Olfactory Learning Supports an Adaptive Sugar-Aversion Gustatory Phenotype in the German Cockroach
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
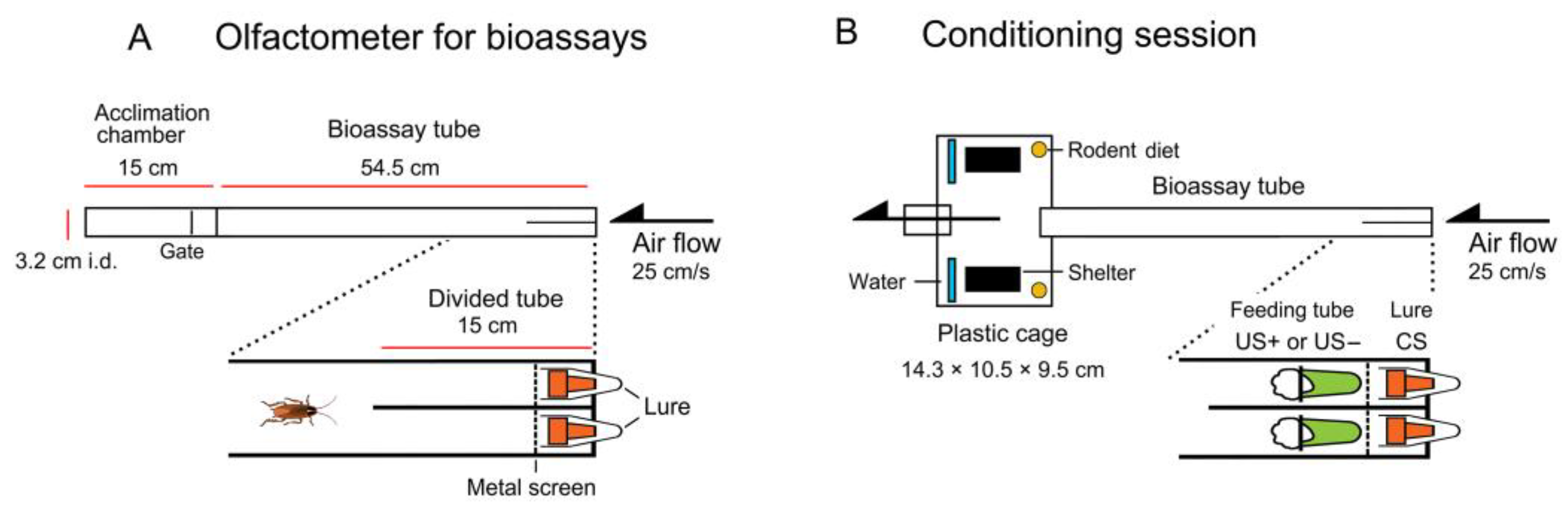
2.2. Olfactometers
2.3. Lures Containing Odors
2.4. Feeding Tubes Containing Tastants
2.5. Chemicals
2.6. Conditioning
2.7. Bioassay Procedures
2.8. Bioassay 1: Unconditioned Odor Preference
2.9. Bioassay 2: Conditioned Odor Preference after Conditioning with a Single Odor
2.10. Bioassay 3: Conditioned Odor Preference after Conditioning with Two Odors
2.11. Bioassay 4: Retention of Olfactory Memory
2.12. Data Analysis
3. Results
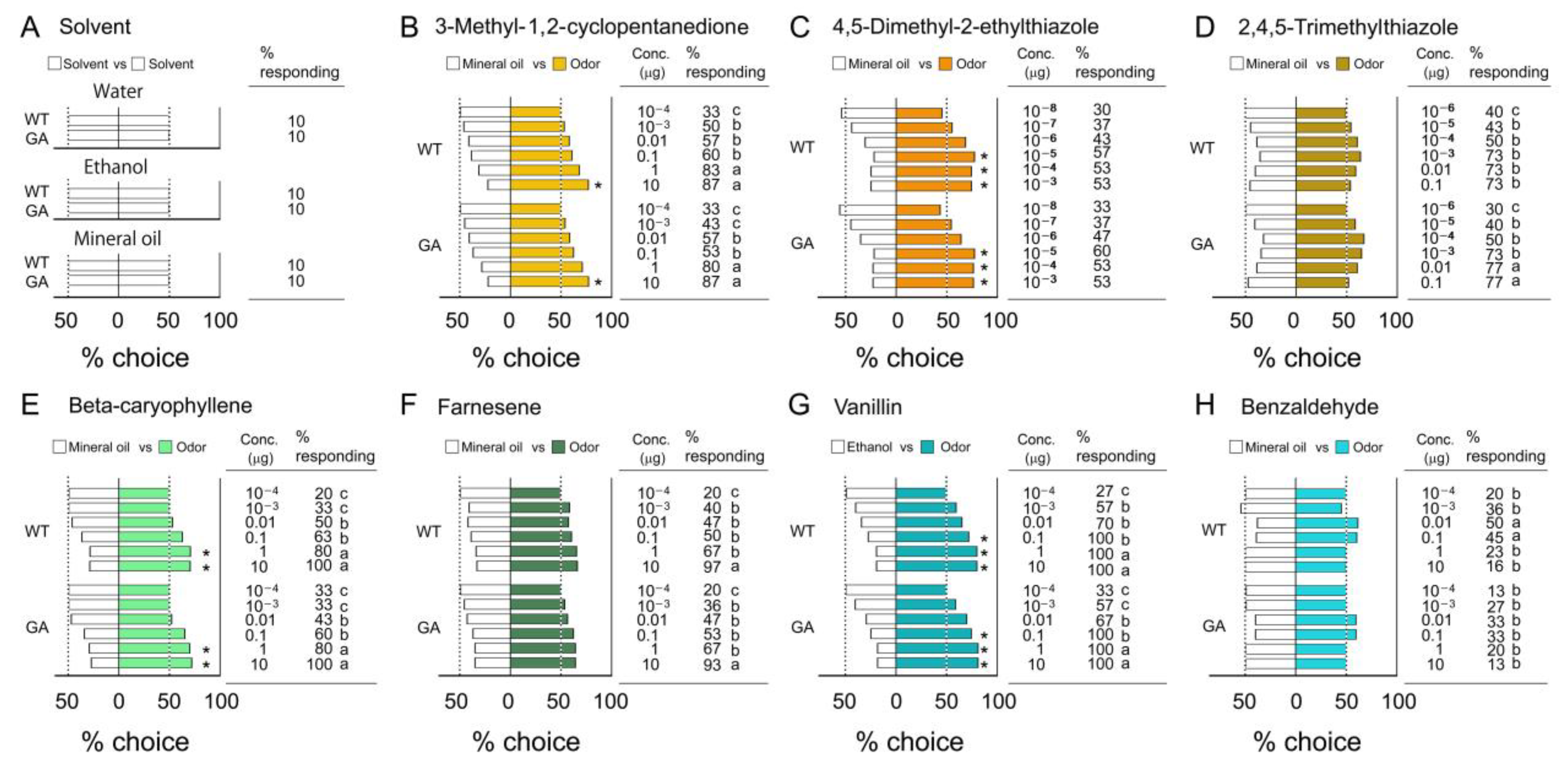
3.1. Innate Odor Preferences
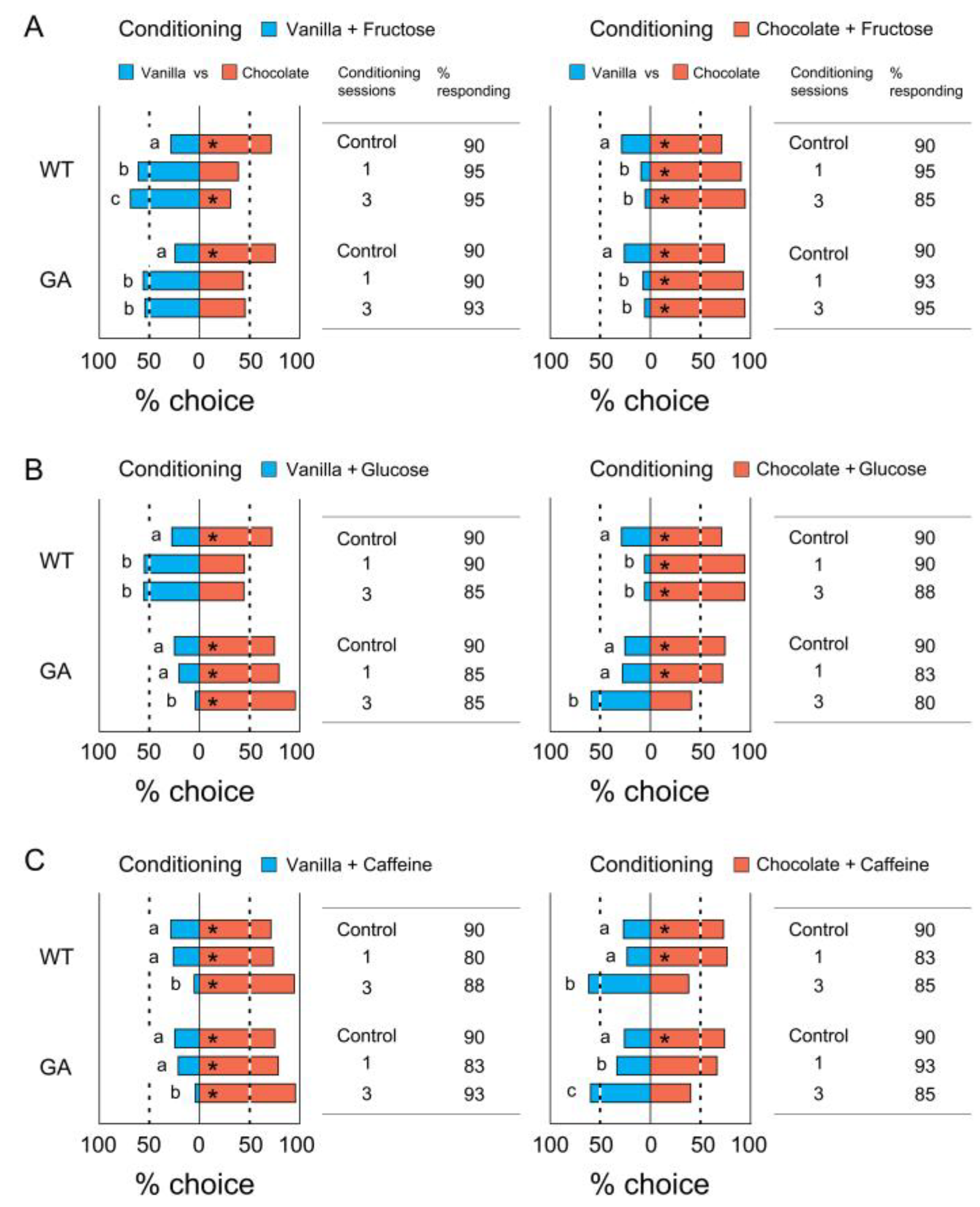
3.2. Association of a Single Odor with Tastants
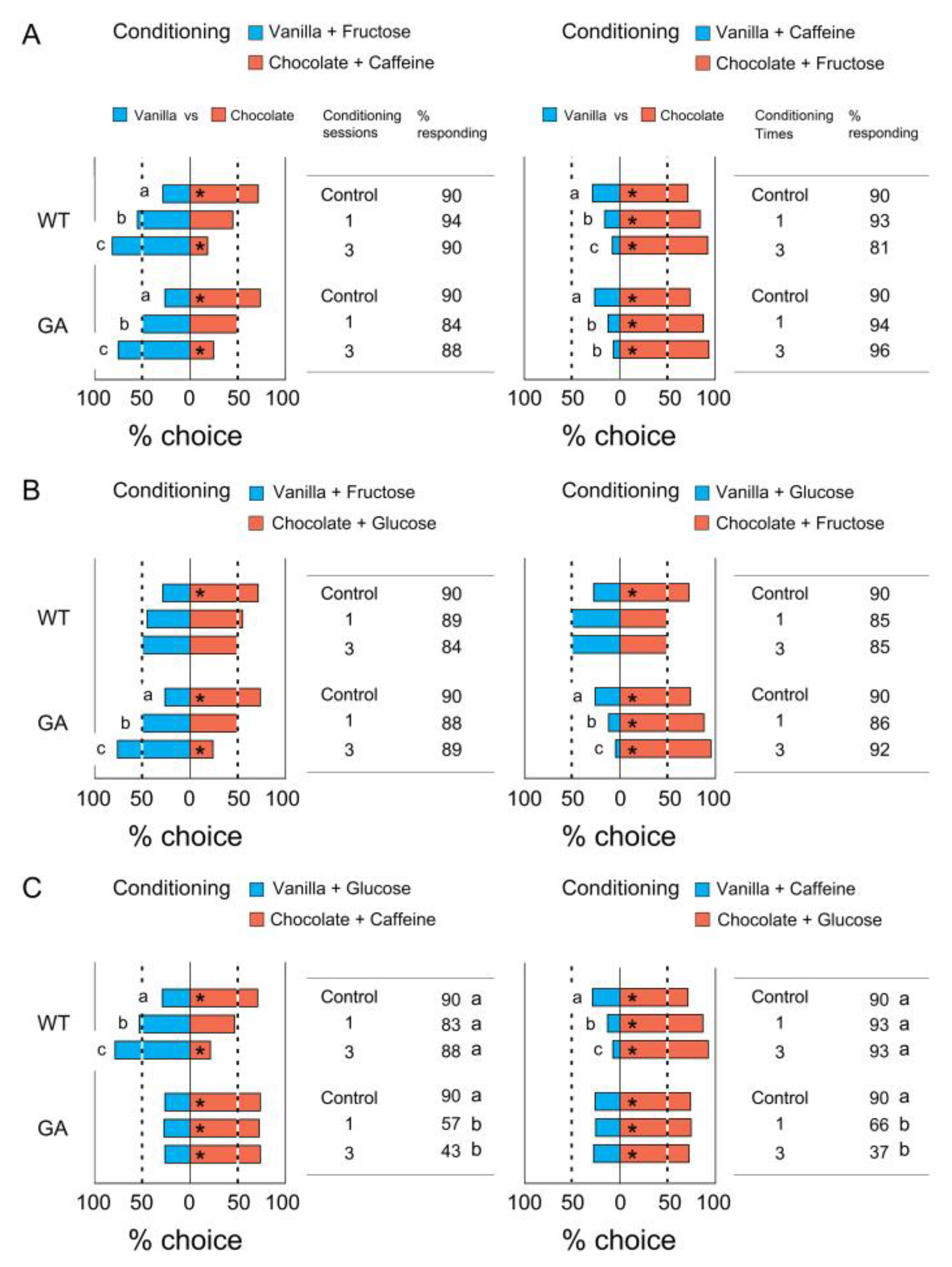
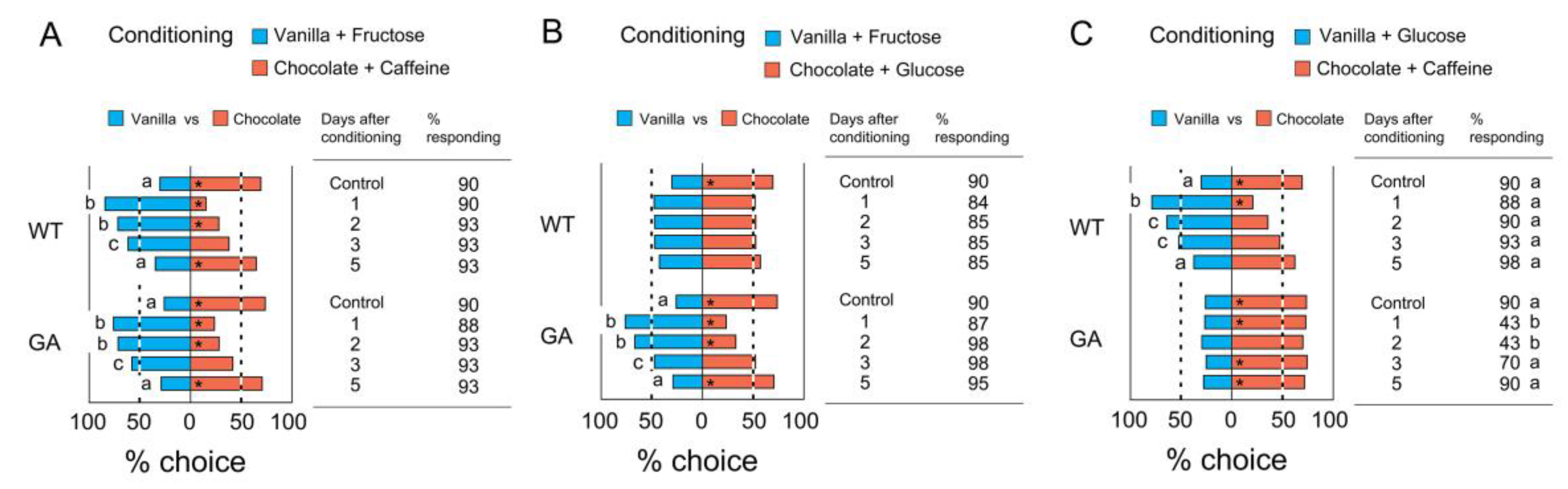
3.3. Association of Two Odors with Tastants
3.4. Retention of Olfactory Memory
4. Discussion
4.1. Innate Odor Preferences
4.2. Modification of Odor Preferences after Self-Training
4.3. Olfactory Learning and Behavioral Resistance in Cockroaches
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearce, J.M. Animal Learning and Cognition: An Introduction, 3rd ed.; Psychology Press: New York, NY, USA; Routledge: Abingdon, UK, 2008; p. 432. [Google Scholar]
- Dukas, R. Evolutionary biology of insect learning. Annu. Rev. Entomol. 2008, 53, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A. Associative learning and animal cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2733–2742. [Google Scholar] [CrossRef]
- Soderstrom, N.C.; Bjork, R.A. Learning versus performance: An integrative review. Perspect. Psychol. Sci. 2015, 10, 176–199. [Google Scholar] [CrossRef]
- Shimaji, K.; Tomida, S.; Yamaguchi, M. Regulation of animal behavior by epigenetic regulators. Front. Biosci. 2019, 24, 1071–1084. [Google Scholar]
