Long-Term Population Dynamics of Namib Desert Tenebrionid Beetles Reveal Complex Relationships to Pulse-Reserve Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
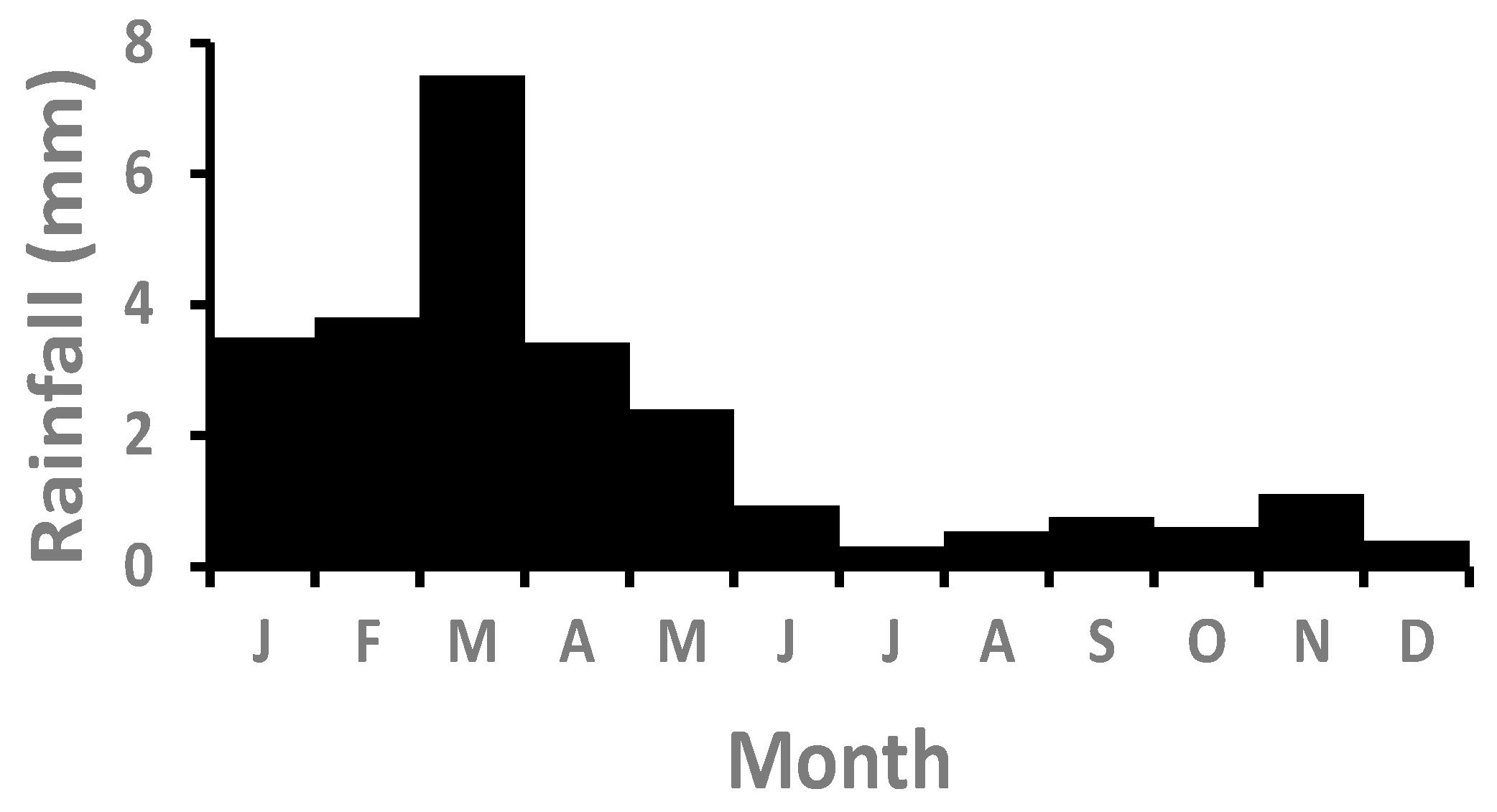
3.1. Rainfall, Fog, and Detritus
3.2. Trapping Data Characteristics
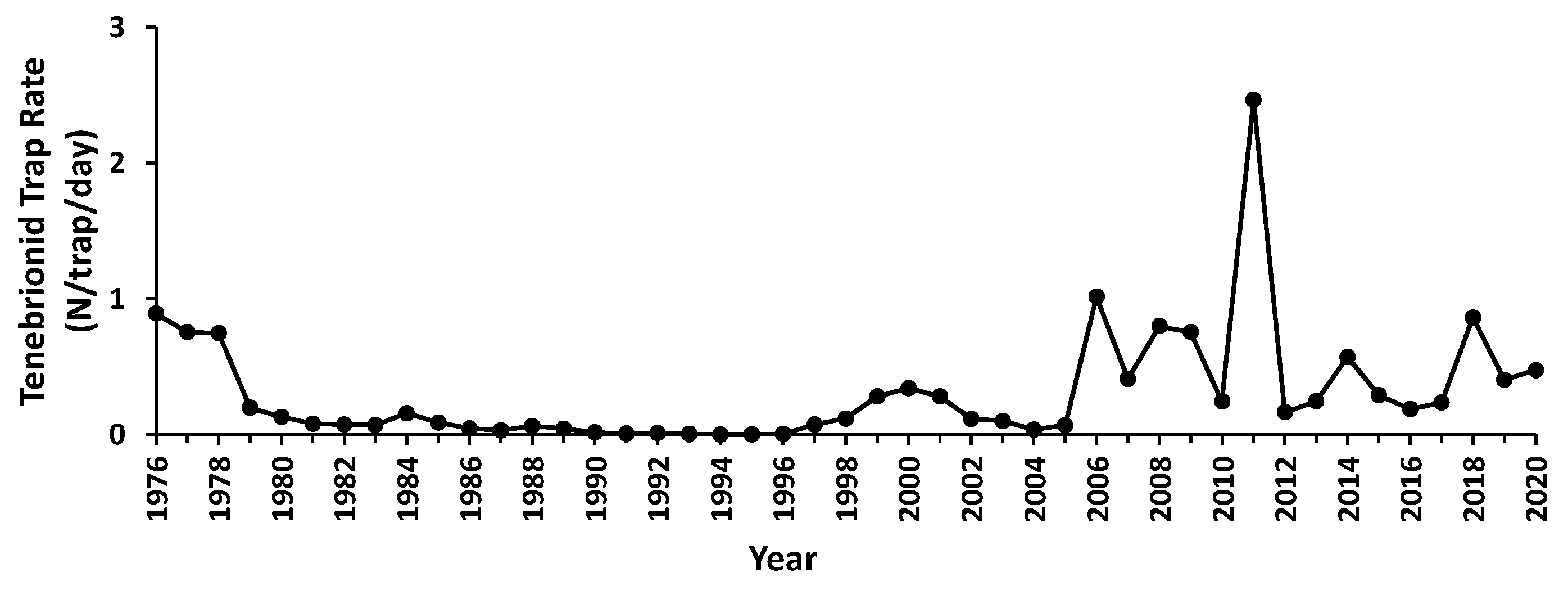
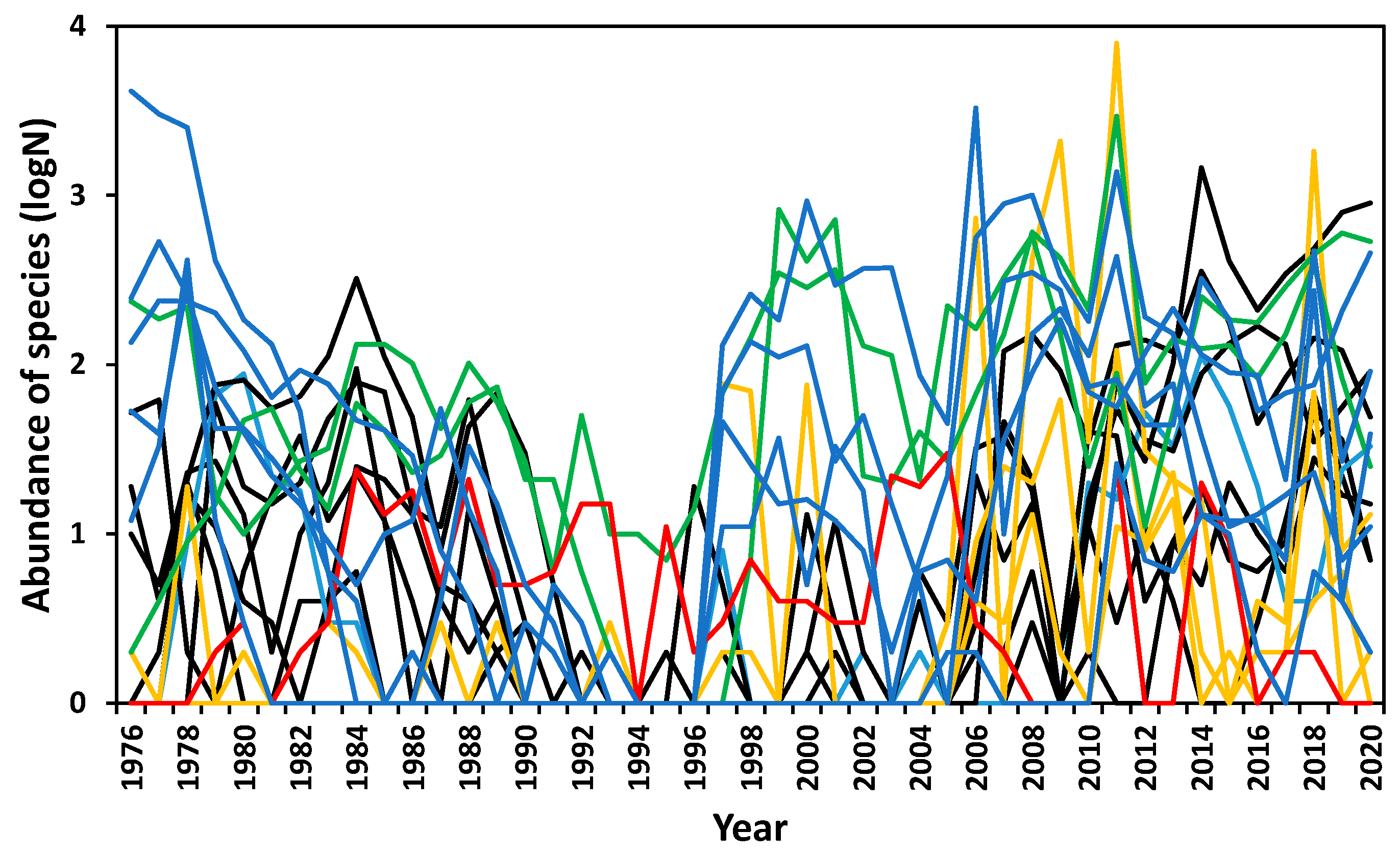
3.3. Abundance Variation
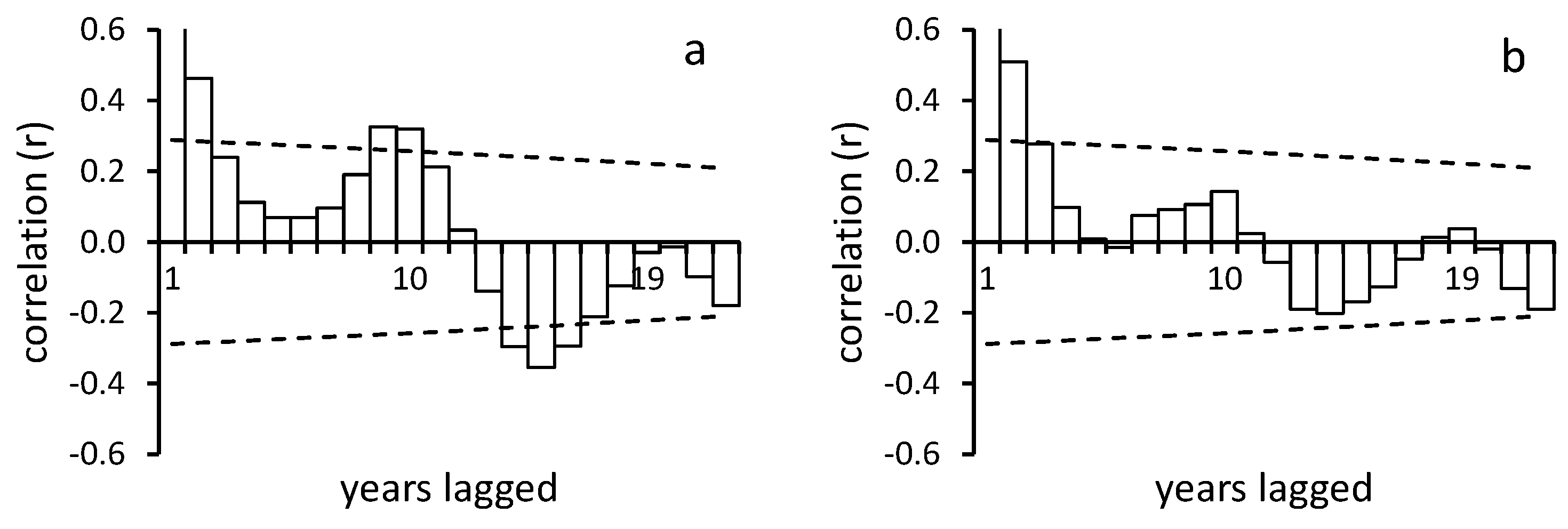
3.4. Responses to Trigger Events
3.5. Community Composition
4. Discussion
4.1. Population Responses to Rainfall
4.2. Population Responses to Fog
4.3. Recovering from Low Abundance
4.4. Population Variability
4.5. Long Term Observations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Tribe | Number Recorded | Focal (F) Other (O) |
|---|---|---|---|
| Zophosis (Gyrosis) moralesi (Koch) | Zophosini | 12,748 | F |
| Eustolopus octoseriatus Gebien | Adesmiini | 9748 | F |
| Metriopus depressus (Haag) | Adesmiini | 7838 | F |
| Cauricara velox (Peringuey) | Adesmiini | 6684 | F |
| Zophosis (Calosis) amabilis (Deyrolle) | Zophosini | 6089 | F |
| Zophosis (Z.) dorsata Peringuey | Zophosini | 4173 | F |
| Zophosis (Gyrosis) devexa Peringuey | Zophosini | 2937 | F |
| Physosterna cribripes (Haag) | Adesmiini | 2504 | F |
| Zophosis (Occidentophosis) cerea Peringuey | Zophosini | 1899 | F |
| Rhammatodes tagenesthoides Koch | Tentyriini | 1840 | F |
| Zophosis (Gyrosis) orbicularis Deyrolle | Zophosini | 853 | F |
| Physadesmia globosa (Haag) | Adesmiini | 709 | F |
| Epiphysa arenicola Penrith | Adesmiini | 556 | F |
| Zophosis (Occidentophosis) damarina Peringuey | Zophosini | 522 | F |
| Parastizopus armaticeps (Peringuey) | Opatrini | 508 | F |
| Rhammatodes subcostatus Koch | Tentyriini | 438 | F |
| Onymacris rugatipennis (Haag) | Adesmiini | 427 | F |
| Rhammatodes longicornis Haag | Tentyriini | 379 | F |
| Eurychora sp. A | Eurychorini | 332 | F |
| Stips dohrni (Haag) | Eurychorini | 322 | F |
| Cauricara eburnea (Pascoe) | Adesmiini | 251 | F |
| Rhammatodes aequalipennis Peringuey | Tentyriini | 231 | F |
| Stenocara gracilipes Solier | Adesmiini | 192 | F |
| Namibomodes serrimargo (Gebien) | Molurini | 190 | F |
| Gonopus tibialis Fabricius | Platynotini | 137 | F |
| Pachynotelus machadoi Koch | Cryptochilini | 127 | F |
| Zophosis mniszechi Deyrolle | Zophosini | 87 | O |
| Vansonium bushmanicum Koch | Calognathini | 69 | O |
| Zophosis kochi Penrith | Zophosini | 67 | O |
| Carchares macer Pascoe | Scaurini | 55 | O |
| Onymacris plana (Peringuey) | Adesmiini | 47 | O |
| Zophosis (Carpiella) latisterna (Koch) | Zophosini | 47 | O |
| Geophanus sp. | Eurychorini | 44 | O |
| Psammodes sp. | Molurini | 42 | O |
| Zophosis (Cerosis) hereoensis Gebien | Zophosini | 40 | O |
| Synhimba melancholicum Koch | Molurini | 35 | O |
| Diestecopus sp. | Drosochrini | 31 | O |
| Caenocrypticus peezi Koch | Caenocrypticini | 16 | O |
| Epiphysa flavicollis (Fabricius) | Adesmiini | 16 | O |
| Nicandra sp. | Drosochrini | 13 | O |
| Planostibes dentipes Koch | Opatrini | 12 | O |
| Zophosis (Dactylocalcar) caecus Gebien | Zophosini | 10 | O |
| Brinckia debilis (Péringuey) | Sepidiini | 4 | O |
| Cyphostete sp. | Tentyriini | 4 | O |
| Onymacris laeviceps Gebien | Adesmiini | 4 | O |
| Asphaltesthes impressipennis Fairmaire | Tentyriini | 2 | O |
| Ennychiatus fitzsimmonsi Koch | Opatrini | 2 | O |
| Phanerotomeina sp. | Molurini | 2 | O |
| Cheirodes sp. | Melanimini | 2 | O |
| Gonocephalum sp. | Opatrini | 2 | O |
| Argenticrinis lossowi (Koch) | Sepidiini | 1 | O |
| Renatiella scrobipennis (Haag-Rutenberg) | Adesmiini | 1 | O |
| Somaticus sp. | Sepidiini | 1 | O |
| Uniungulum hoeschi Koch | Sepidiini | 1 | O |
Appendix B
| Year | Annual Rainfall (mm) | Summer Rainfall (mm) | Winter Rainfall (mm) | Effective Rainfall (mm) | Interpulse Period (y) | Grass Productivity (g m−2) | Detritus Index (g m−2) |
|---|---|---|---|---|---|---|---|
| 1963 | 45.8 | 17.4 | 28.4 | 12.8 | 1.121 | 1.121 | |
| 1964 | 5.4 | 5.3 | 0.1 | 0 | 0 | 0.881 | |
| 1965 | 33.8 | 16.5 | 17.3 | 12.9, 11.9 | 1.5 | 1.770 | 1.911 |
| 1966 | 6.8 | 5.1 | 1.7 | 0 | 0 | 1.475 | |
| 1967 | 25.2 | 23.4 | 1.8 | 19.3 | 1.4 | 4.956 | 5.192 |
