Climate Change and Major Pests of Mediterranean Olive Orchards: Are We Ready to Face the Global Heating?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Climatic Consequences on the Key Olive Pest Bactrocera oleae
2.1. Prediction Models of B. oleae Population Dynamics
2.1.1. Cumulative Degree Day Models
2.1.2. Machine Learning Models
2.1.3. Physiologically Based Demographic Models
2.1.4. Model Based on Exogenous and Endogenous Factors Influencing Insect Population Dynamics
2.1.5. Considerations on the Reliability of Predictive Models
2.2. Climate Influence on B. oleae Parasitoids and Predators
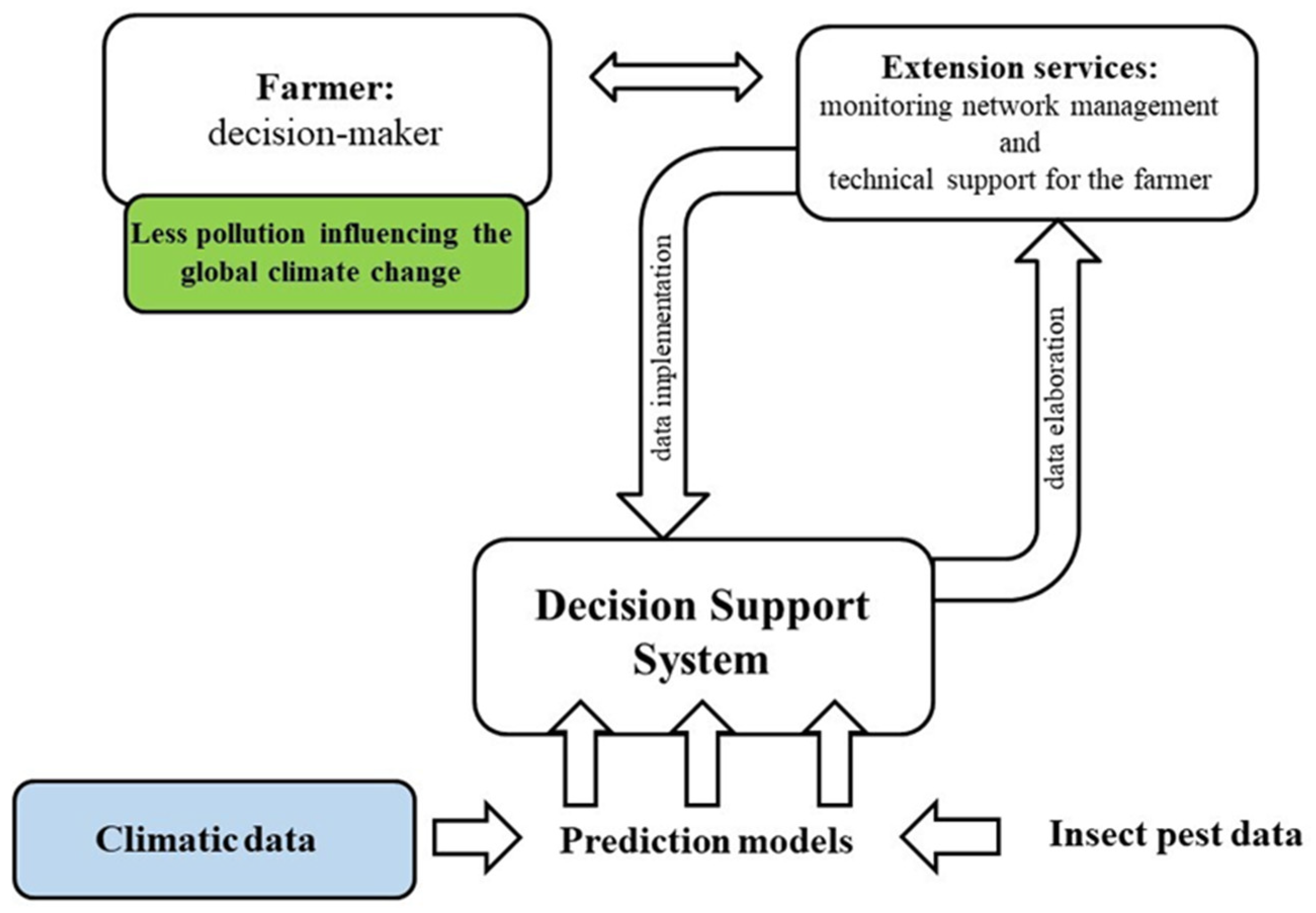
2.3. Control Strategies of B. oleae under Global Warming
3. Influence of Climate on Some of the Major Secondary Pests of O. europaea
3.1. Olive Moths
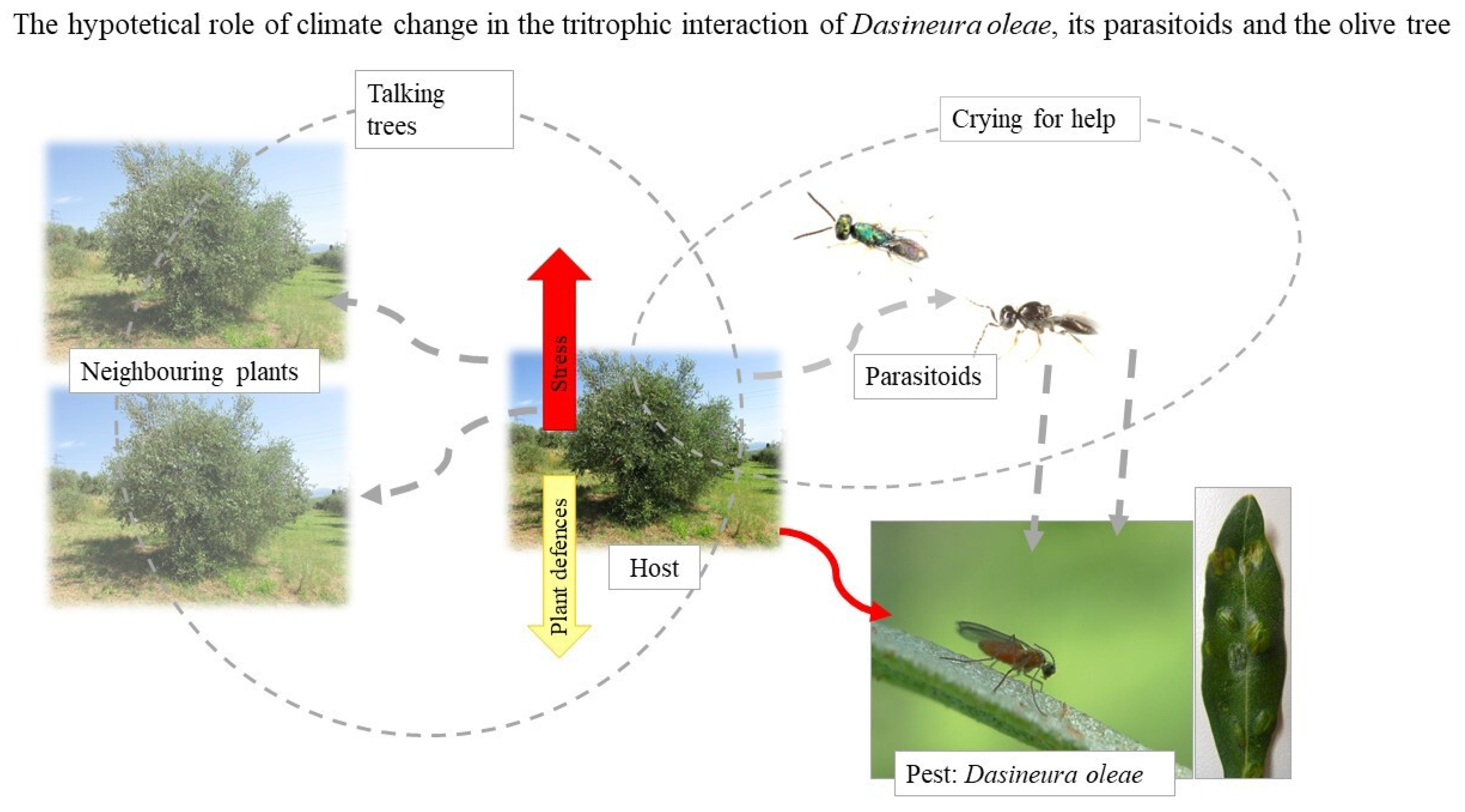
3.2. The Olive Leaf Gall Midge, Dasineura oleae
4. Conclusions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Mgbemene, C.A.; Nnaji, C.C.; Nwozor, C. Industrialization and its backlash: Focus on climate change and its consequences. J. Environ. Sci. Technol. 2016, 9, 301–316. [Google Scholar] [CrossRef] [Green Version]
- War, A.R.; Taggar, G.K.; War, M.Y.; Hussain, B. Impact of climate change on insect pests, plant chemical ecology, tritrophic interactions and food production. Int. J. Clin. Biol. Sci. 2016, 1, 16–29. [Google Scholar]
- Rădoi, M.I. Interconnections between sustainable development, climate change and agriculture. Rev. Stiinte Pol. 2020, 68, 53–61. [Google Scholar]
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef] [Green Version]
- McMichael, A.J. Globalization, climate change, and human health. N. Engl. J. Med. 2013, 368, 1335–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapp, J.; Newell, P.; Brent, Z.W. The global political economy of climate change, agriculture and food systems. J. Peasant Stud. 2018, 45, 80–88. [Google Scholar] [CrossRef]
- Karuppaiah, V.; Sujayanad, G.K. Impact of climate change on population dynamics of insect pests. World J. Agric. Sci. 2012, 8, 240–246. [Google Scholar]
- Huang, J.; Li, J. Effects of climate change on overwintering pupae of the cotton bollworm, Helicoverpa armigera (Hübner) (lepidoptera: Noctuidae). Int. J. Biometerol. 2015, 59, 863–876. [Google Scholar] [CrossRef]
- Wang, X.; Levy, K.; Son, Y.; Johnson, M.W.; Daane, K.M. Comparison of the thermal performance between a population of the olive fruit fly and its co-adapted parasitoids. Biol. Control 2012, 60, 247–254. [Google Scholar] [CrossRef]
- Jaworski, T.; Hilszezański, J. The effect of temperature and humidity changes on insects development and their impact on forest ecosystems in the context of expected climate change. For. Res. Pap. 2013, 74, 345–355. [Google Scholar]
- Harrington, R.; Clark, S.J.; Welham, S.J.; Verrier, P.J.; Denholm, C.H.; Hullé, M.; Maurice, D.; Rounsevell, M.D.; Cocu, N.; European Union Examine Consortium. Environmental change and the phenology pf European aphids. Glob. Chang. Biol. 2007, 13, 1550–1564. [Google Scholar] [CrossRef]
