Growth of Yellowtail (Seriola quinqueradiata) Fed on a Diet Including Partially or Completely Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Defatting of Black Soldier Fly Larvae Meal
2.3. Feed Formulation of Experimental Diets
2.4. Feeding Trials
2.5. Proximate Composition, Amino Acid, and Fatty Acid Analysis
2.6. Statistical Analysis
3. Results
3.1. Analysis of Proximate Composition, Amino Acid Profiles, and Fatty Acid Profiles
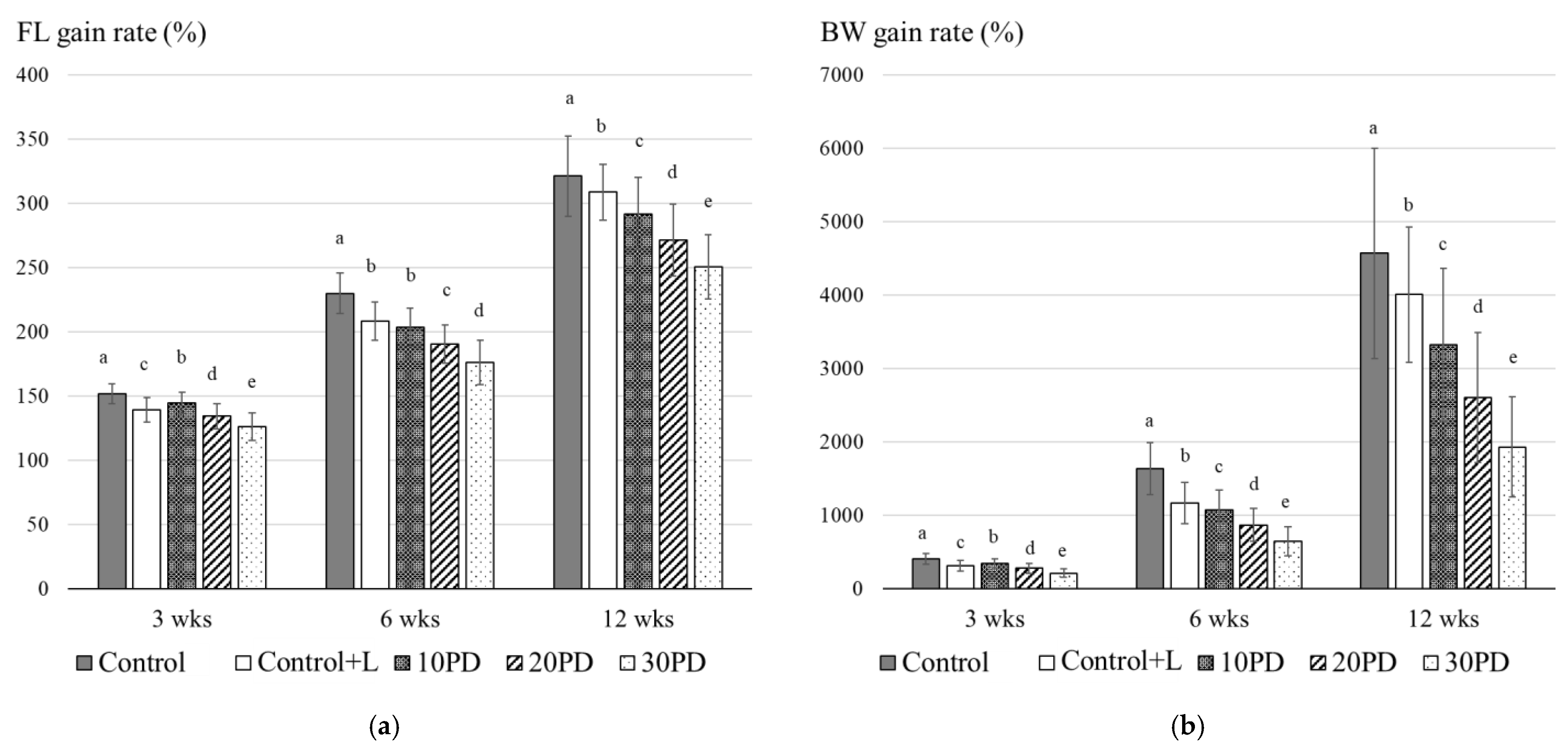
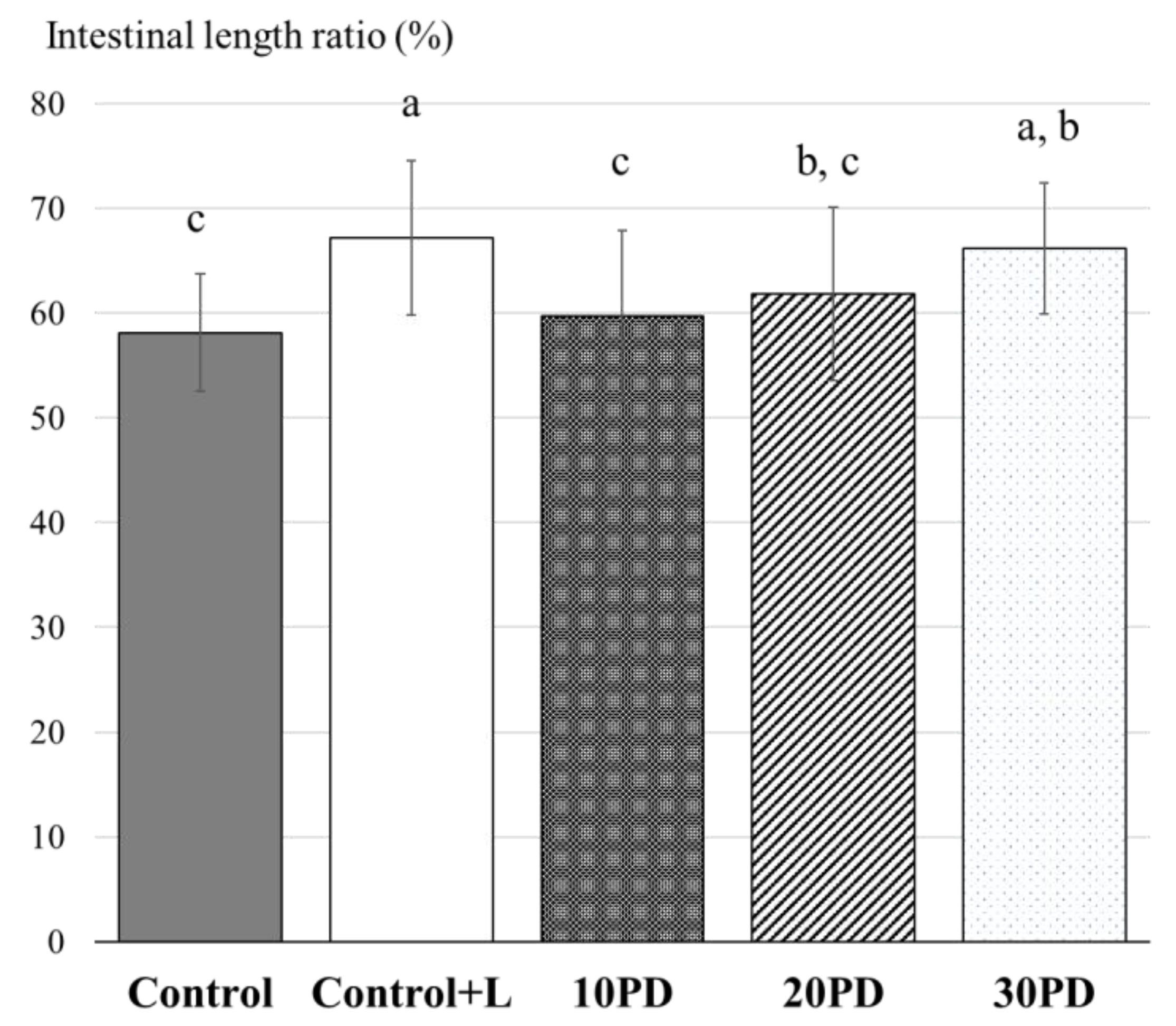
3.2. Feeding Trials with Juvenile Yellowtail
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tacon, A.G.J.; Metian, M. Feed Matters: Satisfying the Feed Demand of Aquaculture. Rev. Fish. Sci. Aquac. 2015, 23, 1–10. [Google Scholar] [CrossRef]
- Alder, J.; Campbell, B.; Karpouzi, V.; Kaschner, K.; Pauly, D. Forage Fish: From Ecosystems to Markets. Annu. Rev. Environ. Resour. 2008, 33, 153–166. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Shafique, L.; Abdel-Latif, H.M.R.; Hassan, F.; Alagawany, M.; Naiel, M.A.E.; Dawood, M.A.O.; Yilmaz, S.; Liu, Q. The Feasibility of Using Yellow Mealworms (Tenebrio molitor): Towards a Sustainable Aquafeed Industry. Animals 2021, 11, 811. [Google Scholar] [CrossRef]
- Ido, A.; Iwai, T.; Ito, K.; Ohta, T.; Mizushige, T.; Kishida, T.; Miura, C.; Miura, T. Dietary effects of housefly (Musca domestica) (Diptera: Muscidae) pupae on the growth performance and the resistance against bacterial pathogen in red sea bream (Pagrus major) (Perciformes: Sparidae). Appl. Entomol. Zool. 2015, 50, 213–221. [Google Scholar] [CrossRef]
- Ali, M.F.Z.; Ohta, T.; Ido, A.; Miura, C.; Miura, T. The Dipterose of Black Soldier Fly (Hermetia illucens) Induces Innate Immune Response through Toll-Like Receptor Pathway in Mouse Macrophage RAW264.7 Cells. Biomolecules 2019, 9, 677. [Google Scholar] [CrossRef] [Green Version]
- Chaklader, M.R.; Siddik, M.A.B.; Fotedar, R.; Howieson, J. Insect larvae, Hermetia illucens in poultry by-product meal for barramundi, Lates calcarifer modulates histomorphology, immunity and resistance to Vibrio harveyi. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Ghetas, H.A.; Khallaf, M.A. Black soldier fly (Hermetia illucens) larvae meal in diets of European seabass: Effects on antioxidative capacity, non-specific immunity, transcriptomic responses, and resistance to the challenge with Vibrio alginolyticus. Fish. Shellfish Immunol. 2021, 111, 111–118. [Google Scholar] [CrossRef]