- Anton, S.; Rössler, W. Plasticity and modulation of olfactory circuits in insects. Cell Tissue Res. 2021, 383, 149–164. [Google Scholar] [CrossRef]
- Menzel, R. Learning, memory and “cognition” in honeybees. In Neurobiology of Comparative Cognition; Kesner, R.P., Olton, D.S., Eds.; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1990; pp. 237–292. [Google Scholar]
- Giurfa, M.; Sandoz, J.C. Invertebrate learning and memory: Fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn. Mem. 2012, 9, 54–66. [Google Scholar] [CrossRef]
- Lemanski, N.J.; Cook, C.N.; Smith, B.H.; Pinter-Wollman, N.A. Multiscale review of behavioral variation in collective foraging behavior in honey bees. Insects 2019, 10, 370. [Google Scholar] [CrossRef]
- McGuire, T.R. Learning in three species of Diptera: The blow fly Phormia regina, the fruit fly Drosophila melanogaster, and the house fly Musca domestica. Behav. Genet. 1984, 14, 479–526. [Google Scholar] [CrossRef]
- Quinn, W.G.; Harris, W.A.; Benzer, S. Conditioned behavior in Drosophila. Proc. Natl. Acad. Sci. USA 1974, 71, 708–712. [Google Scholar] [CrossRef]
- Busto, G.U.; Cervantes-Sandoval, I.; Davis, R.L. Olfactory learning in Drosophila. Physiology 2010, 25, 338–346. [Google Scholar] [CrossRef]
- Hige, T. What can tiny mushrooms in fruit flies tell us about learning and memory? Neurosci. Res. 2018, 129, 8–16. [Google Scholar] [CrossRef]
- Cognigni, P.; Felsenberg, J.; Waddell, S. Do the right thing: Neural network mechanisms of memory formation, expression and update in Drosophila. Curr. Opin. Neurobiol. 2018, 49, 51–58. [Google Scholar] [CrossRef]
- Kinoshita, M.; Stewart, F.J.; Ômura, H. Multisensory integration in Lepidoptera: Insights into flower-visitor interactions. Bioessays 2017, 39, 4. [Google Scholar] [CrossRef]
- Haupt, S.S.; Sakurai, T.; Namiki, S.; Kazawa, T.; Kanzaki, R. Chapter 3 olfactory information processing in moths. In The Neurobiology of Olfaction; Menini, A., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 71–121. [Google Scholar]