- Boullis, A.; Francis, F.; Verheggen, F.J. Climate change and tritrophic interactions: Will modifications to greenhouse gas emissions increase the vulnerability of herbivorous insects to natural enemies? Environ. Entomol. 2015, 44, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Enkegaard, A.; Sørensen, J.G. Temperature affects biological control efficacy: A microcosm study of Trichogramma achaeae. Insects 2021, 12, 95. [Google Scholar] [CrossRef]
- Menéndez, R. How are insects responding to global warming? Tijd. Entomol. 2007, 150, 355–365. [Google Scholar]
- Jönsson, A.M.; Harding, S.; Bärring, L.; Ravn, H.P. Impact of climate change on the population dynamics of Ips typographus in southern Sweden. Agric. For. Meteorol. 2007, 146, 70–81. [Google Scholar] [CrossRef]
- Milano, M.; Ruelland, D.; Fernandez, S.; Dezetter, A.; Fabre, J.; Servat, E.; Fritsch, J.M.; Ardoin-Bardin, S.; Thivet, G. Current state of Mediterranean water resources and future trends under climatic and anthropogenic changes. Hydrol. Sci. J. 2013, 58, 498–518. [Google Scholar] [CrossRef]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate change and interconnected risks to suitable development in the Mediterranen. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Ponti, L.; Gutierrez, A.P.; Ruti, P.M.; Dell’Aquila, A. Fine-scale ecological and economic assessment of climate change on olive in the Mediterranean Basin reveals winner and losers. Proc. Natl. Acad. Sci. USA 2014, 111, 5598–5603. [Google Scholar] [CrossRef] [Green Version]
- Michalopoulos, G.; Kasapi, K.A.; Koubouris, G.; Psarras, G.; Arampatzis, G.; Hatzigiannakis, E.; Kavvadis, V.; Xiloyannis, C.; Montanaro, G.; Malliaraki, S.; et al. Adaptation of Mediterranean olive groves to climate change through sustainable cultivation practices. Climate 2020, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Vossen, P. Olive oil: History, production, and characteristics of the world’s classic oils. HortScience 2007, 42, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Giunti, G.; Benelli, G.; Conte, G.; Mele, M.; Caruso, G.; Gucci, R.; Flamini, G.; Canale, A. VOCs-mediated location of olive fly larvae by the braconid parasitoid Psyttalia concolor. A multivariate comparison among VOC bouquets from three olive cultivars. BioMed Res. Int. 2016, 2016, 7827615. [Google Scholar] [CrossRef]
- Picchi, M.S.; Marchi, S.; Albertini, A.; Petacchi, R. Organic management of olive orchards increases the predation rate of overwintering pupae of Bactrocera oleae (Diptera: Tephritidae). Biol. Control 2017, 108, 9–15. [Google Scholar] [CrossRef]
- Fraga, H.; Pinto, J.G.; Viola, F.; Santos, J.A. Climate change projections for olive yields in the Mediterranean Basin. Int. J. Climatol. 2020, 40, 769–781. [Google Scholar] [CrossRef] [Green Version]
- Julca, I.; Marcet-Houben, M.; Cruz, F.; Gómez-Garrido, J.; Gaut, B.S.; Díez, C.M.; Gut, I.G.; Alioto, T.S.; Vargas, P.; Gabaldón, T. Genomic evidence for recurrent genetic admixture during the domestication of Mediterranean olive trees (Olea europaea L.). BMC Biol. 2020, 18, 148. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 2021, 11, 56. [Google Scholar] [CrossRef]