- Li, Y.; Kortner, T.M.; Chikwati, E.M.; Belghit, I.; Lock, E.J.; Krogdahl, Å. Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar). Aquaculture 2020, 520, 734967. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.-G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Wang, G.; Peng, K.; Hu, J.; Yi, C.; Chen, X.; Wu, H.; Huang, Y. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture 2019, 507, 144–154. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M.R. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Sicuro, B.; Luzzana, U. The State of Seriola spp. Other Than Yellowtail (S. quinqueradiata) Farming in the World. Rev. Fish. Sci. Aquac. 2016, 24, 314–325. [Google Scholar] [CrossRef]
- Nakada, M. Capture-based aquaculture of yellowtail. Capture Based Aquac. Glob. Overv. FAO Fish. Tech. Pap. 2008, 508, 199–215. [Google Scholar]
- Miura, C.; Yoshihara, Y.; Shimizu-Yamaguchi, S.; Hayashi, D.; Hamada, K.; Takeda, Y.; Miura, M.; Miura, T. Controlled feeding alleviates the reduced growth associated with spawning in farmed yellowtail (Seriola quinqueradiata). Aquaculture 2014, 424–425, 10–17. [Google Scholar] [CrossRef]
- Watanabe, T.; Aoki, H.; Shimamoto, K.; Hadzuma, M.; Maita, M.; Yamagata, Y.; Kiron, V.; Satoh, S. A Trial to Culture Yellowtail with Non-fishmeal Diets. Fish. Sci. 1998, 64, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.S.A.; Satoh, S.; Kamata, K.; Haga, Y.; Yamamoto, Y. Partial replacement of fish meal with plant protein sources using organic acids to practical diets for juvenile yellowtail, Seriola quinqueradiata. Aquac. Nutr. 2012, 18, 81–89. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Khaoian, P.; Furutani, T.; Nagano, J.; Fukada, H.; Masumoto, T. Effects of alcohol extract of defatted soybean meal on growth performance and digestive physiology of yellowtail Seriola quinqueradiata. Fish. Sci. 2017, 83, 99–106. [Google Scholar] [CrossRef]
- Murashita, K.; Matsunari, H.; Fukada, H.; Suzuki, N.; Furuita, H.; Oku, H.; Rønnestad, I.; Yoshinaga, H.; Yamamoto, T. Effect of a plant-based low-fishmeal diet on digestive physiology in yellowtail Seriola quinqueradiata. Aquaculture 2019, 506, 168–180. [Google Scholar] [CrossRef]