| 1968 | 6.1 | 5.3 | 0.8 | 0 | 0 | 4.045 | |
| 1969 | 21.0 | 20.7 | 0.3 | 13.2 | 2.0 | 1.357 | 3.905 |
| 1970 | 0.5 | 0.5 | 0.0 | 0 | 0 | 3.261 | |
| 1971 | 21.9 | 18.5 | 3.4 | 0 | 0 | 2.025 | |
| 1972 | 9.9 | 5.5 | 4.4 | 0 | 0 | 1.500 | |
| 1973 | 7.6 | 0.9 | 6.7 | 0 | 0 | 1.146 | |
| 1974 | 23.2 | 17.4 | 5.8 | 0 | 0 | 0.697 | |
| 1975 | 5.3 | 4.3 | 1.0 | 0 | 0 | 0.549 | |
| 1976 | 105.8 | 105.4 | 0.4 | 94.1 | 6.8 | 49.088 | 49.176 |
| 1977 | 10.1 | 2.7 | 7.4 | 0 | 0 | 36.341 | |
| 1978 | 84.9 | 73.2 | 11.7 | 82.6 | 1.8 | 42.303 | 48.118 |
| 1979 | 33.3 | 6.0 | 27.3 | 21.8 | 1.1 | 6.431 | 14.130 |
| 1980 | 8.9 | 1.6 | 7.3 | 0 | 0 | 10.611 | |
| 1981 | 1.8 | 0.0 | 1.8 | 0 | 0 | 8.723 | |
| 1982 | 10.9 | 5.2 | 5.7 | 0 | 0 | 6.376 | |
| 1983 | 10.2 | 1.7 | 8.5 | 0 | 0 | 4.706 | |
| 1984 | 5.4 | 2.2 | 3.2 | 0 | 0 | 3.699 | |
| 1985 | 8.2 | 7.3 | 0.9 | 0 | 0 | 2.804 | |
| 1986 | 4.9 | 2.6 | 2.3 | 0 | 0 | 2.218 | |
| 1987 | 23.5 | 0.0 | 23.5 | 12.3, 11.2 | 7.8 | 1.003 | 1.358 |
| 1988 | 9.2 | 8.6 | 0.6 | 0 | 0 | 1.016 | |
| 1989 | 14.5 | 1.5 | 13.0 | 11.7 | 1.8 | 0.472 | 1.178 |
| 1990 | 13.4 | 12.8 | 0.6 | 12.1 | 0.7 | 0.708 | 1.540 |
| 1991 | 16.8 | 9.8 | 7.0 | 0 | 0 | 1.035 | |
| 1992 | 0.0 | 0.0 | 0.0 | 0 | 0 | 0.869 | |
| 1993 | 12.9 | 12.9 | 0.0 | 0 | 0 | 0.618 | |
| 1994 | 12.0 | 5.3 | 6.7 | 0 | 0 | 0.445 | |
| 1995 | 12.2 | 11.2 | 1.0 | 0 | 0 | 0.319 | |
| 1996 | 12.2 | 10.2 | 2.0 | 0 | 0 | 0.229 | |
| 1997 | 78.0 | 45.5 | 32.5 | 40.9, 32.5 | 6.9 | 30.444 | 30.481 |
| 1998 | 2.6 | 2.6 | 0.0 | 0 | 0 | 24.811 | |
| 1999 | 5.1 | 5.1 | 0.0 | 0 | 0 | 19.576 | |
| 2000 | 34.0 | 34.0 | 0.0 | 34.0 | 2.8 | 13.629 | 16.761 |
| 2001 | 9.1 | 0.0 | 9.1 | 0 | 0 | 12.554 | |
| 2002 | 31.0 | 31.0 | 0.0 | 27.0 | 1.9 | 9.499 | 11.508 |
| 2003 | 1.0 | 1.0 | 0.0 | 0 | 0 | 9.551 | |
| 2004 | 23.6 | 11.5 | 12.1 | 11.0 | 2.7 | 0.059 | 5.828 |
| 2005 | 2.8 | 2.8 | 0.0 | 0 | 0 | 4.732 | |
| 2006 | 116.5 | 72.4 | 44.1 | 108.00 | 1.2 | 57.289 | 58.046 |
| 2007 | 5.5 | 5.5 | 0.0 | 0 | 0 | 45.566 | |
| 2008 | 86.0 | 85.0 | 1.0 | 76.0 | 1.9 | 38.409 | 45.700 |
| 2009 | 51.7 | 50.8 | 0.9 | 45.4 | 0.9 | 20.355 | 27.667 |
| 2010 | 17.1 | 5.6 | 11.5 | 0 | 0 | 18.509 | |
| 2011 | 171.9 | 97.8 | 74.1 | 163.1 | 1.9 | 89.798 | 92.759 |
| 2012 | 16.3 | 12.3 | 4.0 | 0 | 0 | 62.798 | |
| 2013 | 64.5 | 43.8 | 20.7 | 38.2, 20.7 | 1.9 | 21.889 | 31.937 |
| 2014 | 3.2 | 0.2 | 3.0 | 0 | 0 | 25.805 | |
| 2015 | 12.1 | 11.2 | 0.9 | 11.2 | 1.3 | 0.177 | 18.731 |
| 2016 | 24.7 | 6.7 | 18.0 | 18.0 | 1.4 | 4.189 | 7.186 |
| 2017 | 25.3 | 25.3 | 0.0 | 11.3 | 0.8 | 0.236 | 4.454 |
| 2018 | 59.1 | 9.2 | 49.9 | 39.6 | 1.1 | 16.933 | 17.646 |
| 2019 | 4.9 | 3.8 | 1.1 | 0 | 0 | 13.958 | |
| 2020 | 0.2 | 0.0 | 0.2 | 0 | 0 | 11.697 | |
| Mean | 25.3 | 17.0 | 8.4 | 36.8 | 2.3 | 7.105 | 14.5 |
| SD | 33.0 | 24.8 | 14.0 | 37.3 | 2.0 | 17.048 | 19.2 |
| Minimum | 0.0 | 0.0 | 0.0 | 11.0 | 0.7 | 0 | 0.2 |
| Q1 | 5.7 | 2.6 | 0.5 | 12.1 | 1.2 | 0 | 1.5 |
| Median | 12.2 | 6.4 | 2.2 | 20.7 | 1.8 | 0 | 5.5 |
| Q3 | 25.3 | 17.4 | 9.0 | 40.3 | 2.0 | 1.667 | 18.7 |
| Maximum | 171.9 | 105.4 | 74.1 | 163.1 | 7.8 | 89.798 | 92.8 |
| CV (%) | 130 | 146 | 168 | 101 | 88 | 240 | 132 |
| N | 58 | 58 | 58 | 58 | 58 | 58 | 58 |
Appendix C
References
- Illius, A.W.; O’Connor, T.G. On the relevance of nonequilibrium concepts to arid and semiarid grazing systems. Ecol. Appl. 1999, 9, 798–813. [Google Scholar] [CrossRef]
- Noy-Meir, I. Desert ecosystems: Environment and producers. Annu. Rev. Ecol. Syst. 1973, 4, 25–51. [Google Scholar] [CrossRef]
- Noy-Meir, I. Desert ecosystems: Higher trophic levels. Annu. Rev. Ecol. Syst. 1974, 5, 195–214. [Google Scholar] [CrossRef]
- Collins, S.L.; Belnap, J.; Grimm, N.B.; Rudgers, J.A.; Dahm, C.N.; D’Odorico, P.; Litvak, M.; Natvig, D.O.; Peters, D.C.; Pockman, W.T.; et al. A multiscale, hierarchical model of pulse dynamics in arid-land ecosystems. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 397–419. [Google Scholar] [CrossRef]
- Ward, D. The Biology of Deserts, 2nd ed.; Oxford University Press: Oxford, UK, 2016; pp. 1–370. [Google Scholar]
- Dean, W.R.J. Nomadic Desert Birds, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 25, pp. 1–185. [Google Scholar]
