Arthropods as the Engine of Nutrient Cycling in Arid Ecosystems
Abstract
Simple Summary
Abstract
1. Introduction
2. Macro-Arthropods in Deserts
3. Regulation of Plant Litter Removal
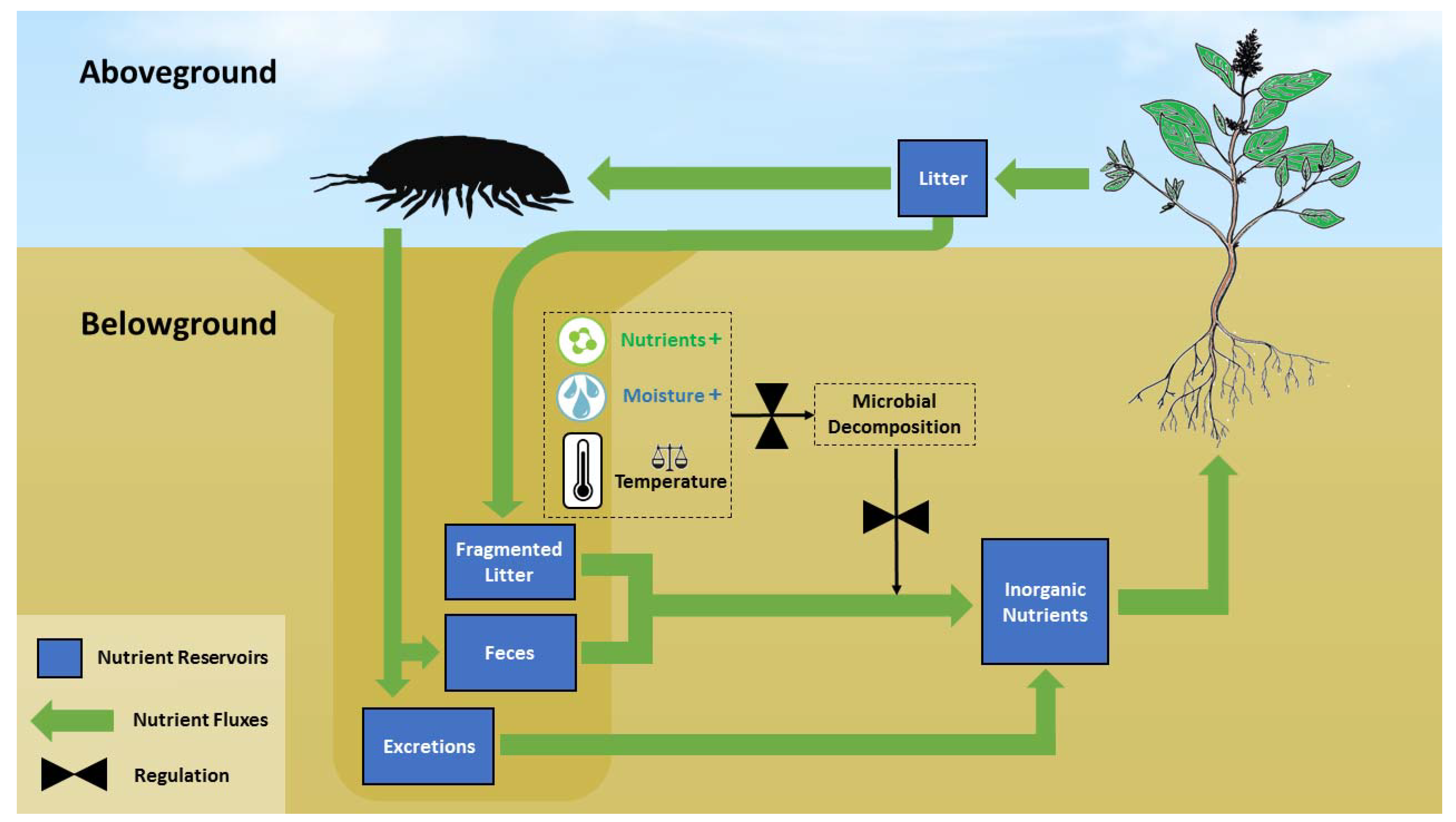
4. Regulation of Plant Litter Decomposition via Ingestion and Fragmentation
5. Regulation of Plant Litter Decomposition via Engineering Effects
6. Desert Arthropods and the Vertical Nutrient Recycling Loop (VRL)
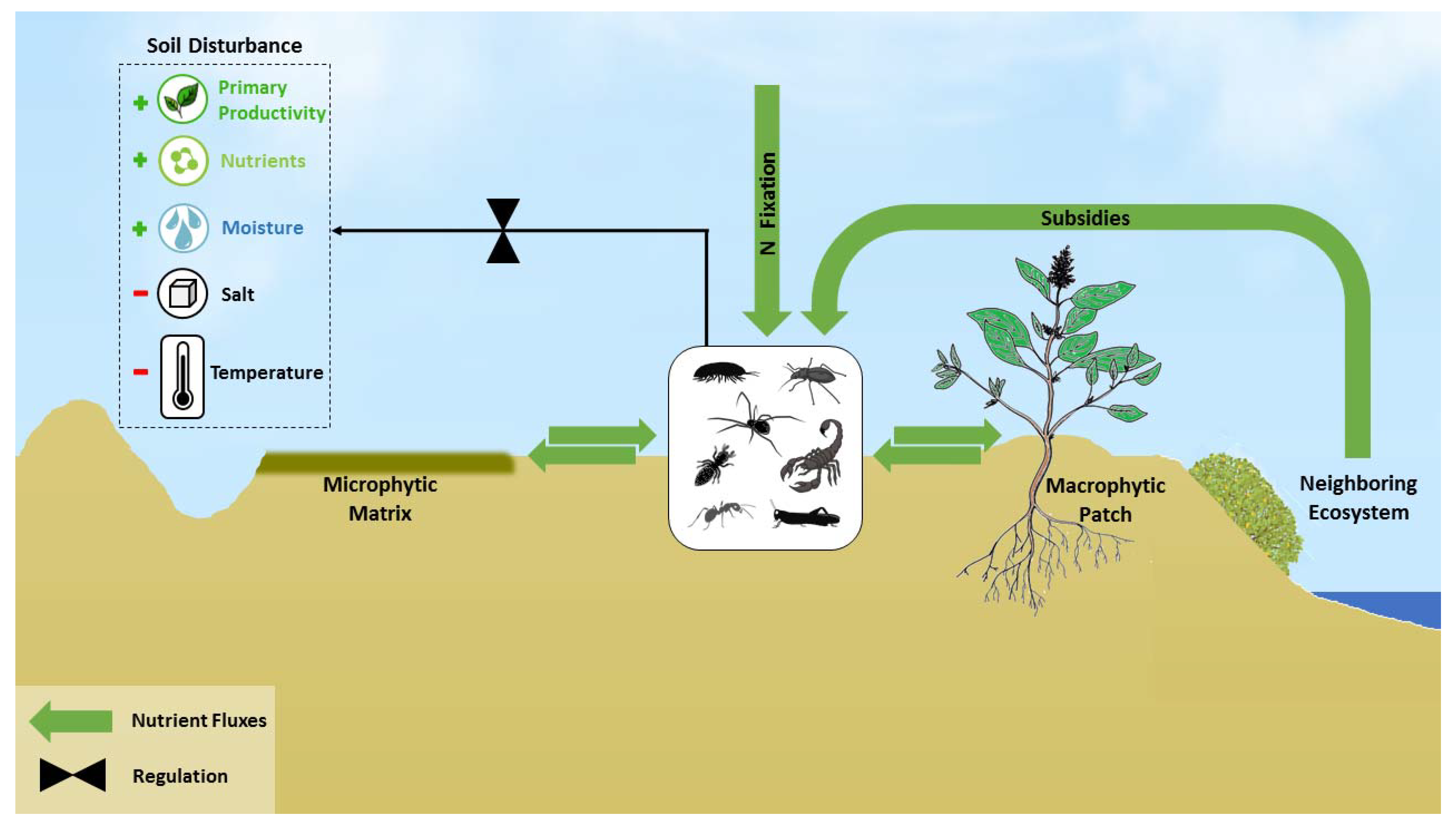
7. Regulation of Nutrient Spatial Distribution within Ecosystems
8. Regulation of Nutrient Spatial Distribution between Ecosystems
9. Soil Desalination via Burrowing Activity
10. Regulation of Primary Production via Soil Disturbances
11. Regulation of Nitrogen Fixation
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Prăvălie, R. Drylands extent and environmental issues. A global approach. Earth-Sci. Rev. 2016, 161, 259–278. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123858740. [Google Scholar]
- Noy-Meir, I. Desert Ecosystems: Environment and Producers. Annu. Rev. Ecol. Syst. 1973, 4, 25–51. [Google Scholar] [CrossRef]
- Noy-Meir, I. Desert ecosystems: Higher trophic levels. Annu. Rev. Ecol. Syst. 1974, 5, 195–214. [Google Scholar] [CrossRef]
- Collins, S.L.; Sinsabaugh, R.L.; Crenshaw, C.; Green, L.; Porras-Alfaro, A.; Stursova, M.; Zeglin, L.H. Pulse dynamics and microbial processes in aridland ecosystems. J. Ecol. 2008, 96, 413–420. [Google Scholar] [CrossRef]
- Austin, A.T. Has water limited our imagination for aridland biogeochemistry. Trends Ecol. Evol. 2011, 26, 229–235. [Google Scholar] [CrossRef]
- Meentemeyer, V. Macroclimate and lignin controls of litter decomposition rates. Ecology 1978, 59, 465–472. [Google Scholar] [CrossRef]
- Whitford, W.G. Exceptions to the AET model: Deserts and clear-cut forest. Ecology 1981, 62, 275–277. [Google Scholar] [CrossRef]
- Fowler, H.G.; Whitford, W.G. Termites, microarthropods and the decomposition of the senescent and fresh creosotebush (Larrea tridentata) leaf litter. J. Arid Environ. 1980, 3, 63–68. [Google Scholar] [CrossRef]
- MacKay, W.P.; Silva, S.I.; Loring, S.J.; Whitford, W.G. The role of subterranean termites in the decomposition of above ground creosotebush litter. Sociobiology 1987, 13, 235–239. [Google Scholar]