- Ido, A.; Hashizume, A.; Ohta, T.; Takahashi, T.; Miura, C.; Miura, T. Replacement of Fish Meal by Defatted Yellow Mealworm (Tenebrio molitor) Larvae in Diet Improves Growth Performance and Disease Resistance in Red Seabream (Pargus major). Animals 2019, 9, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashizume, A.; Ido, A.; Ohta, T.; Thiaw, S.T.; Morita, R.; Nishikawa, M.; Takahashi, T.; Miura, C.; Miura, T. Housefly (Musca domestica) Larvae Preparations after Removing the Hydrophobic Fraction Are Effective Alternatives to Fish Meal in Aquaculture Feed for Red Seabream (Pagrus major). Fishes 2019, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- AOAC international. Official Methods of Analysis of AOAC International, 20th ed.; Latimer, W.G., Ed.; AOAC International: Gaithersburg, MD, USA, 2016; ISBN 0935584870. [Google Scholar]
- Tippayadara, N.; Dawood, M.A.O.; Krutmuang, P.; Hoseinifar, S.H.; Van Doan, H.; Paolucci, M. Replacement of Fish Meal by Black Soldier Fly (Hermetia illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef]
- Fawole, F.J.; Adeoye, A.A.; Tiamiyu, L.O.; Ajala, K.I.; Obadara, S.O.; Ganiyu, I.O. Substituting fishmeal with Hermetia illucens in the diets of African catfish (Clarias gariepinus): Effects on growth, nutrient utilization, haemato-physiological response, and oxidative stress biomarker. Aquaculture 2020, 518, 734849. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Tang, T.; Zhong, L.; Chu, W.; Dai, Z.; Chen, K.; Hu, Y. Effect of partial black soldier fly (Hermetia illucens L.) larvae meal replacement of fish meal in practical diets on the growth, digestive enzyme and related gene expression for rice field eel (Monopterus albus). Aquac. Rep. 2020, 17, 100345. [Google Scholar] [CrossRef]
- Vongvichith, B.; Morioka, S.; Sugita, T.; Phousavanh, N.; Phetsanghanh, N.; Chanthasone, P.; Pommachan, P.; Nakamura, S. Evaluation of the efficacy of aquaculture feeds for the climbing perch Anabas testudineus: Replacement of fishmeal by black soldier fly Hermetia illucens prepupae. Fish. Sci. 2020, 86, 145–151. [Google Scholar] [CrossRef]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Caimi, C.; Renna, M.; Lussiana, C.; Bonaldo, A.; Gariglio, M.; Meneguz, M.; Dabbou, S.; Schiavone, A.; Gai, F.; Elia, A.C.; et al. First insights on Black Soldier Fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture 2020, 515, 734539. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cardinaletti, G.; Randazzo, B.; Messina, M.; Zarantoniello, M.; Giorgini, E.; Zimbelli, A.; Bruni, L.; Parisi, G.; Olivotto, I.; Tulli, F. Effects of Graded Dietary Inclusion Level of Full-Fat Hermetia illucens Prepupae Meal in Practical Diets for Rainbow Trout (Oncorhynchus mykiss). Animals 2019, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Hua, K. A meta-analysis of the effects of replacing fish meals with insect meals on growth performance of fish. Aquaculture 2021, 530, 735732. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Matsui, T.; Shimizu, C. Effect of Chitin, Chitosan, and Cellulose as Diet Supplements on the Growth of Cultured Fish. Nippon Suisan Gakkaish 1987, 53, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Huyben, D.; Vidaković, A.; Werner Hallgren, S.; Langeland, M. High-throughput sequencing of gut microbiota in rainbow trout (Oncorhynchus mykiss) fed larval and pre-pupae stages of black soldier fly (Hermetia illucens). Aquaculture 2019, 500, 485–491. [Google Scholar] [CrossRef]
- Cláudia Figueiredo-Silva, A.; Kaushik, S.; Terrier, F.; Schrama, J.W.; Médale, F.; Geurden, I. Link between lipid metabolism and voluntary food intake in rainbow trout fed coconut oil rich in medium-chain TAG. Br. J. Nutr. 2012, 107, 1714–1725. [Google Scholar] [CrossRef] [Green Version]
- Ballestrazzi, R.; Rainis, S.; Maxia, M. The replacement of fish oil with refined coconut oil in the diet of large rainbow trout (Oncorhynchus mykiss). Ital. J. Anim. Sci. 2006, 5, 155–164. [Google Scholar] [CrossRef]
- Belghit, I.; Waagbø, R.; Lock, E.-J.; Liland, N.S. Insect-based diets high in lauric acid reduce liver lipids in freshwater Atlantic salmon. Aquac. Nutr. 2019, 25, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Xu, N.; Liu, Y.; Li, X.; Xiang, X.; Xu, D.; Yao, C.; Liu, Q.; Yin, Z.; Mai, K.; et al. Optimal amounts of coconut oil in diets improve the growth, antioxidant capacity and lipid metabolism of large yellow croaker (Larimichthys crocea). Mar. Life Sci. Technol. 2020, 2, 376–385. [Google Scholar] [CrossRef]
- Kumar, V.; Fawole, F.J.; Romano, N.; Hossain, M.S.; Labh, S.N.; Overturf, K.; Small, B.C. Insect (black soldier fly, Hermetia illucens) meal supplementation prevents the soybean meal-induced intestinal enteritis in rainbow trout and health benefits of using insect oil. Fish. Shellfish Immunol. 2021, 109, 116–124. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | Control | Control+L | 10PD | 20PD | 30PD |
|---|---|---|---|---|---|
| Fish meal (65% CP) 1 | 64.00 | 64.00 | 56.87 | 49.75 | 42.62 |
| PDBM | - | - | 10.00 | 20.00 | 30.00 |
| Fish oil | 8.00 | 8.00 | 8.76 | 9.51 | 10.27 |