- Le Gall, M.; Overson, R.; Cease, A. A global review on locusts (Orthoptera: Acrididae) and their interactions with livestock grazing practices. Front. Ecol. Evol. 2019, 7, 1–24. [Google Scholar] [CrossRef]
- Throop, H.L.; Belnap, J. Connectivity dynamics in dryland litter cycles: Moving decomposition beyond spatial stasis. BioScience 2019, 69, 602–614. [Google Scholar] [CrossRef]
- Moore, J.C.; Berlow, E.L.; Coleman, D.C.; de Ruiter, P.C.; Dong, Q.; Hastings, A.; Collins Johnson, N.; McCann, K.S.; Melville, K.; Morin, P.J.; et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004, 7, 584–600. [Google Scholar] [CrossRef]
- Adair, E.C.; Parton, W.J.; Del Grosso, S.J.; Silver, W.L.; Harmon, M.E.; Hall, S.A.; Burke, I.C.; Hart, S.C. Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob. Chang. Biol. 2008, 14, 2636–2660. [Google Scholar] [CrossRef]
- Throop, H.L.; Archer, S.R. Resolving the dryland decomposition conundrum: Some new perspectives on potential drivers. In Progress in Botany; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 70. [Google Scholar]
- Louw, G.N.; Seely, M.K. Ecology of Desert Organisms, 1st ed.; Longman Group Limited: London, UK; New York, NY, USA, 1982; pp. 1–194. [Google Scholar]
- Wall, D.H.; Bradford, M.A.; St. John, M.G.; Trofymow, J.A.; Behan-Pelletier, V.; Bignell, D.E.; Dangerfield, M.; Parton, W.J.; Rusek, J.; Voigt, W.; et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Chang. Biol. 2008, 14, 2661–2677. [Google Scholar] [CrossRef]
- Crawford, C.S. Nutrition and habitat selection in desert detritivores. J. Arid Environ. 1988, 14, 111–122. [Google Scholar]
- Sagi, N.; Grunzweig, J.M.; Hawlena, D. Burrowing detritivores regulate nutrient cycling in a desert ecosystem. Proc. Biol. Sci. 2019, 286, 20191647. [Google Scholar] [CrossRef]
- Crawford, C.S.; Seely, M.K. Dunefield detritus: Its potential for limiting population size in Lepidochora discoidalis (Gebien) (Coleoptera: Tenebrionidae) in the Namib Desert. J. Afr. Zool. 1993, 107, 527–533. [Google Scholar]
- Seely, M.K.; Louw, G.N. First approximation of the effects of rainfall on the ecology and energetics of a Namib Desert dune ecosystem. J. Arid Environ. 1980, 3, 25–54. [Google Scholar] [CrossRef]
- Eckardt, F.D.; Soderberg, K.; Coop, L.J.; Muller, A.A.; Vickery, K.J.; Grandin, R.D.; Jack, C.; Kapalanga, T.S.; Henschel, J.R. The nature of moisture at Gobabeb, in the central Namib Desert. J. Arid Environ. 2013, 93, 7–19. [Google Scholar] [CrossRef]
- Ramond, J.B.; Woodborne, S.; Hall, G.; Seely, M.K.; Cowan, D.A. Namib Desert primary productivity is driven by cryptic microbial community N-fixation. Sci. Rep. 2018, 8, 6921. [Google Scholar] [CrossRef]
- Jacobson, K.M.; Jacobson, P.J. Rainfall regulates decomposition of buried cellulose in the Namib Desert. J. Arid Environ. 1998, 38, 571–583. [Google Scholar] [CrossRef]
- Logan, J.R.; Jacobson, K.M.; Jacobson, P.J.; Evans, S.E. Fungal communities on standing litter are structured by moisture type and constrain decomposition in a hyper-arid grassland. Front. Microbiol. 2021, 12, 596517. [Google Scholar] [CrossRef] [PubMed]
- Günster, A. Grass cover distribution in the central Namib—A rapid method to assess regional and local rainfall patterns of arid regions? J. Arid Environ. 1995, 29, 107–114. [Google Scholar] [CrossRef]
- Seely, M.K. Grassland productivity: The desert end of the curve. S. Afr. J. Sci. 1978, 74, 295–297. [Google Scholar]
- Henschel, J.R.; Burke, A.; Seely, M. Temporal and spatial variability of grass productivity in the central Namib Desert. Afr. Study Monogr. 2005, 30, 43–56. [Google Scholar]
- Koch, C. Some aspects of abundant life in the vegetationless sand of the Namib Desert dunes positive psammotropism in tenebrionid-beetles. J. SWA Sci. Soc. 1961, 15, 9–34. [Google Scholar]
- Holm, E.; Scholtz, C.H. Structure and pattern of the Namib Desert dune ecosystem at Gobabeb. Madoqua 1980, 12, 3–39. [Google Scholar]
- Polis, G.A.; Hurd, S.D. Linking marine and terrestrial food webs: Allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am. Nat. 1996, 147, 396–423. [Google Scholar] [CrossRef]