- Silva, S.I.; MacKay, W.P.; Whitford, W.G. The relative contributions of termites and microarthropods to fluff grass litter disappearance in the Chihuahuan Desert. Oecologia 1985, 67, 31–34. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Reynolds, J.F. Mechanisms of surface litter mass loss in the northern Chihuahuan Desert: A reinterpretation. J. Arid Environ. 1989, 16, 157–163. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Adair, E.C.; Parton, W.J.; King, J.Y.; Brandt, L.A.; Lin, Y. Accounting for photodegradation dramatically improves prediction of carbon losses in dryland systems. Ecosphere 2017, 8, e01892. [Google Scholar] [CrossRef]
- Evans, S.; Todd-Brown, K.E.O.; Jacobson, K.; Jacobson, P. Non-rainfall moisture: A key driver of microbial respiration from standing litter in arid, semiarid, and mesic Grasslands. Ecosystems 2020, 23, 1154–1169. [Google Scholar] [CrossRef]
- Day, T.A.; Bliss, M.S.; Placek, S.K.; Tomes, A.R.; Guénon, R. Thermal abiotic emission of CO2 and CH4 from leaf litter and its significance in a photodegradation assessment. Ecosphere 2019, 10, e02745. [Google Scholar] [CrossRef]
- Ayal, Y.; Polis, G.A.; Lubin, Y.; Goldberg, D. How can high animal diversity be supported in low-productivity deserts? The role of macrodetritivory and habitat physiognomy. In Biodiversity in Drylands: Towards a Unified Framework; Shachack, M., Gosz, J.R., Perevolotsky, A., Pickett, S.T.A., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 34–45. ISBN 9786610481309. [Google Scholar]
- Ayal, Y. Trophic structure and the role of predation in shaping hot desert communities. J. Arid Environ. 2007, 68, 171–187. [Google Scholar] [CrossRef]
- Ward, D. Morphological, physiological, and behavioural adaptations of desert animals to the abiotic environment. In The Biology of Deserts; Ward, D., Ed.; Oxford University Press: New York, NY, USA, 2009; pp. 66–101. [Google Scholar]
- Whitford, W.G. Adaptations. In Ecology of Desert Systems; Elsevier Science: Burlington, MA, USA, 2002; pp. 123–155. [Google Scholar]
- Raza, M.B.; Bhoi, T.K.; Samal, I. Soil arthropods as a nutrient enhancer. Int. J. Chem. Stud. 2019, 7, 1687–1692. [Google Scholar]
- Mackay, W.P.; Loring, S.J.; Zak, J.C.; Silva, S.I.; Fisher, F.M.; Whitford, W.G. Factors affecting loss in mass of creosotebush leaf-litter on the soil surface in the northern Chihuahuan Desert. Southwest. Nat. 1994, 39, 78–82. [Google Scholar] [CrossRef]
- Whitford, W.G.; Steinberger, Y.; Ettershank, G. Contributions of subterranean termites to the “economy” of Chihuahuan Desert ecosystems. Oecologia 1982, 55, 298–302. [Google Scholar] [CrossRef]
- Megías, A.G.; Sánchez-Piñero, F.; Hódar, J.A. Trophic interactions in an arid ecosystem: From decomposers to top-predators. J. Arid Environ. 2011, 75, 1333–1341. [Google Scholar] [CrossRef]