- Orlandi, F.; Avolio, E.; Bonofiglio, T.; Federico, S.; Romano, V.; Fornaciari, M. Potential shifts in olive flowering according to climate variations in Southern Italy. Meteorol. Appl. 2012, 20, 497–503. [Google Scholar] [CrossRef]
- Moriondo, M.; Ferrise, R.; Trombi, G.; Brilli, L.; Dibari, C.; Bindi, M. Modelling olive trees and grapevines in a changing climate. Environ. Model. Softw. 2015, 72, 387–401. [Google Scholar] [CrossRef]
- Ponti, L.; Cossu, A.; Gutierrez, A.P. Climate warming effects on the Olea europaea-Bactrocera oleae system in Mediterranean islands: Sardinia as an example. Glob. Chang. Biol. 2009, 15, 2874–2884. [Google Scholar] [CrossRef]
- Obsborne, C.P.; Chuine, I.; Viner, D.; Woodward, F.I. Olive phenology as a sensitive indicator of future climatic warning in the Mediterranean. Plant Cell Environ. 2000, 23, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Besnard, G.; Casas, R.R.D.; Vargas, P. Plastid and nuclear DNA polymorphism reveals historical processes of isolation and reticulation in the olive tree complex (Olea europaea). J. Biogeogr. 2007, 34, 736–752. [Google Scholar] [CrossRef]
- Ayerza, R.; Sibbett, G.S. Thermal adaptability of olive (Olea europaea L.) to the Arid Chaco of Argentina. Agric. Ecosyst. Environ. 2001, 84, 277–285. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ponti, L.; d‘Oultremont, T.; Ellis, C.K. Climate change effects on poikilotherm tritrophic interactions. Clim. Chang. 2008, 87 (Suppl. S1), S167–S192. [Google Scholar] [CrossRef]
- Montiel-Bueno, A.; Jones, O. Alternative methods for controlling the olive fly, Bactrocera oleae, involving semiochemicals. Use of pheromones and other semiochemicals in integrated production. IOBC WPRS Bull. 2002, 25, 147–156. [Google Scholar]
- Haniotakis, E.G. Olive pest control: Present status and prospects. Integrated Protection of Olive Crops. IOBC WPRS Bull. 2005, 28, 1–9. [Google Scholar]
- Cornara, D.; Saponari, M.; Zeilinger, A.R.; de Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Martelli, G.P.; Almeida, R.P.P.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest. Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochat, J.; Gutierrez, A.P. Weather-mediated regulation of olive scale by two parasitoids. J. Anim. Ecol. 2001, 70, 476–490. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, O.J.; Post, E.; Burns, C.E.; Johnston, K.M. Ecosystem responses to global climate change: Moving beyond color mapping. Bioscience 2003, 53, 1199–1205. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Pitcairn, M.J.; Ellis, C.K.; Carruthers, N.; Ghezelbash, R. Evaluating biological control of yellow starthistle (Centaurea solstitialis) in California: A GIS based supply-demand demographic model. Biol. Control 2005, 34, 115–131. [Google Scholar] [CrossRef]
- Merrill, R.M.; Gutierrez, D.; Lewis, O.T.; Gutierrez, J.; Diez, S.B.; Wilson, R.J. Combined effects of climate and biotic interactions on the elevational range of a phytophagous insect. J. Anim. Ecol. 2008, 77, 145–155. [Google Scholar] [CrossRef]
- Marchi, S.; Guidotti, D.; Ricciolini, M.; Petacchi, R. Towards understanding temporal and spatial dynamics of Bactrocera oleae (Rossi) infestations using decade-long agrometeorological time series. Int. J. Biometeorol. 2016, 60, 1681–1694. [Google Scholar] [CrossRef]