- Koch, C. The Tenebrionidae of southern Africa XXXI. comprehensive notes on the tenebrionid fauna of the Namib Desert. Ann. Transvaal Mus. 1962, 24, 61–106. [Google Scholar]
- Hanrahan, S.A.; Seely, M.K. Food and habitat use by three tenebrionid beetles (Coleoptera) in a riparian desert environment. In Namib Ecology 25 Years of Namib Research, 1st ed.; Seely, M.K., Ed.; Transvaal Museum Monograph: Pretoria, South Africa, 1990; Volume 7, pp. 143–147. [Google Scholar]
- Coaton, W.G.H.; Sheasby, J.L. Preliminary Report on a Survey of the Termites (Isoptera) of South West Africa. Cimbebasia: Memoir; State Museum: Windhoek, Namibia, 1972; Volume 2, pp. 7–129. [Google Scholar]
- Watson, R.T.; Irish, J. An introduction to the Lepismatidae (Thysanura: Insecta) of the Namib Desert sand dunes. Madoqua 1988, 15, 285–293. [Google Scholar]
- Collins, G.E.; Hogg, I.D.; Baxter, J.R.; Maggs-Kolling, G.; Cowan, D.A. Ancient landscapes of the Namib Desert harbor high levels of genetic variability and deeply divergent lineages for Collembola. Ecol. Evol. 2019, 9, 4969–4979. [Google Scholar] [CrossRef] [PubMed]
- Prendini, L. Substratum specialization and speciation in southern African scorpions: The Effect Hypothesis revisited. In Scorpions 2001: In Memoriam Gary A. Polis; Fet, V., Selden, P.A., Eds.; British Arachnological Society: Plymouth, UK, 2001; pp. 113–138. [Google Scholar]
- Polis, G.A. Food webs in desert communities: Complexity via diversity and omnivory. In The Ecology of Desert Communities; Polis, G.A., Ed.; University of Arizona Press: Tucson, AZ, USA, 1991; pp. 383–429. [Google Scholar]
- Ayal, Y.; Polis, G.A.; Lubin, Y.; Goldberg, D.E. How can high animal diversity be supported in low-productivity deserts? The role of macrodetritivory and habitat physiognomy. In Biodiversity in Drylands—Towards a Unified Framework; Shachak, M., Gosz, J.R., Pickett, S.T.A., Perevolotsky, A., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 15–29. [Google Scholar]
- Seely, M.K. The Namib’s place among the deserts of the world. S. Afr. J. Sci. 1984, 80, 155–158. [Google Scholar]
- Henschel, J.R.; Seely, M.K. Ecophysiology of atmospheric moisture in the Namib Desert. Atmos. Res. 2008, 87, 362–368. [Google Scholar] [CrossRef]
- Kaseke, K.F.; Wang, L.; Seely, M.K. Nonrainfall water origins and formation mechanisms. Sci. Adv. 2017, 3, e1603131. [Google Scholar] [CrossRef]
- Mitchell, D.; Henschel, J.R.; Hetem, R.S.; Wassenaar, T.D.; Strauss, W.M.; Hanrahan, S.A.; Seely, M.K. Fog and fauna of the Namib Desert: Past and future. Ecosphere 2020, 11, 1–40. [Google Scholar] [CrossRef]
- Tschinkel, W.R. The sorption of water vapor by windborne plant debris in the Namib Desert. Madoqua Ser. II 1973, 2, 21–24. [Google Scholar]
- Evans, S.; Todd-Brown, K.E.O.; Jacobson, K.; Jacobson, P. Non-rainfall moisture: A key driver of microbial respiration from standing litter in arid, semiarid, and mesic grasslands. Ecosystems 2019, 23, 1154–1169. [Google Scholar] [CrossRef]
- Singh, B.K.; Liu, H.; Trivedi, P. Eco-holobiont: A new concept to identify drivers of host-associated microorganisms. Environ. Microbiol. 2020, 22, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Besler, H.; Gut, S. Untersuchungen zum Feuchtegehalt in Dünen am Beispiel der Namib/Namibia. Köln. Geogr. Arb. Sonderfolge Afr. 1997, 13, 39–47. [Google Scholar]
- Li, B.; Wang, L.; Kaseke, K.F.; Li, L.; Seely, M.K. The impact of rainfall on soil moisture dynamics in a foggy desert. PLoS ONE 2016, 11, e0164982. [Google Scholar] [CrossRef]
- Seely, M.K. Factors controlling reproduction of certain Namib Desert tenebrionids. Madoqua Ser. II 1973, 2, 63–65. [Google Scholar]
- De Villiers, P.S. Aspects of the Reproductive Behaviour of the Namib Tenebrionid Beetle, Onymacris Unguicularis (Haag). Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 1984. [Google Scholar]
- Heg, D.; Rasa, O.A.E. Effects of parental body condition and size on reproductive success in a tenebrionid beetle with biparental care. Ecol. Entomol. 2004, 29, 410–419. [Google Scholar] [CrossRef]
- Rössl, R. Faktoren, die das Eiablageverhalten und die Ovulation Wüsten bewohnender Tenebrioniden (Coleoptera: Insecta) beeinflussen. Mitt. Der Dtsch. Ges. Angew. Entomol. 2006, 15, 421–423. [Google Scholar]