- Sagi, N.; Grünzweig, J.M.; Hawlena, D. Burrowing detritivores regulate nutrient cycling in a desert ecosystem. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191647. [Google Scholar] [CrossRef]
- García-Palacios, P.; Maestre, F.T.; Kattge, J.; Wall, D.H. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol. Lett. 2013, 16, 1045–1053. [Google Scholar] [CrossRef]
- van Geffen, K.G.; Berg, M.P.; Aerts, R. Potential macro-detritivore range expansion into the subarctic stimulates litter decomposition: A new positive feedback mechanism to climate change? Oecologia 2011, 167, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.I.; Yahdjian, L.; Austin, A.T. Do soil organisms affect aboveground litter decomposition in the semiarid Patagonian steppe, Argentina? Oecologia 2012, 168, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.F.; Whitford, W.G. The effects of microarthropods on litter decomposition in a Chihuahuan Desert ecosystem. Ecology 1981, 62, 654–663. [Google Scholar] [CrossRef]
- Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2018, 332, 161–172. [Google Scholar] [CrossRef]
- Joly, F.X.; Coq, S.; Coulis, M.; Nahmani, J.; Hättenschwiler, S. Litter conversion into detritivore faeces reshuffles the quality control over C and N dynamics during decomposition. Funct. Ecol. 2018, 32, 2605–2614. [Google Scholar] [CrossRef]
- David, J.F. The role of litter-feeding macroarthropods in decomposition processes: A reappraisal of common views. Soil Biol. Biochem. 2014, 76, 109–118. [Google Scholar] [CrossRef]
- Joly, F.X.; Coq, S.; Coulis, M.; David, J.F.; Hättenschwiler, S.; Mueller, C.W.; Prater, I.; Subke, J.A. Detritivore conversion of litter into faeces accelerates organic matter turnover. Commun. Biol. 2020, 3, 660. [Google Scholar] [CrossRef]
- Whitford, W.G. Decomposition and nutrient cycling. In Ecology of Desert Systems; Whitford, W.G., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 236–272. ISBN 9780127472614. [Google Scholar]
- Anderson, J.M. Invertebrate-mediated transport processes in soils. Agric. Ecosyst. Environ. 1988, 24, 5–19. [Google Scholar] [CrossRef]
- Jouquet, P.; Dauber, J.; Lagerlöf, J.; Lavelle, P.; Lepage, M. Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. Appl. Soil Ecol. 2006, 32, 153–164. [Google Scholar] [CrossRef]
- Eldridge, D.J. Effect of ants on sandy soils in semi-arid eastern australia: Local distribution of nest entrances and their effect on infiltration of water. Aust. J. Soil Res. 1993, 31, 509–518. [Google Scholar] [CrossRef]