- Matsumoto, Y.; Matsumoto, C.S.; Mizunami, M. Signaling pathways for long-term memory formation in the cricket. Front. Psychol. 2018, 9, 1014. [Google Scholar] [CrossRef]
- Mizunami, M. What is learned in Pavlovian conditioning in crickets? Revisiting the S-S and S-R learning theories. Front. Behav. Neurosci. 2021, 15, 114. [Google Scholar] [CrossRef]
- Lubinski, A.J.; Page, T.L. The Optic lobes regulate circadian rhythms of olfactory learning and memory in the cockroach. J. Biol. Rhythms. 2016, 31, 161–169. [Google Scholar] [CrossRef]
- Sakura, M.; Mizunami, M. Olfactory learning and memory in the cockroach Periplaneta americana. Zool. Sci. 2001, 18, 21–28. [Google Scholar] [CrossRef]
- Watanabe, H.; Kobayashi, Y.; Sakura, M.; Matsumoto, Y.; Mizunami, M. Classical olfactory conditioning in the cockroach Periplaneta americana. Zool. Sci. 2003, 20, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Matsumoto, Y.; Sakura, M.; Mizunami, M. Contextual olfactory learning in cockroaches. Neuroreport 2006, 17, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Sato, C.; Kuramochi, T.; Nishino, H.; Mizunami, M. Salivary conditioning with antennal gustatory unconditioned stimulus in an insect. Neurobiol. Learn. Mem. 2008, 90, 245–254. [Google Scholar] [CrossRef]
- Garren, M.V.; Sexauer, S.B.; Page, T.L. Effect of circadian phase on memory acquisition and recall: Operant conditioning vs. classical conditioning. PLoS ONE 2013, 8, e58693. [Google Scholar] [CrossRef]
- Matsumoto, C.S.; Kuramochi, T.; Matsumoto, Y.; Watanabe, H.; Nishino, H.; Mizunami, M. Participation of NO signaling in formation of long-term memory in salivary conditioning of the cockroach. Neurosci. Lett. 2013, 541, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Hosono, S.; Matsumoto, Y.; Mizunami, M. Interaction of inhibitory and facilitatory effects of conditioning trials on long-term memory formation. Learn. Mem. 2016, 23, 669–678. [Google Scholar] [CrossRef]