- Seely, M.K.; Henschel, J.R.; Hamilton, W.J.I. Long-term data show behavioural fog collection adaptations determine Namib Desert beetle abundance. S. Afr. J. Sci. 2005, 101, 570–572. [Google Scholar]
- Polis, G.A.; Barnes, J.D.; Seely, M.K.; Henschel, J.R.; Enders, M.M. Predation as a major cost of reproduction in Namib Desert tenebrionid beetles. Ecology 1998, 79, 2560–2566. [Google Scholar] [CrossRef]
- Wharton, R.A. Dispersal, diel periodicity, and longevity of Stips stali (Haag) (Coleoptera: Tenebrionidae). Coleopt. Bull. 1983, 37, 27–33. [Google Scholar]
- Rössl, R. Reproduktionsbiologie, Entwicklungsbiologie und Ökologie von Sympatrischen Tenebrionidae (Coleoptera: Insecta) des Kuiseb bei Gobabeb in der Namib Wüste/Namibia. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-Universität, Bonn, Germany, 2000. [Google Scholar]
- Hinds, W.T.; Rickard, W.H. Correlations between climatological fluctuations and a population of Philolithus densicollis (Horn) (Coleoptera: Tenebrionidae). J. Anim. Ecol. 1973, 42, 341–351. [Google Scholar] [CrossRef]
- Rogers, L.E.; Rickard, W.H. A survey of darkling beetles in desert steppe vegetation after a decade. Ann. Entomol. Soc. Am. 1975, 68, 1069–1070. [Google Scholar] [CrossRef]
- Thomas, D.B.J. Patterns in the abundance of some tenebrionid beetles in the Mojave Desert. Environ. Entomol. 1979, 8, 568–574. [Google Scholar] [CrossRef]
- Barrows, C.W. Temporal patterns of abundance of arthropods on sand dunes. Southwest. Nat. 2012, 57, 262–266. [Google Scholar] [CrossRef]
- Seely, M.K. Effective use of the desert dune environment as illustrated by the Namib tenebrionids. In Proceedings of the VIII. Intl. Colloquium of Soil Zoology, Louvain-la-Neuve, Belgium, 30 August–2 September 1982; pp. 357–368. [Google Scholar]
- Rasa, O.A.E. Behavioural adaptations to moisture as an environmental constraint in a nocturnal burrow-inhabiting Kalahari detritivore Parastizopus armaticeps Peringuey (Coleoptera: Tenebrionidae). Koedoe 1994, 37, 57–66. [Google Scholar] [CrossRef]
- Henschel, J.R.; Mtuleni, V.; Pallett, J.; Seely, M.K. The surface-dwelling arthropod fauna of Gobabeb with a description of the long-term pitfall trapping project. J. Namib. Sci. Soc. 2003, 51, 65–92. [Google Scholar]
- Lancaster, J.; Lancaster, N.; Seely, M.K. Climate of the central Namib Desert. Madoqua 1984, 14, 5–61. [Google Scholar]
- Schemenauer, R.S.; Cereceda, P. A proposed standard fog collector for use in high-elevation regions. J. Appl. Meteorol. 1994, 33, 1313–1322. [Google Scholar] [CrossRef]
- Juvik, J.O.; Nullet, D. Comments on “A Proposed Standard Fog Collector for Use in High-Elevation Regions”. J. Appl. Meteorol. 1995, 34, 2108–2110. [Google Scholar] [CrossRef]
- Throop, H.L.; Seely, M.K.; Marufu, V.J. Multiple scales of spatial heterogeneity control soil respiration responses to precipitation across a dryland rainfall gradient. Plant Soil 2020, 453, 423–443. [Google Scholar] [CrossRef]
- Wharton, R.A.; Seely, M.K. Species composition of and biological notes on Tenebrionidae of the lower Kuiseb River and adjacent gravel plain. Madoqua 1982, 13, 5–25. [Google Scholar]
- Penrith, M.-L. The Zophosini (Coleoptera: Tenebrionidae) of western southern Africa. Cimbebasia Mem. 1977, 3, 1–291. [Google Scholar]
- Penrith, M.-L. Revision of the Western Southern African Adesmiini (Coleoptera: Tenebrionidae). Cimbebasia A 1979, 5, 1–94. [Google Scholar]
- Robinson, M.D.; Seely, M.K. Physical and biotic environments of the southern Namib dune ecosystem. J. Arid Environ. 1980, 3, 183–203. [Google Scholar] [CrossRef]
- Penrith, M.-L.; Endrödy-Younga, S. Revision of the Subtribe Cryptochilina, 1st ed.; Transvaal Museum Monograph: Pretoria, South Africa, 1994; Volume 9, pp. 1–144. [Google Scholar]
- Gobabeb Namib Research Institute. Gravel plain pitfall trapping data (Namib Desert) 1976 to 2020. In SASSCAL Southern African Science Service Centre for Climate Change and Adaptive Land Management; 2021; Available online: http://data.sasscal.org/metadata/view.php?view=ts_timeseries&id=7235&ident=416501860743123172 (accessed on 6 September 2021).
