann, E.J.; Venugopal, D.; Martinson, H.; Raupp, M.J.; Shrewsbury, P.M. Host Plant Use by the Invasive Halyomorpha halys (Stål) on Woody Ornamental Trees and Shrubs. PLoS ONE 2016, 11, e0149975. [Google Scholar] [CrossRef]
- Milonas, P.G.; Partsinevelos, G.K. First report of brown marmorated stink bug Halyomorpha halys Stål (Hemiptera: Penta-tomidae) in Greece. EPPO Bull. 2014, 44, 183–186. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Navrozidis, E.I.; Farmakis, A.; Pisalidis, A. First evidence of Halyomorpha halys (Hemiptera: Pentatomidae) infesting kiwi fruit (Actinidia chinensis) in Greece. J. Entomol. Sci. 2018, 53, 402–405. [Google Scholar] [CrossRef]
- Hoffman, W.E. A pentatomid pest of growing beans in South China. Peking Nat. Hist. Bull. 1931, 5, 25–26. [Google Scholar]
- Nielsen, A.L.; Hamilton, G.C.; Matadha, D. Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environ. Entomol. 2008, 37, 348–355. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Karpun, N.N.; Zakharchenko, V.Y.; Shoshina, Y.I.; Dolgovskaya, M.Y.; Saulich, A.K.; Musolin, D.L. To Every Thing There Is a Season: Phenology and Photoperiodic Control of Seasonal Development in the Invasive Caucasian Population of the Brown Marmorated Stink Bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae). Insects 2022, 13, 580. [Google Scholar] [CrossRef]
- Inkley, D.B. Characteristics of Home Invasion by the Brown Marmorated Stink Bug (Hemiptera: Pentatomidae). J. Entomol. Sci. 2012, 47, 125–130. [Google Scholar] [CrossRef]
- Bergh, J.C.; Morrison, W.R.; Joseph, S.V.; Leskey, T.C. Characterizing spring emergence of adult Halyomorpha halys using ex-perimental overwintering shelters and commercial pheromone traps. Entomol. Exp. Appl. 2017, 162, 336–345. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, Ecology, and Management of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Cira, T.M.; Venette, R.C.; Aigner, J.; Kuhar, T.; Mullins, D.E.; Gabbert, S.E.; Hutchison, W.D. Cold Tolerance of Halyomorpha halys (Hemiptera: Pentatomidae) Across Geographic and Temporal Scales. Environ. Entomol. 2016, 45, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Toyama, M.; Ihara, F.; Yaginuma, K. Photo-response of the brown marmorated stink bug, Halyomorpha halys (Stål) (Heterop-tera: Pentatomidae), and its role in the hiding behavior. Appl. Entomol. Zool. 2010, 46, 37–40. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Chen, S.; Fleischer, S.J. Coupling Developmental Physiology, Photoperiod, and Temperature to Model Phenology and Dynamics of an Invasive Heteropteran, Halyomorpha halys. Front. Physiol. 2016, 7, 165. [Google Scholar] [CrossRef]
- Watanabe, M. Ecology and extermination of Halyomorpha halys. 4. The relationship between day length and ovarian devel-opment. Annu. Rep. Toyama Inst. Health 1979, 3, 33–37. [Google Scholar]
- Cira, T.M.; Koch, R.L.; Burkness, E.C.; Hutchison, W.D.; Venette, R.C. Effects of diapause on Halyomorpha halys (Hemiptera: Pentatomidae) cold tolerance. Environ. Entomol. 2018, 47, 997–1004. [Google Scholar]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Denlinger, D.L. Molecular signaling pathways that regulate diapause. In Insect Diapause; Cambridge University Press: Cambridge, UK, 2022; pp. 240–292. [Google Scholar]
- Taylor, C.M.; Coffey, P.L.; Hamby, K.A.; Dively, G.P. Laboratory rearing of Halyomorpha halys: Methods to optimize survival and fitness of adults during and after diapause. J. Pest Sci. 2017, 90, 1069–1077. [Google Scholar] [CrossRef]
- Haye, T.; Abdallah, S.; Gariepy, T.; Wyniger, D. Phenology, life table analysis and temperature requirements of the invasive brown marmorated stink bug, Halyomorpha halys, in Europe. J. Pest Sci. 2014, 87, 407–418. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Moysiadis, T.; Gogolashvili, N.E.; Koutsogeorgiou, E.I.; Fifis, G.T.; Tsaliki, E. Quality degradation of industrial hemp due to infestation by Halyomorpha halys (Hemiptera: Pentatomidae). Int. J. Pest Manag. 2022, 1–8. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Fleischer, S.; Hamilton, G.C.; Hancock, T.; Krawczyk, G.; Lee, J.C.; Ogburn, E.; Pote, J.M.; Raudenbush, A.; Rucker, A.; et al. Phenology of brown marmorated stink bug described using female reproductive development. Ecol. Evol. 2017, 7, 6680–6690. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 29 June 2021).