- Laundre, J.W. Effects of small mammal burrows on water infiltration in a cool desert environment. Oecologia 1993, 94, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.D. Development of texture contrast soils by a combination of bioturbation and translocation. Catena 2007, 70, 92–104. [Google Scholar] [CrossRef]
- Butler, D.R.; Butler, W.D. The geomorphic effects of gophers on soil characteristics and sediment compaction: A case study from alpine treeline, Sangre de Cristo mountains, Colorado, USA. Open Geol. J. 2009, 3, 82–89. [Google Scholar] [CrossRef]
- Coombes, M.A.; Viles, H.A. Population-level zoogeomorphology: The case of the Eurasian badger (Meles meles L.). Phys. Geogr. 2015, 36, 215–238. [Google Scholar] [CrossRef]
- Kinlaw, A. A review of burrowing by semi-fossorial vertebrates in arid environments. J. Arid Environ. 1999, 41, 127–145. [Google Scholar] [CrossRef]
- Whittington-Jones, G.M.; Bernard, R.T.F.; Parker, D.M. Aardvark burrows: A potential resource for animals in arid and semi-arid environments. Afr. Zool. 2011, 46, 362–370. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms, 3rd ed.; Chapman & Hall: London, UK, 1996; ISBN 0412561603. [Google Scholar]
- Gullan, P.J.; Cranston, P.S. The Insects: An Outline of Entomology, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Moore, D.; Stow, A.; Kearney, M.R. Under the weather?—The direct effects of climate warming on a threatened desert lizard are mediated by their activity phase and burrow system. J. Anim. Ecol. 2018, 87, 660–671. [Google Scholar] [CrossRef]
- Sagi, N.; Zaguri, M.; Hawlena, D. Macro-detritivores assist resolving the Dryland Decomposition Conundrum by engineering an underworld heaven for decomposers. Ecosystems 2021, 24, 56–67. [Google Scholar] [CrossRef]
- Groffman, P.M.; Bohlen, P.J.; Fisk, M.C.; Fahey, T.J. Exotic earthworm invasion and microbial biomass in temperate forest soils. Ecosystems 2004, 7, 45–54. [Google Scholar] [CrossRef]
- Nutting, W.L.; Haverty, M.I.; Lafage, J.P. Physical and chemical alteration of soil by two subterranean termite species in Sonoran Desert grassland. J. Arid Environ. 1987, 12, 233–239. [Google Scholar] [CrossRef]
- Evans, T.A.; Dawes, T.Z.; Ward, P.R.; Lo, N. Ants and termites increase crop yield in a dry climate. Nat. Commun. 2011, 2, 262. [Google Scholar] [CrossRef]
- Schaefer, D.A.; Whitford, W.G. Nutrient cycling by the subterranean termite Gnathamitermes tubiformans in a Chihuahuan Desert ecosystem. Oecologia 1981, 48, 277–283. [Google Scholar] [CrossRef]