- Wolda, H. Fluctuations in abundance of tropical insects. Am. Nat. 1978, 112, 1017–1045. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Henschel, J.R.; Seely, M.K. Mass-length relationships of Namib tenebrionids. Madoqua 1997, 19, 159–160. [Google Scholar]
- Rickard, W.H.; Haverfield, L.E. A pitfall trapping survey of darkling beetles in desert steppe vegetation. Ecology 1965, 46, 873–875. [Google Scholar] [CrossRef]
- Wise, D.H. Seasonal and yearly patterns in the density of darkling beetles (Coleoptera: Tenebrionidae) in a Montane Community. Environ. Entomol. 1981, 10, 350–358. [Google Scholar] [CrossRef]
- Faragalla, A.A.; Adam, E.E. Pitfall trapping of tenebrionid and carabid beetles (Coleoptera) in different habitats of the central region of Saudi Arabia. Z. Angew. Entomol. 1985, 99, 466–471. [Google Scholar] [CrossRef]
- Stafford Smith, D.M.; Morton, S.R. A framework for the ecology of arid Australia. J. Arid Environ. 1990, 18, 255–278. [Google Scholar] [CrossRef]
- Ayal, Y.; Merkl, O. Spatial and temporal distribution of tenebrionid species (Coleoptera) in the Negev Highlands. J. Arid Environ. 1994, 27, 347–362. [Google Scholar] [CrossRef]
- Deslippe, R.J.; Salazar, J.R.; Guo, Y.-J. A darkling beetle population in West Texas during the 1997-1998 El Niño. J. Arid Environ 2001, 49, 711–721. [Google Scholar] [CrossRef]
- Gibb, H.; Grossman, B.F.; Dickman, C.R.; Decker, O.; Wardle, G.M. Long-term responses of desert ant assemblages to climate. J. Anim. Ecol. 2019, 88, 1549–1563. [Google Scholar] [CrossRef]
- Kwok, A.B.C.; Wardle, G.M.; Greenville, A.C.; Dickman, C.R. Long-term patterns of invertebrate abundance and relationships to environmental factors in arid Australia. Austral Ecol. 2016, 41, 480–491. [Google Scholar] [CrossRef]
- Walter, H. Die ökologischen Verhältnisse in der Nebelwüste Namib (Deutsch-Südwestafrika). Ber. Dtsch. Bot. Ges. 1936, 54, 39–44. [Google Scholar]
- Seely, M.K. Is there anything special about the Namib Desert? S. Afr. J. Sci. 1989, 85, 215. [Google Scholar]
- Southgate, R.I.; Masters, P.; Seely, M.K. Precipitation and biomass changes in the Namib Desert dune ecosystem. J. Arid Environ. 1996, 33, 267–280. [Google Scholar] [CrossRef]
- Hachfeld, B. Rain, fog and species richness in the Central Namib Desert in the exceptional rainy season of 1999/2000. Dinteria 2000, 26, 113–146. [Google Scholar]
- Aushiku, N.N.L.; Buschbeck, L.; Christian, A.; Handjaba, M.; Haraes, L.; Ithindi, J.; Johannes, K.; Kandjai, A.; Ndjomba, E.; Chicoine, T.; et al. Changes in rainfall and biomass in the Namib Sand Sea dune ecosystem over 52 years. J. Namib. Sci. Soc. 2015, 63, 219–237. [Google Scholar]
- Henschel, J.R.; Wassenaar, T.D.; Kanandjembo, A.; Kilbourn-Louw, M.; Neef, G.; Shuuya, T.; Soderberg, K. Roots point to water sources of Welwitschia mirabilis in a hyperarid desert. Ecohydrology 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Jacobson, K.M. Moisture and substrate stability determine VA-mycorrhizal fungal community distribution and structure in an arid grassland. J. Arid Environ. 1997, 35, 59–75. [Google Scholar] [CrossRef]
- Rasmussen, J.L.; Seely, M.K.; Pietruszka, R.D. The reproductive behavior of six species of Namib Desert tenebrionid beetles (Coleoptera: Tenebrionidae). J. Insect Behav. 1991, 4, 567–582. [Google Scholar] [CrossRef]
- Milton, S.J. Spatial and temporal patterns in the emergence and survival of seedlings in arid Karoo shrubland. J. Appl. Ecol. 1995, 32, 145–156. [Google Scholar] [CrossRef]
- Schwinning, S.; Sala, O.E.; Loik, M.E.; Ehleringer, J.R. Thresholds, memory, and seasonality: Understanding pulse dynamics in arid/semi-arid ecosystems. Oecologia 2004, 141, 191–193. [Google Scholar] [CrossRef]
- Craven, P. Phytogeographic Study of the Kaokoveld Centre of Endemism; Stellenbosch University: Stellenbosch, South Africa, 2009. [Google Scholar]
- Reynolds, J.F.; Kemp, P.R.; Ogle, K.; Fernandez, R.J. Modifying the ‘pulse-reserve’ paradigm for deserts of North America: Precipitation pulses, soil water, and plant responses. Oecologia 2004, 141, 194–210. [Google Scholar] [CrossRef]
- Barrows, C.W. Tenebrionid species richness and distribution in the Coachella Valley sand dunes (Coleoptera: Tenebrionidae). Southwest. Nat. 2000, 45, 306–312. [Google Scholar] [CrossRef]
- Hamilton, W.J.I.; Seely, M.K. Fog basking by the Namib Desert beetle, Onymacris unguicularis. Nature 1976, 262, 284–285. [Google Scholar] [CrossRef]
- Seely, M.K.; Hamilton, W.J.I. Fog catchment sand trenches constructed by tenebrionid beetles, Lepidochora, from the Namib Desert. Science 1976, 193, 484–486. [Google Scholar] [CrossRef]
- Seely, M.K.; Lewis, C.J.; O’Brien, K.A.; Suttle, A.E. Fog response of tenebrionid beetles in the Namib Desert. J. Arid Environ. 1983, 6, 135–143. [Google Scholar] [CrossRef]
- Seely, M.K.; de Vos, M.P.; Louw, G.N. Fog imbibition, satellite fauna and unusual leaf structure in a Namib Desert dune plant Trianthema hereroensis. S. Afr. J. Sci. 1977, 73, 169–172. [Google Scholar]
- Louw, G.N.; Seely, M.K. Exploitation of fog water by a perennial Namib dune grass, Stipagrostis sabulicola. S. Afr. J. Sci. 1980, 76, 38–39. [Google Scholar]
- Nott, K. An Autecological Study of a Namib Dune Succulent, Trianthema hereroensis. Master’s Thesis, University of Natal, Pietermaritzburg, South Africa, 1985. [Google Scholar]
