The Imaginal Characters of Neoephemera projecta Showing Its Plesiomorphic Position and a New Genus Status in the Family (Ephemeroptera: Neoephemeridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
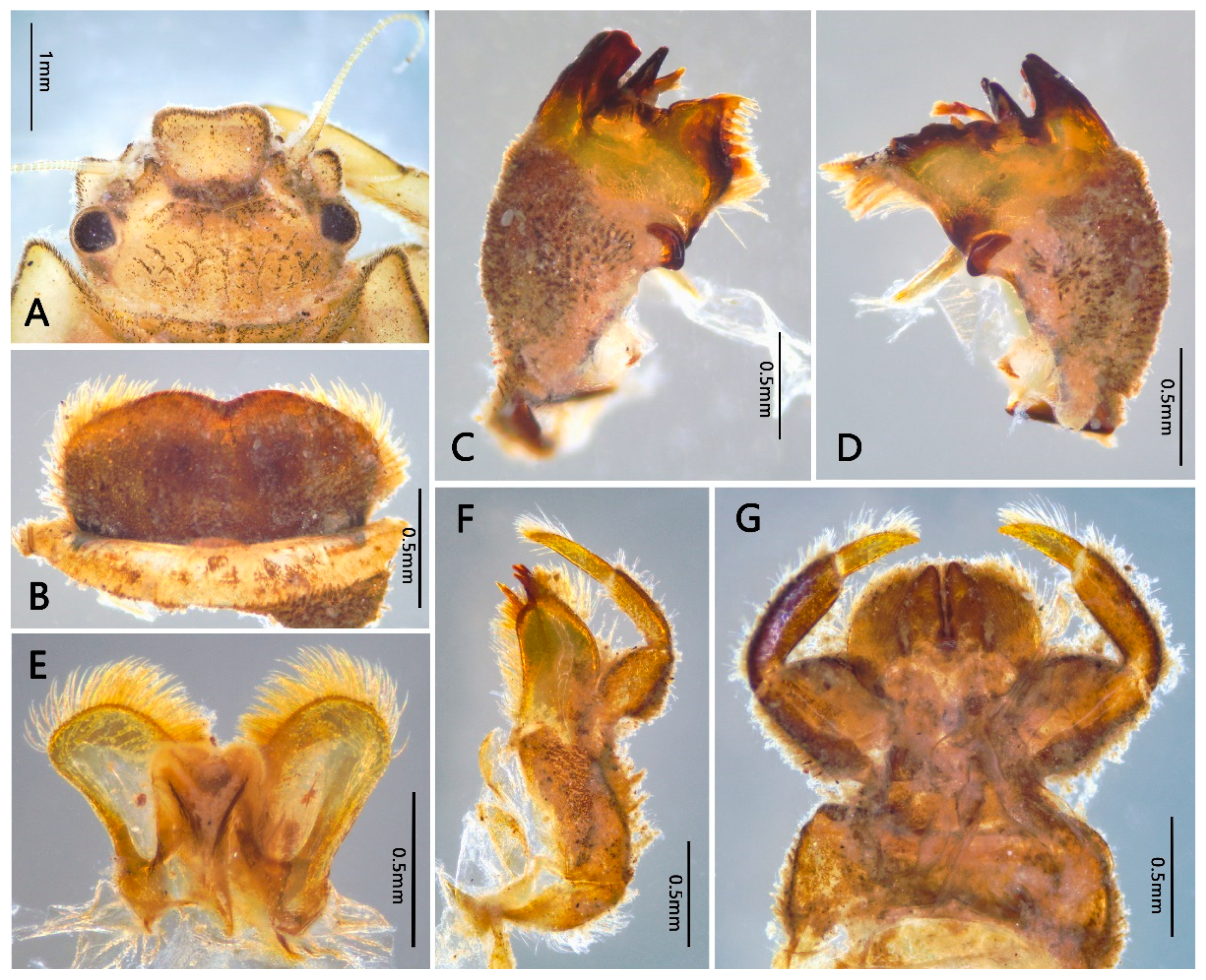
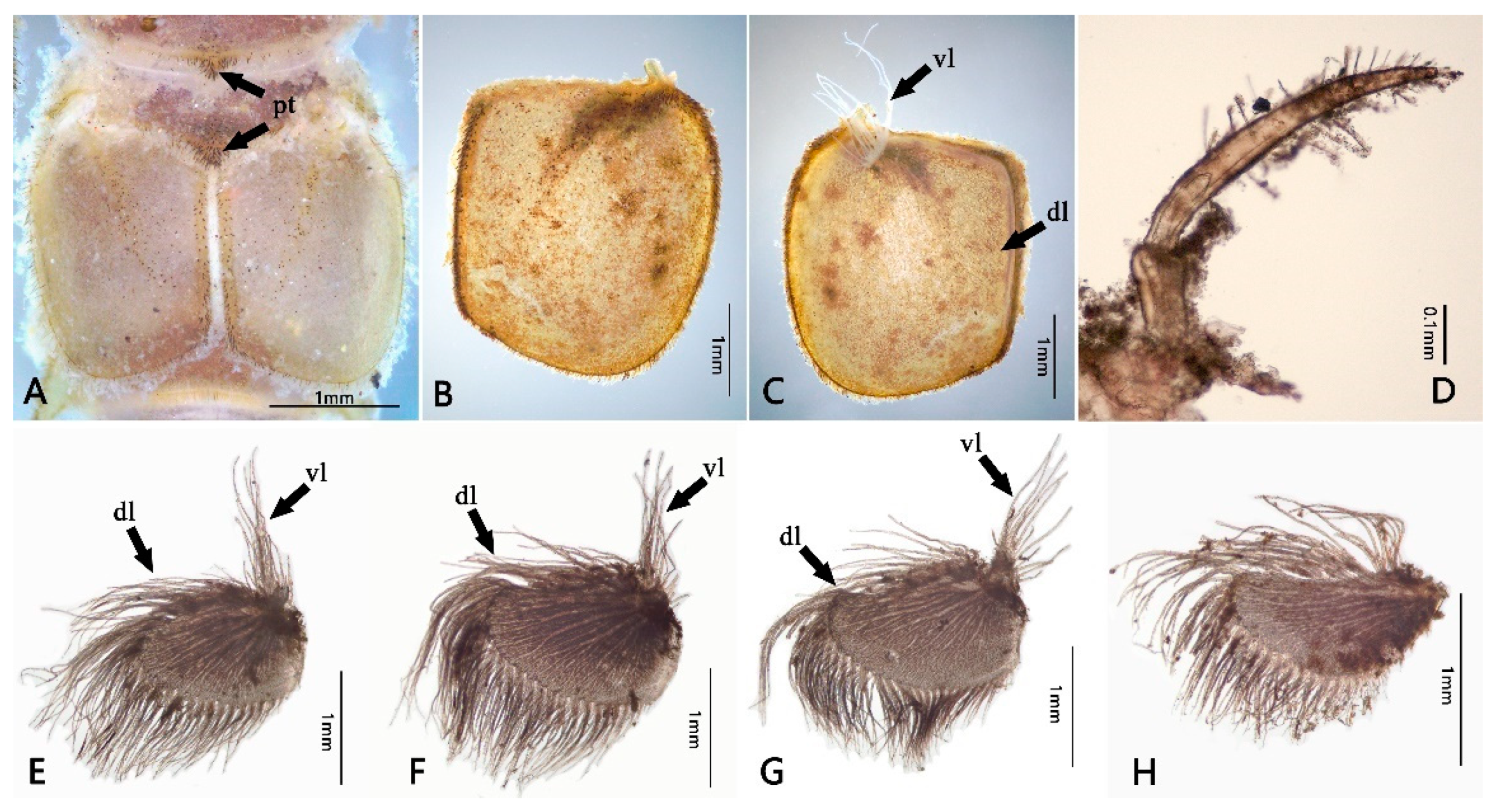
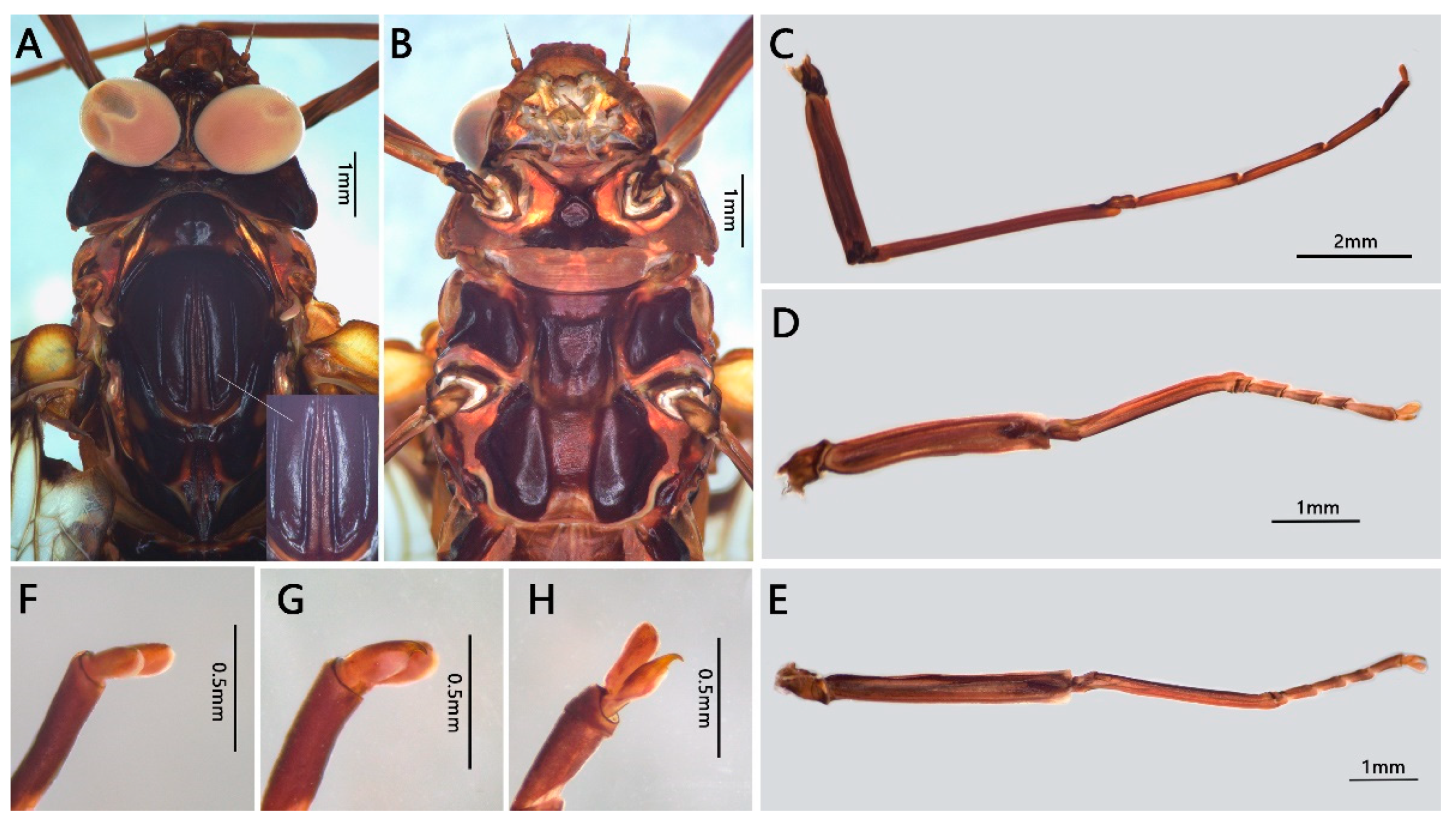
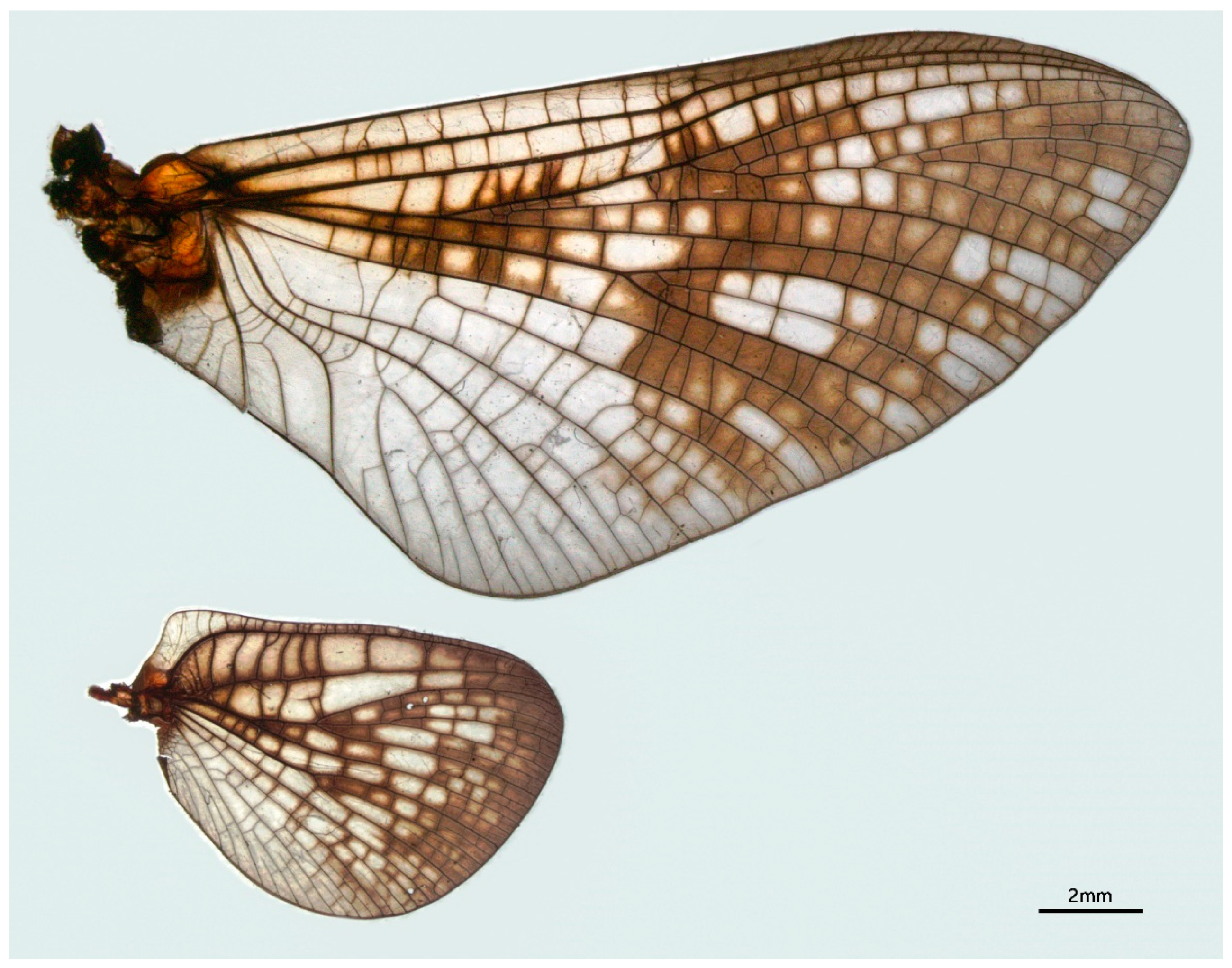
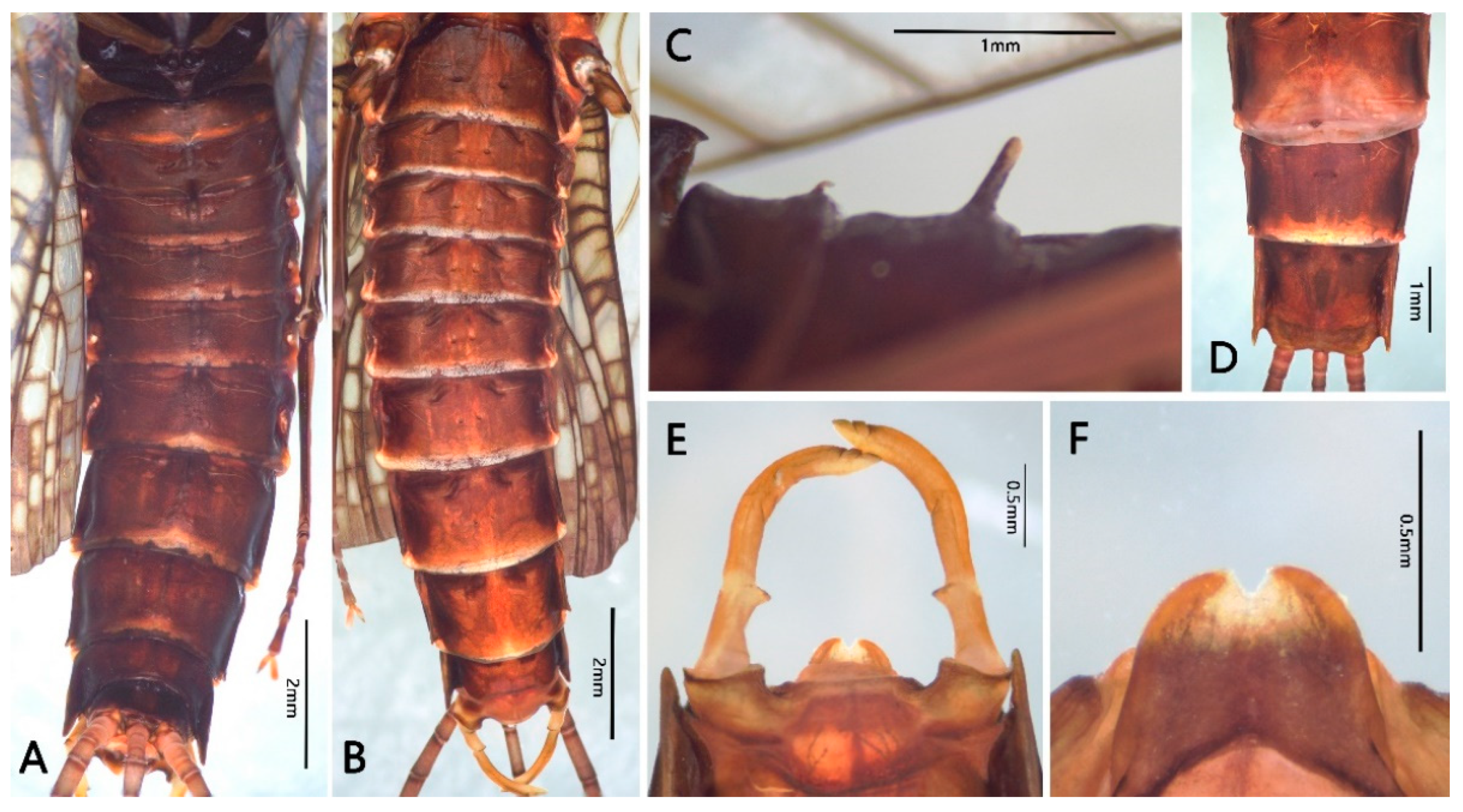
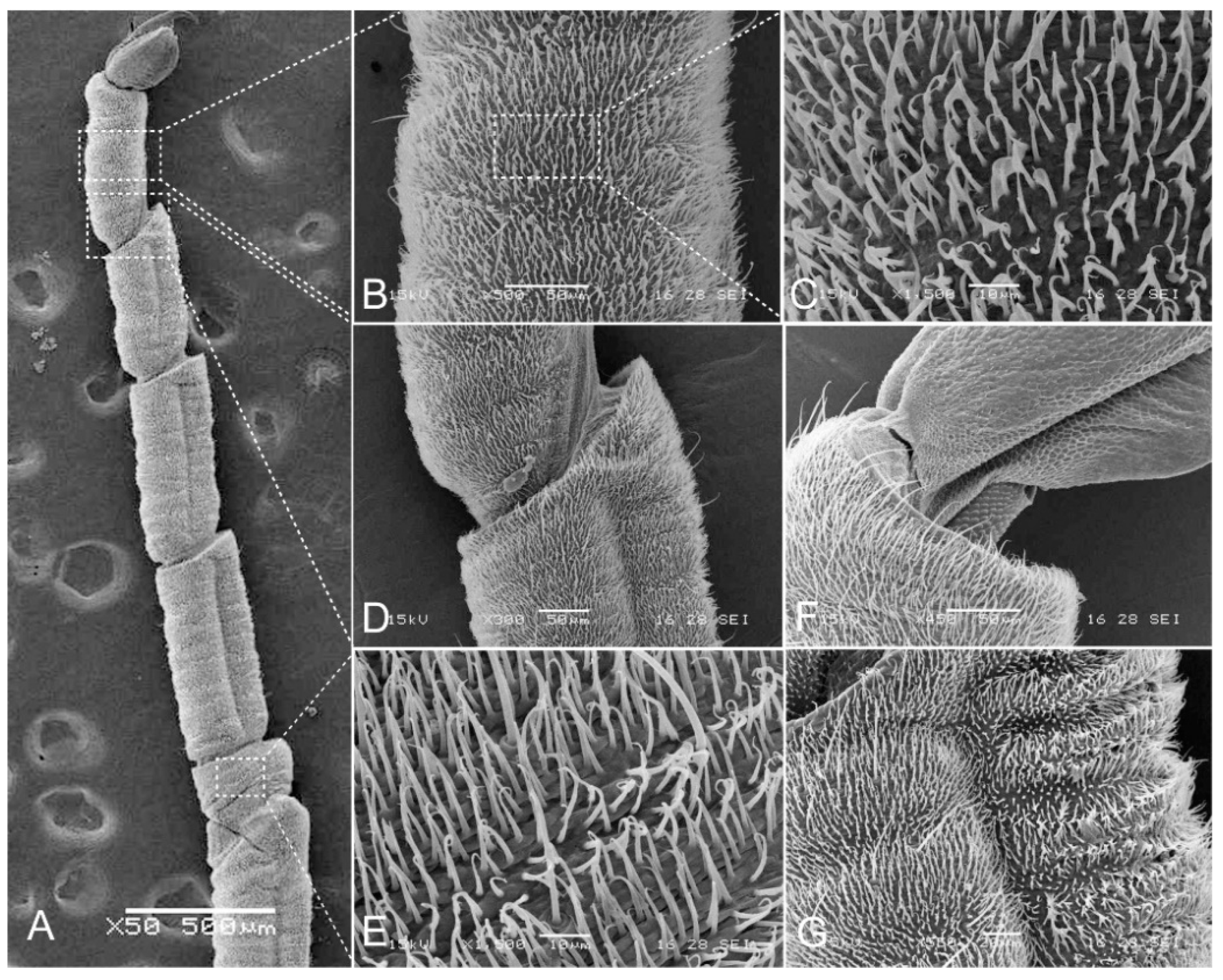
3.1. Description
3.2. Biology and Remarks
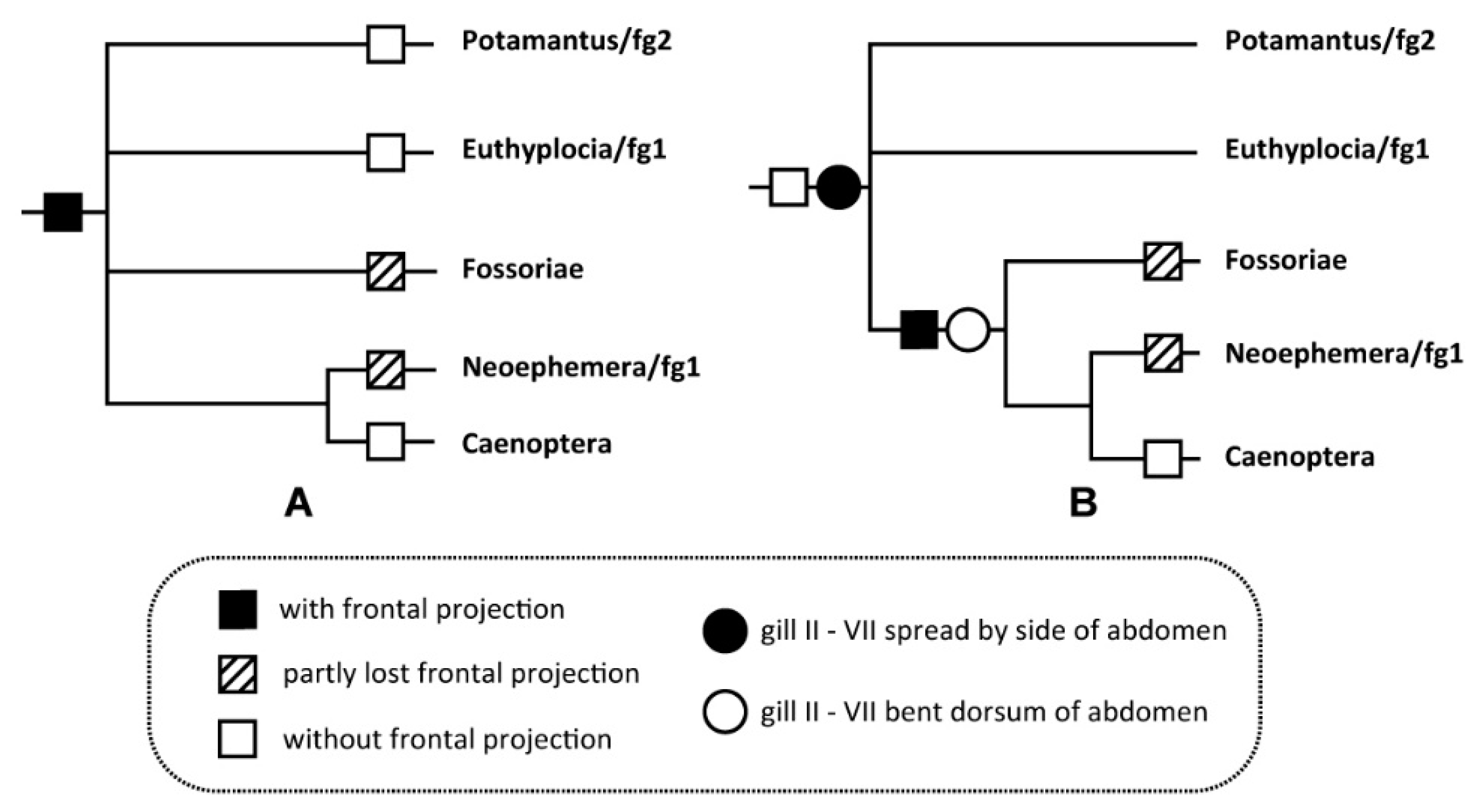
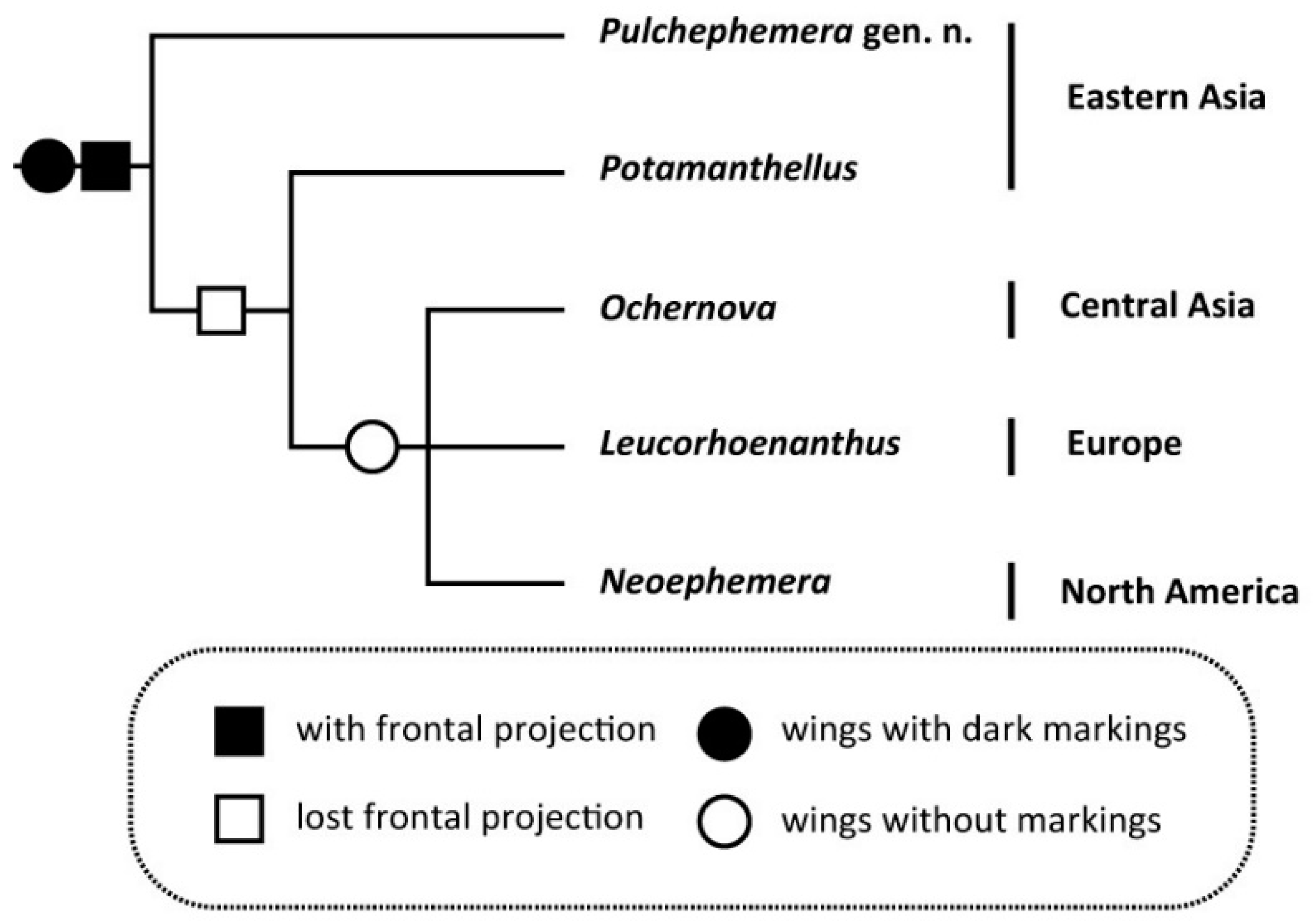
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bae, Y.; McCafferty, W. Phylogenetic Systematics and Biogeography of the Neoephemeridae (Ephemeroptera: Pannota). Aquat. Insects 1998, 20, 35–68. [Google Scholar] [CrossRef]
- Zhou, C.-F.; Zheng, L.-Y. A New Species of the Genus Neoephemera McDunnough from China (Ephemeroptera: Neoephemeridae). Aquat. Insects 2001, 23, 327–332. [Google Scholar] [CrossRef]
- Holland, V.B.; Beaty, S.R.; Jacobus, L.M. A new species of Neoephemera McDunnough, 1925 (Ephemeroptera: Neoephemeridae) from North Carolina and Virginia. Zootaxa 2016, 