- Iofrida, N.; De Luca, A.I.; Gulisano, G.; Strano, A. An application of Q-methodology to Mediterranean olive production-stakeholders’ understanding of sustainability issues. Agric. Syst. 2018, 162, 46–55. [Google Scholar] [CrossRef]
- Nardi, F.; Carapelli, A.; Dallai, R.; Roderick, G.K.; Frati, F. Population structure and colonization history of the olive fly, Bactrocera oleae (Diptera: Tephritidae). Mol. Ecol. 2005, 14, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A review of Bactrocera oleae (Rossi) impact in olive products: From the tree to the table. Trends Food Sci. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Abd El-Salam, A.M.E.; Salem, S.A.W.; Abdel-Rahman, R.S.; El-Behery, H.H.; Magd Elden, M.A. Effects of climatic changes on olive fly, Bactrocera oleae (Rossi) population dynamic with respect to the efficacy of its larval parasitoid in Egyptian olive trees. Bull. Natl. Res. Cent. 2019, 43, 173. [Google Scholar] [CrossRef]
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Aggressive behavior and territoriality in the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae): Role of residence and time of day. J. Insect Behav. 2014, 27, 145–161. [Google Scholar] [CrossRef]
- Naz Eti, C.; Dogac, E.; Taskin, B.G.; Gokdere, G.; Taskin, V. Population structure and patterns of geographic differentiation on Bactrocera oleae (Diptera: Tephritidae) in Eastern Mediterranean Basin. Mitochondrial DNA A 2018, 29, 1051–1062. [Google Scholar]
- Kounatidis, N.T.; Papadopoulos, P.; Mavragani-Tsipidou, P.; Cohen, Y.; Tertivanidis, K.; Nomikou, M.; Nestel, D. Effect of elevation on spatio-temporal patterns of olive fly (Bactrocera oleae) populations in northern Greece. J. Appl. Entomol. 2008, 132, 722–733. [Google Scholar] [CrossRef]
- Petacchi, R.; Marchi, S.; Federici, S.; Ragaglini, G. Large-scale simulation of temperature-dependent phenology in wintering populations of Bactrocera oleae (Rossi). J. Appl. Entomol. 2015, 139, 496–509. [Google Scholar] [CrossRef]
- Ragaglini, G.; Tomassone, D.; Petacchi, R. Can spring-preventive adulticide treatments be assumed to improve Bactrocera oleae (Rossi) management? IOBC WPRS Bull. 2005, 30, 309–314. [Google Scholar]
- Gutierrez, A.P.; Ponti, L.; Cossu, Q.A. Effects of climate warming on olive and olive fly (Bactrocera oleae (Gmelin)) in California and Italy. Clim. Chang. 2009, 95, 195–217. [Google Scholar] [CrossRef]
- Wang, X.G.; Johnson, M.W.; Daane, K.M.; Opp, S.B. Combined effects of heat stress and food supply on the flight performance of olive fruit fly (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2009, 102, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.H.; Parry, M.; Carter, T.R. The potential effects of climatic change on agricultural insect pests. Agric. For. Meteorol. 1991, 57, 221–240. [Google Scholar] [CrossRef]
- Park, Y.L.; Tollefson, J.J. Spatial prediction of corn root-worm (Coleoptera: Chrysomelidae) adult emergence in Iowa corn fields. J. Econ. Entomol. 2005, 98, 121–128. [Google Scholar] [CrossRef]