- Ebner, M.; Miranda, T.; Roth-Nebelsick, A. Efficient fog harvesting by Stipagrostis sabulicola (Namib dune bushman grass). J. Arid Environ. 2011, 75, 524–531. [Google Scholar] [CrossRef]
- Gottlieb, T.R.; Eckardt, F.D.; Venter, Z.S.; Cramer, M.D. The contribution of fog to water and nutrient supply to Arthraerua leubnitziae in the central Namib Desert, Namibia. J. Arid Environ. 2019, 161, 35–46. [Google Scholar] [CrossRef]
- Wessels, D.C.J.; Wessels, L.A.; Holzapfel, W.H. Preliminary report on lichen-feeding Coleoptera occurring on Teloschistes capensis in the Namib Desert, South West Africa. Bryologist 1979, 82, 270–273. [Google Scholar] [CrossRef]
- Fattorini, S. Insect rarity, extinction and conservation in urban Rome (Italy): A 120-year-long study of tenebrionid beetles. Insect Conserv. Divers. 2011, 4, 307–315. [Google Scholar] [CrossRef]
- Fattorini, S. Spatial variations in rarity in the Aegean tenebrionid beetles (Coleoptera, Tenebrionidae). Fragm. Entomol. 2006, 38, 227–249. [Google Scholar] [CrossRef][Green Version]
- Didham, R.K.; Hammond, P.M.; Lawton, J.H.; Eggleton, P.; Stork, N.E. Beetle species responses to tropical forest fragmentation. Ecol. Monogr. 1998, 68, 295–323. [Google Scholar] [CrossRef]
- Kaseke, K.F.; Mills, A.J.; Esler, K.; Henschel, J.R.; Seely, M.K.; Brown, R. Spatial variation of “non-rainfall” water input and the effect of mechanical soil crusts on input and evaporation. Pure Appl. Geophys. 2012, 169, 2217–2229. [Google Scholar] [CrossRef]
- Roer, H. Zur Lebensweise des Namibwüstenkäfers Onymacris plana Peringuey (Col., Tenebrionidae, Adesmiini) unter besonderer Berücksichtigung seines Migrationsverhaltens. Bonn. Zoöl. Beitr. 1975, 26, 239–256. [Google Scholar]
- Roer, H. Dispersion dynamics of tenebrionids of the genus Onymacris (Col.: Tenebrionidae, Adesmiini) in the Namib Desert. J. SWA Sci. Soc. 1985, 39, 65–70. [Google Scholar]
- Crawford, C.S.; Hanrahan, S.A.; Seely, M.K. Scale-related habitat use by Physadesmia globosa (Coleoptera: Tenebrionidae) in a riparian desert environment. In Namib Ecology 25 Years of Namib Research, 1st ed.; Seely, M.K., Ed.; Transvaal Museum Monograph: Pretoria, South Africa, 1990; Volume 7, pp. 135–142. [Google Scholar]
- Chesson, P.I.; Warner, R.R. Environmental variability promotes coexistence in lottery competitive systems. Am. Nat. 1981, 117, 923–943. [Google Scholar] [CrossRef]
- Chesson, P.I. Multispecies competition in variable environments. Theor. Popul. Biol. 1994, 45, 227–276. [Google Scholar] [CrossRef]
- Hopper, K.R. Risk-spreading and bet-hedging in insect population biology. Annu. Rev. Entomol. 1999, 44, 535–560. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.J.I.; Buskirk, R.E.; Buskirk, W.H. Social organization of the Namib Desert tenebrionid beetle Onymacris rugatipennis. Can. Entomol. 1976, 108, 305–316. [Google Scholar] [CrossRef]
- Marden, J.H. In pursuit of females: Following and contest behavior by males of a Namib Desert tenebrionid beetle, Physadesmia globosa. Ethology 1987, 75, 15–24. [Google Scholar] [CrossRef]
- Ferguson, J.W.H. Mate following in desert tenebrionid beetles revisited: Why do males spend so much time in courtship? J. Kans. Entomol. Soc. 1992, 65, 125–133. [Google Scholar]
- Enders, M.M. Size-assortative mating in a tenebrionid beetle of the Namib Desert. J. Arid Environ. 1995, 29, 469–484. [Google Scholar] [CrossRef]
- Enders, M.M.; Schüle, H.; Henschel, J.R. Sexual dimorphism, fighting success and mating tactics of male Onymacris plana Péringuey (Coleoptera: Tenebrionidae) in the Namib Desert. Ethology 1998, 104, 1003–1019. [Google Scholar] [CrossRef]
- Penrith, M.L. Revision of the Zophosini (Coleoptera: Tenebrionidae) part 10: Key to the subgenera, supplement, evolution and biogeography of the tribe, and catalogue. Cimbebasia 1986, 6, 417–502. [Google Scholar]
- Endrödy-Younga, S. Dispersion and translocation of dune specialist tenebrionids in the Namib area. Cimbebasia A 1982, 5, 257–271. [Google Scholar]
- Holm, E.; Scholtz, C.H.; Louw, S.M.; Penrith, M.J. Notes on the Coleopterous fauna of the Kalahari. 1. The boundaries of the Kalahari region as derived from the distribution maps of three beetle genera. 2. Comparison of the faunistics and ecological patterns of the ground-living beetle faunas of the Kalahari and Namib Desert. 3. Origin of the sand-adapted tenebrionid beetles of the Kalahari. Koedoe 1984, 1984, 153–165. [Google Scholar]
- Steckel, J.; Penrith, M.L.; Henschel, J.R.; Brandl, R.; Meyer, J. A preliminary molecular phylogeny of the Namib Desert darkling beetles (Tenebrionidae). Afr. Zoöl. 2010, 45, 107–114. [Google Scholar] [CrossRef]
- Lamb, T.; Bond, J.E. A multilocus perspective on phylogenetic relationships in the Namib darkling beetle genus Onymacris (Tenebrionidae). Mol. Phylogenet. Evol. 2013, 66, 757–765. [Google Scholar] [CrossRef]
- Mirtl, M.; Borer, E.T.; Djukic, I.; Forsius, M.; Haubold, H.; Hugo, W.; Jourdan, J.; Lindenmayer, D.; McDowell, W.H.; Muraoka, H.; et al. Genesis, goals and achievements of Long-Term Ecological Research at the global scale: A critical review of ILTER and future directions. Sci. Total Environ. 2018, 626, 1439–1462. [Google Scholar] [CrossRef]