- Saulich, A.K.; Musolin, D.L. Diapause in the seasonal cycle of stink bugs from the temperate zone. Entomol. Rev. 2012, 92, 1–26. [Google Scholar] [CrossRef]
- Šlachta, M.; Vambera, J.; Zahradníčková, H.; Košťál, V. Entering diapause is a prerequisite for successful cold-acclimation in adult Graphosoma lineatum (Heteroptera: Pentatomidae). J. Insect Physiol. 2002, 48, 1031–1039. [Google Scholar] [CrossRef]
- Bradshaw, W.E. Insects at not so low temperatures: Climate change in the temperate zone and its biotic consequences. In Low Temperature Biology of Insects; Denlinger, D.L., Lee, R.E., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 242–275. [Google Scholar]
- Wyatt, I.J.; Brown, S.J. The Influence of Light Intensity, Daylength and Temperature on Increase Rates of Four Glasshouse Aphids. J. Appl. Ecol. 1977, 14, 391. [Google Scholar] [CrossRef]
- Leather, S.R.; Dixon, A.F.G. Secondary host preferences and reproductive activity of the bird cherry-oat aphid, Rhopalosiphum padi. Ann. Appl. Biol. 1982, 101, 219–228. [Google Scholar] [CrossRef]
- Kaakeh, W.; Dutcher, J.D. Rates of increase and probing behavior of Acyrthosiphon pisum (Homoptera: Aphididae) on pre-ferred and nonpreferred host cover crops. Environ. Entomol. 1993, 22, 1016–1021. [Google Scholar] [CrossRef]
- Ju, R.-T.; Wang, F.; Li, B. Effects of Temperature on the Development and Population Growth of the Sycamore Lace Bug, Corythucha ciliata. J. Insect Sci. 2011, 11, 1–12. [Google Scholar] [CrossRef]
- Dreyer, H.; Baumgärtner, J. Temperature influence on cohort parameters and demographic characteristics of the two cowpea coreids Clavigralla tomentosicollis and C. shadabi. Entomol. Exp. Appl. 1996, 78, 201–213. [Google Scholar] [CrossRef]
- Santos, Í.T.B.F.; Pinheiro, H.S.S.; Santos, V.B.; De Sanatana, L.K.N.; Poderoso, J.C.M.; Riberio, G.T. Effects of temperature on the development of Podisus nigrispinus (Heteroptera: Pentatomidae): Implications for mass rearing. Fla. Entomol. 2018, 101, 458–463. [Google Scholar] [CrossRef]
- Barrigossi, J.A.F.; Da Silva, C.V.; Alonso, J.D.D.S.; Hirose, E. Notes on Biology of the Stink Bug Cyptocephala alvarengai Rolston (Hemiptera: Pentatomidae) Feeding on Rice Panicles. Fla. Entomol. 2017, 100, 823–825. [Google Scholar] [CrossRef]
- Baek, S.; Hwang, A.; Kim, H.; Lee, H.; Lee, J.H. Temperature-dependent development and oviposition models of Halyomor-pha halys (Hemiptera: Pentatomidae). J. Asia-Pac. Entomol. 2017, 20, 367–375. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Lucini, T.; Possebom, T. Development of Dichelops furcatus (Hemiptera: Heteroptera: Pentatomidae) Reared on Spring Cereals Versus Soybean. J. Insect Sci. 2018, 18, 17. [Google Scholar] [CrossRef]
- Dingha, B.N.; Jackai, L.E. Laboratory rearing of the brown marmorated stink bug (Hemiptera: Pentatomidae) and the impact of single and combination of food substrates on development and survival. Can. Entomol. 2016, 149, 104–117. [Google Scholar] [CrossRef]
- Govindan, B.N.; Hutchison, W.D. Influence of Temperature on Age-Stage, Two-Sex Life Tables for a Minnesota-Acclimated Population of the Brown Marmorated Stink Bug (Halyomorpha halys). Insects 2020, 11, 108. [Google Scholar] [CrossRef]
- Musolin, D.L.; Dolgovskaya, M.Y.; Zakharchenko, V.Y.; Karpun, N.N.; Haye, T.; Saulich, A.K.; Reznik, S.Y. Flying over Eurasia: Geographic Variation of Photoperiodic Control of Nymphal Development and Adult Diapause Induction in Native and Invasive Populations of the Brown Marmorated Stink Bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae). Insects 2022, 13, 522. [Google Scholar] [CrossRef]
- Lowenstein, D.M.; Walton, V.M. Halyomorpha halys (Hemiptera: Pentatomidae) winter survival, feeding activity, and repro-duction rates based on episodic cold shock and winter temperature regimes. J. Econ. Entomol. 2018, 111, 1210–1218. [Google Scholar] [CrossRef]
- Corsini-Foka, M.; Zava, B.; Insacco, G. Occurrence of Halyomorpha halys (Stål, 1855) (Hemiptera: Pentatomidae) in Rhodes Is-land, Greece. J. Insect Biodivers. 2021, 29, 12–15. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutsogeorgiou, E.I.; Kouloussis, N.A.; Koveos, D.S.; Andreadis, S.S. Females of Halyomorpha halys (Hemiptera: Pentatomidae) Experience a Facultative Reproductive Diapause in Northern Greece. Insects 2022, 13, 866. https://doi.org/10.3390/insects13100866
Koutsogeorgiou EI, Kouloussis NA, Koveos DS, Andreadis SS. Females of Halyomorpha halys (Hemiptera: Pentatomidae) Experience a Facultative Reproductive Diapause in Northern Greece. Insects. 2022; 13(10):866. https://doi.org/10.3390/insects13100866
Chicago/Turabian StyleKoutsogeorgiou, Eleni I., Nikos A. Kouloussis, Dimitrios S. Koveos, and Stefanos S. Andreadis. 2022. "Females of Halyomorpha halys (Hemiptera: Pentatomidae) Experience a Facultative Reproductive Diapause in Northern Greece" Insects 13, no. 10: 866. https://doi.org/10.3390/insects13100866
APA StyleKoutsogeorgiou, E. I., Kouloussis, N. A., Koveos, D. S., & Andreadis, S. S. (2022). Females of Halyomorpha halys (Hemiptera: Pentatomidae) Experience a Facultative Reproductive Diapause in Northern Greece. Insects, 13(10), 866. https://doi.org/10.3390/insects13100866