- Carriere, Y.; Ellsworth, P.C.; Dutilleul, P.; Ellers-Kirk, C.; Barkley, V.; Antilla, L. A GIS-based approach for area-wide pest management: The scales of Lygus hesperus movements to cotton from alfalfa, weeds, and cotton. Entomol. Exp. Appl. 2006, 118, 203–210. [Google Scholar] [CrossRef]
- Crovetti, A.; Quaglia, F.; Loi, G.; Rossi, E.; Malfatti, P.; Chesi, F.; Conti, B.; Belcari, A.; Raspi, A.; Paparatti, B. Influence of temperature and humidity on the development of the immature stages of Dacus oleae (Gmelin). Frustula Entomol. 1982, 5, 133–166. [Google Scholar]
- Higley, L.G.; Pedigo, L.P.; Ostlie, K.R. DEGDAY: A program for calculating degree-days and assumptions behind the degree-day approach. Environ. Entomol. 1986, 15, 999–1016. [Google Scholar] [CrossRef] [Green Version]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Volpi, I.; Guidotti, D.; Mammini, M.; Petacchi, R.; Marchi, S. Managing complex datasets to predict Bactrocera oleae infestation at the regional scale. Comput. Electron. Agric. 2020, 179, 105867. [Google Scholar] [CrossRef]
- Moonen, A.C.; Ercoli, L.; Mariotti, M.; Masoni, A. Climate change in Italy indicated by agrometeorological indices over 122 years. Agric. For. Meteorol. 2002, 11, 13–27. [Google Scholar] [CrossRef]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, M.F.; Torres, L.M. The use of cumulative degree-days to predict olive fly, Bactrocera oleae (Rossi), activity in traditional olive groves from the northeast pf Portugal. J. Pest Sci. 2011, 84, 187–197. [Google Scholar] [CrossRef]
- Dey, A. Machine learning algorithms: A review. Int. J. Comput. Sci. Inf. Technol. IJCSIT 2016, 7, 1174–1179. [Google Scholar]
- Ip, R.H.L.; Ang, L.M.; Seng, K.P.; Broster, J.C.; Pratley, J.E. Big data and machine learning for crop protection. Comput. Electron. Agric. 2018, 151, 376–383. [Google Scholar] [CrossRef]
- Hill, M.G.; Connolly, P.G.; Reutemann, P.; Fletcher, D. The use of data mining to assist crop protection decisions on kiwifruit in New Zealand. Comput. Electron. Agric. 2014, 108, 250–257. [Google Scholar] [CrossRef]
- Kornejady, A.; Ownegh, M.; Bahremand, A. Landslide susceptibility assessment using maximum entropy model with two different data sampling method. Catena 2017, 152, 144–162. [Google Scholar] [CrossRef]
- Benhadi-Marín, J.; Santos, S.A.P.; Baptista, P.; Pereira, J.A. Distribution of Bactrocera oleae (Rossi, 1790) throughout the Iberian Peninsula based on a maximum entropy modelling approach. Ann. Appl. Biol. 2020, 177, 112–120. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Mills, N.J.; Ponti, L. Limits to the potential distribution of the light brown apple moth in Arizona-California based on climate suitability and host plant availability. Biol. Invasions 2010, 12, 3319–3331. [Google Scholar] [CrossRef] [Green Version]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Ponti, L.; Gilioli, G.; Biondi, A.; Desneux, N.; Gutierrez, A.P. Physiologically based demographic models streamline identification and collection of data in evidence-based pest risk assessment. Bull. OEPP 2015, 45, 317–322. [Google Scholar] [CrossRef]