4138, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.V.; Bae, Y.J. Taxonomic review of the Vietnamese Neoephemeridae (Ephemeroptera) with description of Potamanthellus unicutibius, new species. Pan-Pacific Entomol. 2004, 79, 230–236. [Google Scholar]
- Kluge, N. The Phylogenetic System of Ephemeroptera; Springer: Singapore, 2004; pp. 271–276. [Google Scholar]
- Bauernfeind, E.; Soldan, T. The Mayflies of Europe (Ephemeroptera); Apollo Books: Ollerup, Denmark, 2012; pp. 513–515. [Google Scholar]
- Mcdunnough, J. New Canadian Ephemeridae with Notes, III. Can. Èntomol. 1925, 57, 168–176. [Google Scholar] [CrossRef]
- Lestage, J.A. Contribution à l’étude des larves des Éphéméroptères. VII. Le groupe Potamanthidien. Mémories Soc. Entomol. Belg. 1931, 23, 73–146. [Google Scholar]
- Kazlauskas, R. New and little-known mayflies (Ephemeroptera) from the USSR. Entomol. Obozr. 1963, 42, 582–593. [Google Scholar]
- Ogden, T.H.; Whiting, M.F. Phylogeny of Ephemeroptera (mayflies) based on molecular evidence. Mol. Phylogen. Evol. 2005, 37, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Ogden, T.H.; Breinholt, J.W.; Bybee, S.M.; Miller, D.B.; Sartori, M.; Shiozawa, D.; Whiting, M.F. Mayfly phylogenomics: Initial evaluation of anchored hybrid enrichment data for the order Ephemeroptera. Zoosymposia 2019, 16, 167–181. [Google Scholar]