- Trudgill, S.T. Soil and Vegetation Systems, 2nd ed.; Oxford University Press: New York, NY, USA, 1988. [Google Scholar]
- Stark, J.M. Causes of soil nutrient heterogeneity at different scales. In Exploitation of Environmental Heterogeneity by Plants: Ecophysiological Processes above and below Ground; Caldwell, M.M., Pearcy, R., Eds.; John Wiley & Sons: New York, NY, USA, 1994; pp. 255–284. [Google Scholar]
- Hodge, A. Roots: The acquisition of water and nutrients from the heterogeneous soil environment. In Progress in Botany 71; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 307–337. [Google Scholar]
- Liu, B.; He, J.; Zeng, F.; Lei, J.; Arndt, S.K. Life span and structure of ephemeral root modules of different functional groups from a desert system. New Phytol. 2016, 211, 103–112. [Google Scholar] [CrossRef]
- McCulley, R.L.; Jobbágy, E.G.; Pockman, W.T.; Jackson, R.B. Nutrient uptake as a contributing explanation for deep rooting in arid and semi-arid ecosystems. Oecologia 2004, 141, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Schenk, H.J.; Jackson, R.B. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. The global biogeography of roots. Ecol. Monogr. 2002, 72, 311–328. [Google Scholar] [CrossRef]
- Cameron, E.K.; Cahill, J.F.; Bayne, E.M. Root foraging influences plant growth responses to earthworm foraging. PLoS ONE 2014, 9, e108873. [Google Scholar] [CrossRef]
- Daleo, P.; Iribarne, O. The burrowing crab Neohelice granulata affects the root strategies of the cordgrass Spartina densiflora in SW Atlantic salt marshes. J. Exp. Mar. Biol. Ecol. 2009, 373, 66–71. [Google Scholar] [CrossRef]
- Sasaki, T.; Kakinuma, K.; Yoshihara, Y. Marmot disturbance drives trait variations among five dominant grasses in a Mongolian grassland. Rangel. Ecol. Manag. 2013, 66, 487–491. [Google Scholar] [CrossRef]
- Villarreal, D.; Clark, K.L.; Branch, L.C.; Hierro, J.L.; Machicote, M. Alteration of ecosystem structure by a burrowing herbivore, the plains vizcacha (Lagostomus maximus). J. Mammal. 2008, 89, 700–711. [Google Scholar] [CrossRef]
- Jackson, R.B.; Caldwell, M.M. Geostatistical patterns of soil heterogeneity around individual perennial plants. J. Ecol. 1993, 81, 683. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Raikks, J.A.; Hartley, A.E.; Cross, A.F. On the spatial pattern of soil nutrients in desert ecosystems. Ecology 1996, 77, 364–374. [Google Scholar] [CrossRef]