- Arican, C.; Bulk, J.; Deisig, N.; Nawrot, M.P. Cockroaches show individuality in learning and memory during classical and operant conditioning. Front. Physiol. 2020, 10, 1539. [Google Scholar] [CrossRef]
- Vinauger, C.; Lahondère, C.; Cohuet, A.; Lazzari, C.R.; Riffell, J.A. Learning and memory in disease vector insects. Trends. Parasitol. 2016, 32, 761–771. [Google Scholar] [CrossRef]
- Vinauger, C.; Lallement, H.; Lazzari, C.R. Learning and memory in Rhodnius prolixus: Habituation and aversive operant conditioning of the proboscis extension response. J. Exp. Biol. 2013, 216, 892–900. [Google Scholar] [CrossRef]
- Stockton, D.G.; Martini, X.; Patt, J.M.; Stelinski, L.L. The influence of learning on host plant preference in a significant phytopathogen vector, Diaphorina citri. PLoS ONE 2016, 11, e0149815. [Google Scholar]
- Little, C.M.; Chapman, T.W.; Hillier, N.K. Considerations for insect learning in integrated pest management. J. Insect Sci. 2019, 19, 6. [Google Scholar] [CrossRef]
- Pomés, A.; Schal, C. Chapter 15 Cockroach and other inhalant insect allergens. In Allergens and Allergen Immunotherapy, 6th ed.; Lockey, R.F., Ledford, D.K., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2020; pp. 237–255. [Google Scholar]
- Silverman, S.; Bieman, D.N. Glucose aversion in the German cockroach, Blattella germanica. J. Insect Physiol. 1993, 39, 925–933. [Google Scholar] [CrossRef]
- Wang, C.; Scharf, M.E.; Bennett, G.W. Genetic basis for resistance to gel baits, fipronil, and sugar-based attractants in German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 2006, 99, 1761–1767. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Silverman, J.; Schal, C. Differential inputs from chemosensory appendages mediate feeding responses to glucose in wild-type and glucose-averse German cockroaches, Blattella germanica. Chem. Senses 2011, 36, 589–600. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Silverman, J.; Schal, C. Changes in taste neurons support the emergence of an adaptive behavior in cockroaches. Science 2013, 340, 972–975. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Robertson, H.M.; Silverman, J.; Schal, C. Changes in the peripheral chemosensory system drive adaptive shifts in food preferences in insects. Front. Cell Neurosci. 2018, 12, 281. [Google Scholar] [CrossRef]
- Ross, M.H.; Silverman, J. Genetic studies of a behavioral mutant, glucose aversion, in the German cockroach (Dictyoptera: Blattellidae). J. Insect. Behav. 1995, 8, 825–834. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Schal, C. Salivary digestion extends the range of sugar-aversions in the German cockroach. Insects 2021, 12, 263. [Google Scholar] [CrossRef]
- Wang, C.; Scharf, M.E.; Bennett, G.W. Behavioral and physiological resistance of the German cockroach to gel baits (Blattodea: Blattellidae). J. Econ. Entomol. 2004, 97, 2067–2072. [Google Scholar] [CrossRef]
- Liang, D. Performance of cockroach gel baits against susceptible and bait averse strains of German cockroach, Blattella germanica—Role of bait base and active ingredient. In Proceedings of the Fifth International Conference on Urban Pests, Suntec, Singapore, 10–13 July 2005; Lee, C.Y., Robinson, W.H., Eds.; P & Y Design Network: Penang, Malaysia, 2005; pp. 107–114. [Google Scholar]
- Kells, S. Bait aversion by German cockroaches (Dictyoptera: Blattellidae): The influence and interference of nutrition. In Proceedings of the Fifth International Conference on Urban Pests, Singapore, 10–13 July 2005; Lee, C.Y., Robinson, W.H., Eds.; P & Y Design Network: Penang, Malaysia, 2005; pp. 419–422. [Google Scholar]