| Palm kernel oil | - | 4.35 | 2.90 | 1.45 | - |
| Corn oil | 4.35 | - | - | - | - |
| Starch | 13.15 | 13.15 | 10.97 | 8.79 | 6.61 |
| Taurine | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin mix 2 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Mineral mix 3 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Choline chloride | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin C derivatives | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| NaH2PO4 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| KH2PO4 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Calcium lactate | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| CMC 4 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition (%) | |||||
| Moisture | 3.3 | 3.2 | 4.0 | 3.7 | 4.8 |
| Crude protein | 46.7 | 46.5 | 46.2 | 46.1 | 45.1 |
| Crude fat | 18.8 | 17.6 | 17.4 | 17.5 | 20.2 |
| Ash | 11.1 | 11.2 | 11.1 | 11.0 | 11.0 |
| NFE + crude fiber 5 | 20.1 | 21.4 | 21.3 | 21.7 | 19.0 |
| Fatty acid composition (% on a dried basis) | |||||
| C12:0 | 0.61 | 2.68 | 2.74 | 2.79 | 2.85 |
| C16:0 | 2.85 | 2.73 | 2.95 | 3.18 | 3.40 |
| C18:1 | 4.21 | 3.60 | 4.39 | 5.18 | 5.97 |
| C22:6 | 2.35 | 2.35 | 2.35 | 2.35 | 2.35 |
| Ingredients | Control | Control+L | 10PD | 20PD | 10CD | 20CD |
|---|---|---|---|---|---|---|
| Fish meal (65% CP) 1 | 64.00 | 64.00 | 57.18 | 50.36 | 57.18 | 50.36 |
| PDBM | - | - | 10.00 | 20.00 | - | - |
| CDBM | - | - | - | - | 8.02 | 16.03 |
| Fish oil | 8.00 | 8.00 | 8.74 | 9.49 | 8.74 | 9.49 |
| Palm kernel oil | - | 4.37 | 2.19 | - | 3.74 | 3.11 |
| Corn oil | 4.37 | - | - | - | - | - |
| Starch | 9.00 | 9.00 | 9.00 | 9.00 | 9.00 | 9.00 |
| Cellulose | 4.13 | 4.13 | 2.39 | 0.65 | 2.82 | 1.51 |
| Taurine | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin mix 2 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Mineral mix 3 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Choline chloride | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin C derivatives | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| NaH2PO4 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| KH2PO4 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Calcium lactate | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| CMC 4 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition (%) | ||||||
| Moisture | 4.3 | 4.6 | 4.0 | 4.0 | 3.8 | 4.2 |
| Crude protein | 44.8 | 44.7 | 46.5 | 46.1 | 45.9 | 44.4 |