- Edmunds, G.F., Jr.; Jensen, S.L.; Berner, L. The Mayflies of North and Central America; University of Minnesota Press: Minneapolis, MN, USA, 1976; pp. 260–263. [Google Scholar]
- Wang, T.Q.; McCafferty, W.P.; Bae, Y.J. Sister relationships of the Neoephemeridae and Caenidae (Ephemeroptera: Pannota). Entomolomol. News 1997, 108, 52–56. [Google Scholar]
- Kluge, N.J.; Novikova, E.A. Systematics of Indobaetis Müller-Liebenau & Morihara 1982, and related implications for some other Baetidae genera (Ephemeroptera). Zootaxa 2014, 3835, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Traver, J.R. A New Mayfly Genus from North Carolina. Can. Èntomol. 1931, 63, 103–109. [Google Scholar] [CrossRef]
- Berner, L. New Mayfly Records from Florida and a Description of a New Species. Fla. Èntomol. 1953, 36, 145. [Google Scholar] [CrossRef]
- Hsu, Y.C. New Chines mayflies from Kiangsi Province (Ephemeroptera). Peking Nat.History Bull. 1936, 10, 319–325. [Google Scholar]
- Berner, L. The Genus Neoephemera in North America (Ephemeroptera: Neoephemeridae)1. Ann. Èntomol. Soc. Am. 1956, 49, 33–42. [Google Scholar] [CrossRef]
- Kazlauskas, R.S. Materials about the mayflies (Ephemeroptera) in Lithuanian SSR with description of new space Eurylophella lithuanica Kazlauskas and imago Neoephemera maxima (Joly). Russ. Lith. Engl. Summ. 1959, 23, 157–174. [Google Scholar]
- Jazdzewska, T. Neoephemera maxima (Joly, 1870) (Ephemeroptera, Neoephemeridae) in Poland. Bull. Entomol. Pol. 1975, 45, 227–238. [Google Scholar]
- Klonowska-Olejnik, M.; Jazdzewska, T. Scanning electron microscopy study of the eggs of some rare mayfly (Ephemerop-tera) species: Ametropus fragilis, Isonychia ignota and Neoephemera maxima. In Research Update on Ephemeroptera & Plecoptera; Gaino, E., Ed.; University Perugia: Perugia, Italy, 2003; pp. 457–462. [Google Scholar]
- Edmunds, G.F., Jr.; Traver, J.R. An outline of a reclassification of the Ephemeroptera. Proc. Entomol. Soc. Wash. 1954, 56, 236–240. [Google Scholar]
- Demoulin, G. Nouveau schema de classification des Archodonates et des Ephéméroptères. Bull. l’Institut R. Sci. Nat. Bel. 1958, 34, 1–19. [Google Scholar]
- Tshernova, O.A. On the classification of fossil and recent Ephemeroptera. Entomol. Obozr. 1970, 49, 124–145. [Google Scholar]