- Burke, I.C.; Lauenroth, W.K.; Vinton, M.A.; Hook, P.B.; Kelly, R.H.; Epstein, H.E.; Aguiar, M.R.; Robles, M.D.; Aguilera, M.O.; Murphy, K.L.; et al. Plant-soil interactions in temperate grasslands. Biogeochemistry 1998, 42, 121–143. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Eldridge, D.J.; Delgado-Baquerizo, M.; Soliveres, S.; Bowker, M.A.; Gross, N.; Le Bagousse-Pinguet, Y.; Quero, J.L.; García-Gómez, M.; Valencia, E.; et al. Soil fungal abundance and plant functional traits drive fertile island formation in global drylands. J. Ecol. 2018, 106, 242–253. [Google Scholar] [CrossRef]
- Brown, G.; Scherber, C.; Ramos, P.; Ebrahim, E.K. The effects of harvester ant (Messor ebeninus Forel) nests on vegetation and soil properties in a desert dwarf shrub community in north-eastern Arabia. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 503–511. [Google Scholar] [CrossRef]
- Wagner, D.; Jones, J.B. The contribution of harvester ant nests, Pogonomyrmex rugosus (Hymenoptera, Formicidae), to soil nutrient stocks and microbial biomass in the Mojave Desert. Environ. Entomol. 2004, 33, 599–607. [Google Scholar] [CrossRef]
- James, A.I.; Eldridge, D.J.; Koen, T.B.; Whitford, W.G. Landscape position moderates how ant nests affect hydrology and soil chemistry across a Chihuahuan Desert watershed. Landsc. Ecol. 2008, 23, 961–975. [Google Scholar] [CrossRef]
- Baubin, C.; Farrell, A.M.; Šťovíček, A.; Ghazaryan, L.; Giladi, I.; Gillor, O. Seasonal and spatial variability in total and active bacterial communities from desert soil. Pedobiologia 2019, 74, 7–14. [Google Scholar] [CrossRef]
- Jones, J.B.; Wagner, D. Microhabitat-specific controls on soil respiration and denitrification in the Mojave Desert: The role of harvester ant nests and vegetation. West. N. Am. Nat. 2006, 66, 426–433. [Google Scholar] [CrossRef]
- Brown, M.F.; Whitford, W.G. The effects of termites and straw mulch on soil nitrogen in a creosotebush (Larrea tridentata) dominated Chihuahuan Desert ecosystem. J. Arid Environ. 2003, 53, 15–20. [Google Scholar] [CrossRef]
- Li, X.R.; Gao, Y.H.; Su, J.Q.; Jia, R.L.; Zhang, Z.S. Ants mediate soil water in arid desert ecosystems: Mitigating rainfall interception induced by biological soil crusts? Appl. Soil Ecol. 2014, 78, 57–64. [Google Scholar] [CrossRef]
- Henschel, J.R.; Lubin, Y.D. A test of habitat selection at two spatial scales in a sit-and-wait predator: A web spider in the Namib Desert dunes. J. Anim. Ecol. 1997, 66, 401. [Google Scholar] [CrossRef]
- Chen, Y.W.; Li, X.R. Spatio-temporal distribution of nests and influence of ant (Formica cunicularia Lat.) activity on soil property and seed bank after revegetation in the Tengger Desert. Arid Land Res. Manag. 2012, 26, 365–378. [Google Scholar] [CrossRef]