- Gondhalekar, A.D.; Song, C.; Scharf, M.E. Development of strategies for monitoring indoxacarb and gel bait susceptibility in the German cockroach (Blattodea: Blattellidae). Pest. Manag. Sci. 2011, 67, 262–270. [Google Scholar] [CrossRef]
- Tsuji, H. Attractive and feeding stimulative effect of some fatty acids and related compounds on three species of cockroaches. Jpn. J. Sanit. Zool. 1966, 17, 89–97. [Google Scholar] [CrossRef][Green Version]
- Wileyto, P.E.; Boush, M.G. Attraction of the German cockroach, Blattella germanica (Orthoptera: Blattellidae), to some volatile food components. J. Econ. Entomol. 1983, 76, 752–756. [Google Scholar] [CrossRef]
- Nalyanya, G.; Schal, C. Evaluation of attractants for monitoring populations of the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 2001, 94, 208–214. [Google Scholar] [CrossRef]
- Lauprasert, P.; Laupraset, P.; Sitthicharoenchai, D.; Thirakhupt, K.; Pradatsudarasar, A.O. Food preference and feeding behavior of the German cockroach, Blattella germanica (Linnaeus). J. Sci. Res. Chulalongkorn Univ. 2006, 31, 121–126. [Google Scholar]
- Karimifar, N.; Gries, R.; Khaskin, G.; Gries, G. General food semiochemicals attract omnivorous German cockroaches, Blattella germanica. J. Agric. Food Chem. 2011, 59, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.; Gries, R.; Gries, G. Rye bread and synthetic bread odorants—Effective trap bait and lure for German cockroaches. Entomol. Exp. Appl. 2017, 166, 81–93. [Google Scholar] [CrossRef]
- Durier, V.; Rivault, C. Learning and foraging efficiency in German cockroaches, Blattella germanica (L.) (Insecta: Dictyoptera). Anim. Cogn. 2000, 3, 139–145. [Google Scholar] [CrossRef]
- Durier, V.; Rivault, C. Effects of spatial knowledge and feeding experience on foraging choices in German cockroaches. Anim. Behav. 2001, 62, 681–688. [Google Scholar] [CrossRef][Green Version]
- Jeanson, R.; Deneubourg, J.L. Path selection in cockroaches. J. Exp. Biol. 2006, 209, 4768–4775. [Google Scholar] [CrossRef][Green Version]