- Landa, V. Comparative anatomy of mayfly larvae (Ephemeroptera). Acta Entomol. Bohemoslov. 1969, 66, 289–316. [Google Scholar]
- McCafferty, W.P. Toward a Phylogenetic Classification of the Ephemeroptera (Insecta): A Commentary on Systematics. Ann. Èntomol. Soc. Am. 1991, 84, 343–360. [Google Scholar] [CrossRef]
- Zhou, C.F.; Peters, J.G. The nymph of Siphluriscus chinensis and additional imaginal description: A living mayfly with Jurassic origin (Siphluriscidae new family: Ephemeroptera). Fla. Entomol. 2003, 86, 345–352. [Google Scholar] [CrossRef]
- Jo, J.; Tojo, K. Molecular analyses of the genus Drunella (Ephemeroptera: Ephemerellidae) in the East Asian region. Limnology 2019, 20, 243–254. [Google Scholar] [CrossRef]
- Zembrzuski, D.C.; Anderson, F.E. Clarifying the phylogenetic relationships and taxonomy of Stenonema, Stenacron and Maccaffertium, three common eastern North American mayfly genera. Mol. Phylogenetics Evol. 2018, 128, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Massariol, F.C.; Takiya, D.M.; Salles, F.F. Global classification and evolution of brushlegged mayflies (Insecta: Ephemerop-tera: Oligoneuriidae): Phylogenetic analyses of morphological and molecular data and dated historical biogeography. Zool. J. Linn. Soc. 2019, 187, 378–412. [Google Scholar] [CrossRef]






| Genera | Neoephemera [1,3,5,18] | Ochernova [1,5,9] | Leucorhoenanthus [1,5,6,19,20] | Pulchephemera gen. n. | Potamanthellus [1,5] | |
|---|---|---|---|---|---|---|
| Characters | ||||||
| Nymph | Frontal projection on head | Absent | Absent | Absent | Present | Absent |
| Mesonotal anterolateral expansions | Greatly or somewhat expanded | Somewhat expanded | Without distinct expansions | Somewhat expanded | Without or somewhat expanded | |
| Maxillary palpi (length ratio of terminal segment/2nd segment) | 0.7–1.3 | 0.35 | 0.78–0.84 | 1.0–1.1 | 1.1–2.0 | |
| Labial palpi (length ratio of terminal segment/2nd segment) | 0.6–1.1 | 0.39 | 0.65 | 0.65 | 1.0–1.5 | |
| Patella-tibial suture of mid- and hindlegs | Present | Absent | Present | Present | Present | |
| Length of mid- and hind femora | Longer than tibiae | Shorter than tibiae | Longer than tibiae | Longer than tibiae | Longer than tibiae | |
| Median tubercles on thoracic terga | Well developed (except N. eatoni) | Absent | Absent | Absent | Absent | |