- Catenazzi, A.; Donnelly, M.A. The Ulva connection: Marine algae subsidize terrestrial predators in coastal Peru. Oikos 2007, 116, 75–86. [Google Scholar] [CrossRef]
- Sanzone, D.M.; Meyer, J.L.; Marti, E.; Gardiner, E.P.; Tank, J.L.; Grimm, N.B. Carbon and nitrogen transfer from a desert stream to riparian predators. Oecologia 2003, 134, 238–250. [Google Scholar] [CrossRef]
- Thomas, D.S.G.; Middleton, N.J. Salinization: New perspectives on a major desertification issue. J. Arid Environ. 1993, 24, 95–105. [Google Scholar] [CrossRef]
- Yair, A. Short and long term effects of bioturbation on soil erosion, water resources and soil development in an arid environment. Geomorphology 1995, 13, 87–99. [Google Scholar] [CrossRef]
- Shabanova, N.P.; Lebedeva-Verba, M.P.; Bykov, A.V. Morphological and chemical properties of the meadow-semidesert soil complexes of the Khaki playa (the Caspian Lowland) and the influence of the biogenic factor on them. Eurasian Soil Sci. 2010, 43, 259–268. [Google Scholar] [CrossRef]
- Throop, H.L.; Belnap, J. Connectivity dynamics in dryland litter cycles: Moving decomposition beyond spatial stasis. BioScience 2019, 69, 602–614. [Google Scholar] [CrossRef]
- Beattie, A.J.; Culver, D.C. The nest chemistry of two seed-dispersing ant species. Oecologia 1983, 56, 99–103. [Google Scholar] [CrossRef]
- Elkins, N.Z.; Sabol, G.V.; Ward, T.J.; Whitford, W.G. The influence of subterranean termites on the hydrological characteristics of a Chihuahuan Desert ecosystem. Oecologia 1986, 68, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Gutterman, Y. Spring and summer daily subsurface temperatures in three microhabitats in a flat natural loess area in the Negev Desert, Israel. J. Arid Environ. 1997, 36, 225–235. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Whitford, W.G.; Duval, B.D. Animal disturbances promote shrub maintenance in a desertified grassland. J. Ecol. 2009, 97, 1302–1310. [Google Scholar] [CrossRef]
- Shachak, M.; Brand, S.; Gutterman, Y. Porcupine disturbances and vegetation pattern along a resource gradient in a desert. Oecologia 1991, 88, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Danin, A. Association of Salsola inermis and scorpion burrows in leached soils in the Judean Desert, Israel. Isr. J. Plant Sci. 1994, 42, 37–40. [Google Scholar] [CrossRef]
- Benemann, J.R. Nitrogen fixation in termites. Science 1973, 181, 164–165. [Google Scholar] [CrossRef]