- Liu, J.L.; Sakuma, M. Olfactory conditioning with single chemicals in the German cockroach, Blattella germanica (Dictyoptera: Blattellidae). Appl. Entomol. Zool. 2013, 48, 387–396. [Google Scholar] [CrossRef]
- Si, A.; Zhang, S.W.; Maleszka, R. Effects of caffeine on olfactory and visual learning in the honey bee (Apis mellifera). Pharmacol. Biochem. Behav. 2005, 82, 664–672. [Google Scholar] [CrossRef]
- Wright, G.A.; Baker, D.D.; Palmer, M.J.; Stabler, D.; Mustard, J.A.; Power, E.F.; Borland, A.M.; Stevenson, P.C. Caffeine in floral nectar enhances a pollinator’s memory of reward. Science 2013, 339, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Schal, C.; Wada-Katsumata, A. Behavior and chemical ecology. In Biology and Management of the German Cockroach; Wang, C., Lee, C.Y., Rust, M., Eds.; CSIRO Publishing: Clayton South, VIC, Australia, 2021; pp. 113–142. [Google Scholar]
- Pamir, E.; Chakroborty, N.K.; Stollhoff, N.; Gehring, K.B.; Antemann, V.; Morgenstern, L.; Felsenberg, J.; Eisenhardt, D.; Menzel, R.; Nawrot, M.P. Average group behavior does not represent individual behavior in classical conditioning of the honeybee. Learn. Mem. 2011, 18, 733–741. [Google Scholar] [CrossRef]
- Verschut, T.A.; Carlsson, M.A.; Hambäck, P.A. Scaling the interactive effects of attractive and repellent odours for insect search behaviour. Sci. Rep. 2019, 9, 15309. [Google Scholar] [CrossRef] [PubMed]
- De Brito Sanchez, M.G.; Serre, M.; Avarguès-Weber, A.; Dyer, A.G.; Giurfa, M. Learning context modulates aversive taste strength in honey bees. J. Exp. Biol. 2015, 218, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Modi, M.; Shuai, Y.; Turner, G.C. The Drosophila mushroom body: From architecture to algorithm in a learning circuit. Annu. Rev. Neurosci. 