| Caudal filaments | Without swimming setae; ca. 0.8–1.0× length of body | Without swimming setae; ca. 1.4× length of body | Without swimming setae; ca. 0.7× length of body | Without swimming setae; ca. 0.45–0.5× length of body | With swimming setae; ca. 0.4–0.7× length of body | |
| Male imago | Ratio of distance between compound eyes/diameter of compound eyes | 0.15–0.5 | / | 1.15 | 0.1 | 0.04–0.15 |
| Claws of forelegs | One sharp, one blunt | / | Two blunt | Two blunt | Two blunt | |
| Forewing length (mm) | 8–17 | 9 | 8–11 | 18–20 | 6–10 | |
| Coloration of wings | Without markings | Without markings | Without markings | with distinct markings | with distinct markings | |
| Basal C-Sc crossveins of forewings | Reduced | Reduced | Reduced | Not reduced | Not reduced | |
| Shape of basal costal projection of hindwings | Acute | Acute | Acute | Rounded | Rounded | |
| Forceps | Well developed, 4-segmented | Well developed, 4-segmented | Well developed, 4-segmented | Well developed, 4-segmented | Vestigial, 3-segmented | |
| Median incision of penis | Small | Small | Small | Small | Wide | |
| Median caudal filament | Well developed | Well developed | Vestigial | Well developed | Vestigial | |
| Length ratio of cerci/body | 1.0–1.5 | / | 2.4 | 2.3–2.5 | 2.2–4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Zhou, C. The Imaginal Characters of Neoephemera projecta Showing Its Plesiomorphic Position and a New Genus Status in the Family (Ephemeroptera: Neoephemeridae). Insects 2021, 12, 723. https://doi.org/10.3390/insects12080723
Ma Z, Zhou C. The Imaginal Characters of Neoephemera projecta Showing Its Plesiomorphic Position and a New Genus Status in the Family (Ephemeroptera: Neoephemeridae). Insects. 2021; 12(8):723. https://doi.org/10.3390/insects12080723
Chicago/Turabian StyleMa, Zhenxing, and Changfa Zhou. 2021. "The Imaginal Characters of Neoephemera projecta Showing Its Plesiomorphic Position and a New Genus Status in the Family (Ephemeroptera: Neoephemeridae)" Insects 12, no. 8: 723. https://doi.org/10.3390/insects12080723
APA StyleMa, Z., & Zhou, C. (2021). The Imaginal Characters of Neoephemera projecta Showing Its Plesiomorphic Position and a New Genus Status in the Family (Ephemeroptera: Neoephemeridae). Insects, 12(8), 723. https://doi.org/10.3390/insects12080723