- Aluja, M.; Ordano, M.; Guillén, L.; Rull, J. Understanding long-term fruit fly (Diptera: Tephritidae) population dynamics: Implications for area wide management. J. Econ. Entomol. 2012, 105, 823–836. [Google Scholar] [CrossRef]
- Hódar, J.A.; Zamora, R.; Cayuela, L. Climate change and the incidence of a forest pest in Mediterranean ecosystem: Can the North Atlantic Oscillation be used as a predictor? Clim. Chang. 2012, 113, 699–711. [Google Scholar] [CrossRef]
- Ordano, M.; Engelhard, I.; Rempoulakis, P.; Nemny-Lavy, E.; Blum, M.; Yasin, S.; Lensky, I.M.; Papadopoulos, N.T.; Nestel, D. Olive fruit fly (Bactrocera oleae) population dynamics in the eastern Mediterranean: Influence of exogenous uncertainty on a monophagous frugivorous insect. PLoS ONE 2015, 10, e0127798. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeol. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, A.; Nichols, J.D.; Campbell Grant, E.H.; Veran, S. Presence-only modelling using MAXENT: When can we trust the inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Bosso, L.; Di Febbraio, M.; Cristinzio, G.; Zoina, A.; Russo, D. Shedding light on the effects of climate change on the potential distribution of Xylella fastidiosa in the Mediterranean basin. Biol. Invasions 2016, 18, 1759–1768. [Google Scholar] [CrossRef]
- Ashraf, U.; Peterson, A.T.; Chaudhry, M.N.; Ashraf, I.; Saqib, Z.; Ahmad, S.R.; Ali, H. Ecological niche model comparison uder different climate scenarios: A case study of Olea sp. in Asia. Ecosphere 2017, 8, e01825. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Wang, R.; Yang, H.; Yang, W.; Yang, C.; Jin, Z. MaxEnt modeling to predict current and future distributions of Batocera lineolata (Coleoptera: Cerambycidae) under climate change in China. Écoscience 2019, 27, 23–31. [Google Scholar] [CrossRef]
- Veloz, S.D.; Williams, J.W.; Blois, J.L.; He, F.; Otto-Bliesner, B.; Liu, Z. No-analog climates and shifting realized niches during the late quaternary: Implications for the 21st-century predictions by species distribution models. Glob. Chang. Biol. 2012, 18, 1698–1713. [Google Scholar] [CrossRef]
- Wang, W.G.; Johnson, M.W.; Yokoyama, V.Y.; Pickett, C.H.; Daane, K.M. Comparative evaluation of two olive fruit fly parasitoids under varying abiotic conditions. BioControl 2011, 56, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Garantonakis, N.; Varikou, K.; Birouraki, A. Parasitism of Psyttalia concolor (Hymenoptera: Braconidae) on Bactrocera oleae (Diptera: Tephritidae) infesting different olive varieties. Phytoparasitica 2017, 45, 461–469. [Google Scholar] [CrossRef]
- Hoelmer, K.A.; Kirk, A.A.; Pickett, C.H.; Daane, K.M.; Johnson, M.W. Prospect for improving biological control of olive fruit fly, Bactrocera oleae (Diptera: Tephritidae), with introduced parasitoids (Hymenoptera). BioControl Sci. Technol. 2011, 21, 1005–1025. [Google Scholar] [CrossRef]
- Orsini, M.M.; Daane, K.M.; Sime, K.R.; Nelson, E.H. Mortality of olive fruit fly pupae in California. BioControl Sci. Technol. 2007, 17, 797–807. [Google Scholar] [CrossRef]