- Henschel, J.R.; Lancaster, N. Gobabeb—50 years of Namib Desert research. J. Arid Environ. 2013, 93, 1–6. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Lister, B.C.; Garcia, A. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. USA 2018, 115, E10397–E10406. [Google Scholar] [CrossRef]
- Henschel, J.R. Locust times—Monitoring populations and outbreak controls in relation to Karoo natural capital. Trans. R. Soc. S. Afr. 2015, 70, 135–143. [Google Scholar] [CrossRef]
- Noy-Meir, I. Structure and function of desert ecosystems. Isr. J. Bot. 1980, 28, 1–19. [Google Scholar]
- Ward, J.D.; Seely, M.K.; Lancaster, N. On the antiquity of the Namib. S. Afr. J. Sci. 1983, 79, 175–183. [Google Scholar]



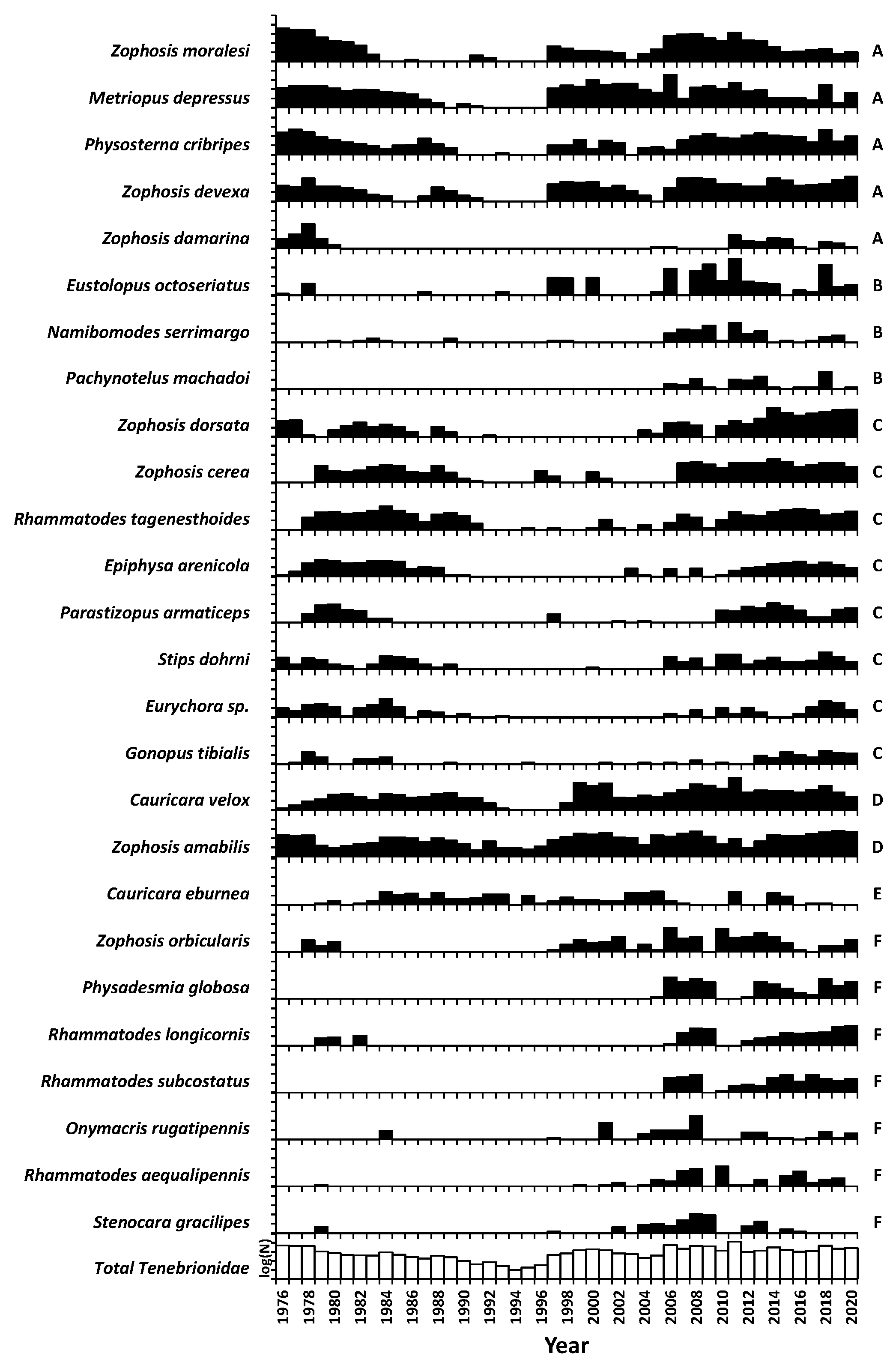
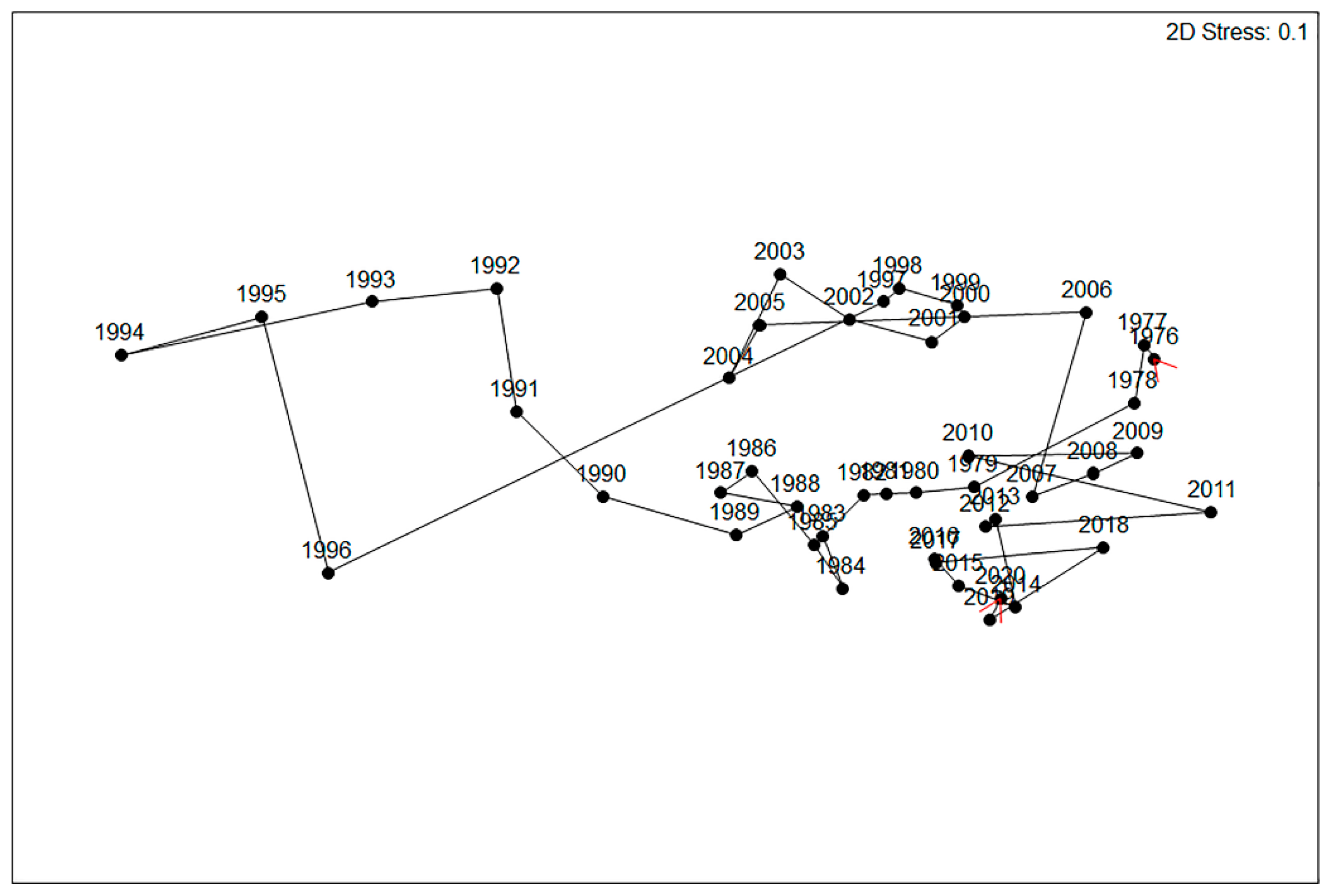

| Gravel Plain Species | Tribe | Group | Capture Rate/1000 Traps | Maximum Capture Rate | Years Recorded | Years Increasing | Years Decreasing | Annual Variability (AV) | Times Irrupted (GC > 1.3) | Rainfall Trigger | Month First Response | Year Peak Response | Duration of Response | Mass (mg) | Diel Activity | Winter Activity (%) | Principal Season |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zophosis (Gyrosis) moralesi (Koch) | Zophosini | A | 57.59 | 753 | 35 | 6 | 16 | 0.516 | 2 | S, H | 1–19 | 1–3 | 3–4 | 33 | D | 30 | S |
| Metriopus depressus (Haag) | Adesmiini | A | 35.41 | 601 | 39 | 13 | 16 | 1.012 | 3 | S, H | 1–10 | 1–2 | 4–10 | 54 | D | 65 | A |
| Zophosis (Gyrosis) devexa Peringuey | Zophosini | A | 13.27 | 83 | 37 | 13 | 16 | 0.525 | 2 | S, H | 1–8 | 1–2 | 3–7 | 12 | D | 38 | A |
| Physosterna cribripes (Haag) | Adesmiini | A | 11.31 | 97 | 38 | 12 | 14 | 0.534 | 3 | S, H | 2–8 | 1–4 | 3–11 | 402 | D | 48 | A |
| Zophosis (Occidentophosis) damarina Peringuey | Zophosini | A | 2.36 | 76 | 16 | 3 | 2 | 1.719 | 1 | H | 1–24 | 1 | 1 | 32 | D | 60 | A |
| Eustolopus octoseriatus Gebien | Adesmiini | B | 44.04 | 1445 | 21 | 8 | 9 | 4.979 | 7 | S, H | 1–4 | 1 | 1–2 | 105 | D | 94 | W |
| Namibomodes serrimargo (Gebien) | Molurini | B | 0.86 | 22 | 19 | 3 | 3 | 2.004 | 1 | H | 2–3 | 1 | 3–4 | 33 | N | 91 | W |
| Pachynotelus machadoi Koch | Cryptochilini | B | 0.57 | 12 | 12 | 4 | 3 | 1.994 | 2 | H | 3–4 | 1 | 1–2 | 71 | C | 86 | W |
| Zophosis (Z.) dorsata Peringuey | Zophosini | C | 18.85 | 266 | 29 | 15 | 13 | 0.641 | 2 | H | 1–7 | 1–2 | 3–4 | 56 | D | 23 | S |
| Zophosis (Occidentophosis) cerea Peringuey | Zophosini | C | 8.58 | 65 | 31 | 12 | 13 | 0.713 | 4 | W | 6–18 | 2–3 | 3–5 | 27 | D | 32 | S |
| Rhammatodes tagenesthoides Koch | Tentyriini | C | 8.31 | 59 | 35 | 16 | 10 | 0.422 | 1 | W | 3–13 | 3 | 3–5 | 3 | N | 48 | A |
| Epiphysa arenicola Penrith | Adesmiini | C | 2.51 | 11 | 30 | 6 | 7 | 0.182 | 0 | H | 23–24 | 4–5 | 10–15 | 384 | C | 30 | S |
| Parastizopus armaticeps (Peringuey) | Opatrini | C | 2.29 | 21 | 21 | 7 | 6 | 0.583 | 1 | H | 3–29 | 3–4 | 7–8 | 160 | N | 34 | A |
| Stips dohrni (Haag) | Eurychorini | C | 1.45 | 12 | 29 | 6 | 7 | 0.851 | 2 | H | 1–6 | 1–2 | 2–5 | 47 | N | 72 | W |
| Eurychora sp. A | Eurychorini | C | 1.50 | 17 | 27 | 5 | 6 | 0.741 | 1 | H | 1–17 | 2–7 | 3–10 | 70 | C | 60 | A |
| Gonopus tibialis Fabricius | Platynotini | C | 0.62 | 5 | 21 | 3 | 3 | 0.455 | 0 | H | 24–25 | 3 | 7–8 | 321 | N | 63 | A |
| Cauricara velox (Peringuey) | Adesmiini | D | 30.20 | 538 | 41 | 14 | 16 | 0.497 | 1 | W, H, L | 1–20 | 1–3 | 3–5 | 35 | D | 96 | W |
| Zophosis (Calosis) amabilis (Deyrolle) | Zophosini | D | 27.51 | 109 | 45 | 18 | 18 | 0.288 | 0 | W, L | 1–10 | 1–3 | 3–4 | 36 | D | 24 | S |
| Cauricara eburnea (Pascoe) | Adesmiini | E | 1.13 | 5 | 32 | 7 | 7 | 1.274 | 3 | W, L | 6–18 | 1–2 | 1–4 | 44 | D | 57 | A |
| Zophosis (Gyrosis) orbicularis Deyrolle | Zophosini | F | 3.85 | 67 | 25 | 8 | 8 | 1.879 | 3 | S, H | 11–24 | 1–3 | 3–4 | 62 | D | 39 | A |
| Physadesmia globosa (Haag) | Adesmiini | F | 3.20 | 35 | 14 | 5 | 6 | 1.586 | 3 | ? | 236 | D | 67 | W | |||
| Rhammatodes longicornis Haag | Tentyriini | F | 1.71 | 24 | 16 | 6 | 2 | 1.457 | 1 | ? | N | 65 | A | ||||
| Rhammatodes subcostatus Koch | Tentyriini | F | 1.98 | 61 | 14 | 7 | 4 | 1.049 | 1 | ? | 7 | N | 47 | A | |||
| Onymacris rugatipennis (Haag) | Adesmiini | F | 1.93 | 61 | 16 | 2 | 2 | 2 | ? | 235 | D | 74 | W | ||||
| Rhammatodes aequalipennis Peringuey | Tentyriini | F | 1.04 | 27 | 18 | 5 | 3 | 2.830 | 2 | ? | N | 58 | A | ||||
| Stenocara gracilipes Solier | Adesmiini | F | 0.87 | 22 | 13 | 3 | 3 | 1.561 | 0 | ? | 110 | D | 70 | W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henschel, J.R. Long-Term Population Dynamics of Namib Desert Tenebrionid Beetles Reveal Complex Relationships to Pulse-Reserve Conditions. Insects 2021, 12, 804. https://doi.org/10.3390/insects12090804
Henschel JR. Long-Term Population Dynamics of Namib Desert Tenebrionid Beetles Reveal Complex Relationships to Pulse-Reserve Conditions. Insects. 2021; 12(9):804. https://doi.org/10.3390/insects12090804
Chicago/Turabian StyleHenschel, Joh R. 2021. "Long-Term Population Dynamics of Namib Desert Tenebrionid Beetles Reveal Complex Relationships to Pulse-Reserve Conditions" Insects 12, no. 9: 804. https://doi.org/10.3390/insects12090804
APA StyleHenschel, J. R. (2021). Long-Term Population Dynamics of Namib Desert Tenebrionid Beetles Reveal Complex Relationships to Pulse-Reserve Conditions. Insects, 12(9), 804. https://doi.org/10.3390/insects12090804