2020, 43, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Eschbach, C.; Fushiki, A.; Winding, M.; Schneider-Mizell, C.M.; Shao, M.; Arruda, R.; Eichler, K.; Valdes-Aleman, J.; Ohyama, T.; Thum, A.S.; et al. Recurrent architecture for adaptive regulation of learning in the insect brain. Nat. Neurosci. 2020, 23, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.W.; Bayer, B.E.; Koehler, P.G.; Pereira, R.M. Bait evaluation methods for urban pest management. In Insecticides; Trdan, S., Ed.; Intech Open: London, UK, 2013; pp. 445–469. [Google Scholar]

| Symbols of Conditioning Session in Figure 1B | ||
|---|---|---|
| Symbols (Terms) | Compounds Used | |
| CS (Conditioning stimulus) | Odors of vanilla and/or chocolate | |
| US + (Appetitive unconditioned stimulus) | Reward tastant (fructose and glucose for WT, fructose for GA) | |
| US − (Aversive unconditioned stimulus) | Punishment tastant (caffeine for WT, caffeine and glucose for GA) | |
| Schedule of experiments | ||
| Conditioning (1 session = 1 h/day, 12–1 p.m. [scotophase]) | Two-choice Bioassay (12–4 p.m. [scotophase]) | |
| Bioassay 1 |
|
|
| Bioassay 2 |
|
|
| Bioassay 3 |
|
|
| Bioassay 4 |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada-Katsumata, A.; Schal, C. Olfactory Learning Supports an Adaptive Sugar-Aversion Gustatory Phenotype in the German Cockroach. Insects 2021, 12, 724. https://doi.org/10.3390/insects12080724
Wada-Katsumata A, Schal C. Olfactory Learning Supports an Adaptive Sugar-Aversion Gustatory Phenotype in the German Cockroach. Insects. 2021; 12(8):724. https://doi.org/10.3390/insects12080724
Chicago/Turabian StyleWada-Katsumata, Ayako, and Coby Schal. 2021. "Olfactory Learning Supports an Adaptive Sugar-Aversion Gustatory Phenotype in the German Cockroach" Insects 12, no. 8: 724. https://doi.org/10.3390/insects12080724
APA StyleWada-Katsumata, A., & Schal, C. (2021). Olfactory Learning Supports an Adaptive Sugar-Aversion Gustatory Phenotype in the German Cockroach. Insects, 12(8), 724. https://doi.org/10.3390/insects12080724