- Albertini, A.; Pizzolotto, R.; Petacchi, R. Carabid patterns in olive orchards and woody semi-natural habitats: First implications for conservation biological control against Bactrocera oleae. BioControl 2017, 62, 71–83. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Dimou, I.; Koutsikopoulos, C.; Economopoulos, A.P.; Lykakis, J. Depth of pupation of the wild olive fruit fly Bactrocera (Dacus) oleae (Gmel.) (Dipt., Tephritidae), as affected by soil abiotic factors. J. Appl. Entomol. 2003, 127, 12–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Smith, S.L.; Riseborough, W.; Cihlar, J. Soil temperature in Canada during the twentieth century: Complex responses to atmospheric climate change. J. Geophys. Res. Atmos. 2005, 110. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Nestel., D.; Rempoulakis, P.; Yanovski, L.; Miranda, M.A.; Papadopoulos, N.T. The evolution of alternative control strategies in a traditional crop: Economy and policy as drivers of olive fly control. Adv. Insect Control Resist. Manag. 2016. [Google Scholar] [CrossRef]
- Petacchi, R.; Ferrali, M.; Valicenti, M. Mosca delle olive, il metodo “push-pull”. Olivo Olio 2021, 4, 28–31. [Google Scholar]
- Marchini, D.; Petacchi, R.; Marchi, S. Bactrocera oleae reproductive biology: New evidence on wintering wild populations in olive groves of Tuscany (Italy). Bull. Insectology 2017, 70, 121–128. [Google Scholar]
- Albertini, A.; Marchi, S.; Ratti, C.; Burgio, G.; Petacchi, R.; Magagnoli, S. Bactrocera oleae pupae predation by Ocypus olens detected by molecular gut content analysis. BioControl 2018, 63, 227–239. [Google Scholar] [CrossRef]
- Miranda, M.A.; Barceló, C.; Valdés, F.; Feliu, J.F.; Nestel, D.; Papadopoulos, N.; Sciarretta, A.; Ruiz, M.; Alorda, B. Developing and implementation of decision support system (DSS) for the control of olive fruit fly, Bactrocera oleae, in Mediterranean olive orchards. Agronomy 2019, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Pontikakos, C.M.; Tsiligiridis, T.A.; Drougka, M.E. Location-aware system for olive fruit fly spray control. Comput. Electron. Agric. 2010, 70, 355–368. [Google Scholar] [CrossRef]
- Fenger, J. Air pollution in the last 50 years-from local to global. Atmos. Environ. 2009, 43, 13–22. [Google Scholar] [CrossRef]
- Ait Mansour, A.; Ouanaimi, F.; Chemseddine, M.; Boumezzough, A. Study of the flight dynamics of Prays oleae (Lepidoptera: Yponomeutidae) using sexual trapping in olive orchards of Essaouira region, Morocco. J. Entomol. Zool. Stud. 2017, 5, 943–952. [Google Scholar]
- Schilling, J.; Freier, K.P.; Hertig, E.; Scheffran, J. Climate change, vulnerability and adaptation in North Africa with focus on Morocco. Agric. Ecosyst. Environ. 2012, 156, 12–26. [Google Scholar] [CrossRef]
- Rosales, R.; Garrido, D.; Ramos, P.; Ramos, J.M. Ethylene can reduce Prays oleae attack in olive trees. Crop Prot. 2006, 25, 140–143. [Google Scholar] [CrossRef]
- Simoglou, K.B.; Karataraki, A.; Roditakis, N.E.; Roditakis, E. Euzophera bigella (Zeller) (Lepidoptera: Pyralidae) and Dasineura oleae (F. Low) (Diptera: Cecidomyiidae): Emerging olive crop pests in the Mediterranean? J. Pest Sci. 2012, 85, 169–177. [Google Scholar] [CrossRef]
- Chaouche, S.T.; Bengouga, K.; Fadlaoui, H. The first detection of the olive leaf moth Palpita vitrealis (Rossi) (Lepidoptera: Pyralidae) as a serious pest in Biskra province (Algeria). Bull. OEPP 2019, 49, 593–596. [Google Scholar] [CrossRef]