- Breznak, J.A.; Brill, W.J.; Mertins, J.W.; Coppel, H.C. Nitrogen fixation in termites. Nature 1973, 244, 577–580. [Google Scholar] [CrossRef]
- Bar-Shmuel, N.; Behar, A.; Segoli, M. What do we know about biological nitrogen fixation in insects? Evidence and implications for the insect and the ecosystem. Insect Sci. 2020, 27, 392–403. [Google Scholar] [CrossRef]
- Nardi, J.B.; Mackie, R.I.; Dawson, J.O. Could microbial symbionts of arthropod guts contribute significantly to nitrogen fixation in terrestrial ecosystems? J. Insect Physiol. 2002, 48, 751–763. [Google Scholar] [CrossRef]
- Deng, M.; Liu, L.; Jiang, L.; Liu, W.; Wang, X.; Li, S.; Yang, S.; Wang, B. Ecosystem scale trade-off in nitrogen acquisition pathways. Nat. Ecol. Evol. 2018, 2, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Bar-Shmuel, N.; Shavit, R.; Behar, A.; Segoli, M. Gut bacteria of weevils developing on plant roots under extreme desert conditions. BMC Microbiol. 2019, 19, 311. [Google Scholar] [CrossRef]
- Cook, G.D.; Dawes-Gromadzki, T.Z. Stable isotope signatures and landscape functioning in banded vegetation in arid-central Australia. Landsc. Ecol. 2005, 20, 649–660. [Google Scholar] [CrossRef]
- Barnes, C.J.; Jacobson, G.; Smith, G.D. The origin of high-nitrate ground waters in the Australian arid zone. J. Hydrol. 1992, 137, 181–197. [Google Scholar] [CrossRef]
- Bar-Shmuel, N.; Rogovin, E.; Rachmilevitch, S.; Friedman, A.L.L.; Shelef, O.; Hoffmann, I.; Rosenberg, T.; Behar, A.; Shavit, R.; Meng, F.; et al. Tripartite symbiosis of plant-weevil-bacteria is a widespread phenomenon in the Negev Desert. Sci. Rep. 2018, 8, 2420. [Google Scholar] [CrossRef]
- Shelef, O.; Helman, Y.; Friedman, A.L.L.; Behar, A.; Rachmilevitch, S. Tri-party underground symbiosis between a weevil, bacteria and a desert plant. PLoS ONE 2013, 8, e76588. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagi, N.; Hawlena, D. Arthropods as the Engine of Nutrient Cycling in Arid Ecosystems. Insects 2021, 12, 726. https://doi.org/10.3390/insects12080726
Sagi N, Hawlena D. Arthropods as the Engine of Nutrient Cycling in Arid Ecosystems. Insects. 2021; 12(8):726. https://doi.org/10.3390/insects12080726
Chicago/Turabian StyleSagi, Nevo, and Dror Hawlena. 2021. "Arthropods as the Engine of Nutrient Cycling in Arid Ecosystems" Insects 12, no. 8: 726. https://doi.org/10.3390/insects12080726
APA StyleSagi, N., & Hawlena, D. (2021). Arthropods as the Engine of Nutrient Cycling in Arid Ecosystems. Insects, 12(8), 726. https://doi.org/10.3390/insects12080726