- Antonelli, R.; Rossi, E. La Palpita unionalis (Lepidoptera: Pyraustidae): Un fitofago di recente importanza negli oliveti toscani. Inf. Fitopatol. 1989, 39, 27–32. [Google Scholar]
- Valicenti, M.; Granchi, P.; Petacchi, R. Olivo, monitoraggio efficace e controllo di fitofagi fillofagi. L’Informatore Agrar. 2021, 4, 57–65. [Google Scholar]
- Proietti, P.; Nasini, L.; Ilarioni, L. Photosynthetic behavior of Spanish Arbequina and Italian Maurino olive (Olea europaea L.) cultivars under super-intensive grove conditions. Photosynthetica 2012, 50, 239–246. [Google Scholar] [CrossRef]
- Doğanlar, M. Parasitoids complex of the olive leaf gall midge, Dasineura oleae (Angelini, 1831) and Lasioptera oleicola Skuhravá (Diptera: Cecidomyiidae) in Hatay Turkey, with descriptions of new genus and species from Tetrastichinae (Hymenoptera: Eulophidae). Türkiye Entomol. Derneği 2011, 35, 245–264. [Google Scholar]
- Tondini, E.; Petacchi, R. First observations on the parasitoids complex and on the biology of Dasineura oleae during an outbreak in Tuscany, Italy. Bull. Insectology 2019, 72, 93–102. [Google Scholar]
- Caselli, A.; Francini, A.; Minnocci, A.; Petacchi, R. Dasineura oleae: Morphological and physiological characterization following the midge attack on olive leaves. J. Plant Dis. Prot. 2021, 128, 173–182. [Google Scholar] [CrossRef]
- Picchi, M.S.; Tondini, E.; Albertarelli, N.; Monteforti, G.; Petacchi, R. Following the outbreak: Preliminary findings on the landscape effect on Dasineura oleae and its parasitoids in Central Italy. Phytoparasitica 2021. [Google Scholar] [CrossRef]
- Damos, P. Modular structure of web-based decision support systems for integrated pest management. A review. Agron. Sustain. Dev. 2015, 35, 1347–1372. [Google Scholar] [CrossRef] [Green Version]
- Guidotti, D.; Ragaglini, G.; Petacchi, R. Analysis of spatio-temportal Bactrocera oleae (Diptera, Tephritidae) infetstation distributions obtained from a large-scale monitoring network and its importance to IPM. IOBC Bull. 2005, 28, 13–18. [Google Scholar]
- Hammann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate change alters plant-herbivore interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caselli, A.; Petacchi, R. Climate Change and Major Pests of Mediterranean Olive Orchards: Are We Ready to Face the Global Heating? Insects 2021, 12, 802. https://doi.org/10.3390/insects12090802
Caselli A, Petacchi R. Climate Change and Major Pests of Mediterranean Olive Orchards: Are We Ready to Face the Global Heating? Insects. 2021; 12(9):802. https://doi.org/10.3390/insects12090802
Chicago/Turabian StyleCaselli, Alice, and Ruggero Petacchi. 2021. "Climate Change and Major Pests of Mediterranean Olive Orchards: Are We Ready to Face the Global Heating?" Insects 12, no. 9: 802. https://doi.org/10.3390/insects12090802
APA StyleCaselli, A., & Petacchi, R. (2021). Climate Change and Major Pests of Mediterranean Olive Orchards: Are We Ready to Face the Global Heating? Insects, 12(9), 802. https://doi.org/10.3390/insects12090802