| Crude fat | 19.4 | 19.1 | 19.8 | 19.7 | 19.1 | 18.6 |
| Ash | 2.1 | 2.0 | 2.2 | 2.1 | 2.0 | 1.9 |
| NFE + crude fiber 5 | 29.5 | 29.6 | 27.6 | 28.1 | 29.3 | 30.9 |
| Fatty acid composition (% on a dried basis) | ||||||
| C12:0 | 0.61 | 2.69 | 2.40 | 2.10 | 2.61 | 2.52 |
| C16:0 | 2.29 | 0.39 | 0.37 | 0.34 | 0.98 | 1.58 |
| C18:1 | 5.05 | 4.99 | 4.98 | 4.98 | 5.06 | 5.14 |
| C22:6 | 2.35 | 2.35 | 2.35 | 2.35 | 2.35 | 2.35 |
| Proximate Composition (% on a Dried Basis) | PDBM | CDBM | FM |
|---|---|---|---|
| Crude protein | 49.0 | 60.6 | 72.5 |
| Crude fat | 23.2 | 8.3 | 8.6 |
| Ash | 1.8 | 2.1 | 18.5 |
| Components (% of Total AAs) | PDBM | FM |
|---|---|---|
| Ala | 8.6 | 7.0 |
| Arg | 4.7 | 6.5 |
| Asp | 9.1 | 9.5 |
| Cys | 1.0 | 1.0 |
| Glu | 13.0 | 13.2 |
| Gly | 6.0 | 7.7 |
| His | 2.7 | 3.3 |
| Ile | 4.6 | 4.3 |
| Leu | 7.4 | 8.2 |
| Lys | 6.5 | 8.3 |
| Met | 1.8 | 3.1 |
| Phe | 4.4 | 4.3 |
| Pro | 6.4 | 5.0 |
| Ser | 4.4 | 4.3 |
| Thr | 4.2 | 4.6 |
| Trp | 1.6 | 1.3 |
| Tyr | 6.8 | 3.3 |
| Val | 6.7 | 5.2 |
| Components (% of Total FAs) | Oil from PDBM | Fish Oil |
|---|---|---|
| Saturated fatty acid | ||
| 10:0 | 0.8 | - |
| 12:0 | 29.2 | 4.1 |
| 14:0 | 7.6 | - |
| 15:0 | 0.1 | 0.5 |
| 16:0 | 14.0 | 15.6 |
| 17:0 | 0.1 | 0.7 |
| 18:0 | 2.7 | 3.6 |
| 20:0 | 0.2 | 0.3 |
| 22:0 | - | - |
| Total | 54.7 | 24.8 |
| Monounsaturated fatty acid | ||
| 14:1 | 0.1 | - |
| 16:1 | 1.2 | 5.0 |
| 17:1 | 0.1 | 0.5 |
| 18:1 | 40.2 | 19.4 |
| 20:1 | - | 5.2 |
| 22:1 | - | 5.0 |
| 24:1 | - | 0.6 |
| Total | 41.5 | 35.7 |
| Polyunsaturated fatty acid | ||
| ω-3 fatty acid | ||
| 16:3n-3 | 0.1 | - |
| 18:3n-3 | 3.5 | 1.0 |
| 20:3n-3 | - | 0.2 |
| 20:4n-3 | - | 0.7 |
| 20:5n-3 | - | 7.5 |
| 21:5n-3 | - | 0.3 |
| 22:5n-3 | - | 1.9 |
| 22:6n-3 | - | 15.7 |
| Total | 3.6 | 27.3 |
| ω-6 fatty acid | ||
| 18:2n-6 | - | 2.8 |
| 20:2n-6 | - | 0.3 |
| 20:3n-6 | - | 0.2 |
| 20:4n-6 | - | 1.1 |
| 22:5n-6 | - | 0.6 |
| Total | - | 5.0 |
| Others | ||
| 16:2 | - | 0.3 |
| 16:3 | - | 0.2 |
| 16:4 | - | 0.3 |
| Total | - | 0.8 |
| Not identified | 0.2 | 4.3 |
| Control | Control+L | 10PD | 20PD | 30PD | ||
|---|---|---|---|---|---|---|
| N | Initial | 60 | 60 | 60 | 60 | 60 |
| 3 wks | 60 | 60 | 59 | 60 | 60 | |
| 6 wks | 60 | 58 | 57 | 43 | 57 | |
| 12 wks | 59 | 58 | 54 | 41 | 56 | |
| FL (cm) | Initial | 6.6 ± 0.3 a | 6.6 ± 0.4 a | 6.6 ± 0.3 a | 6.5 ± 0.3 a | 6.6 ± 0.3 a |
| 3 wks | 10.0 ± 0.5 a | 9.2 ± 0.6 c | 9.5 ± 0.6 b | 8.8 ± 0.7 d | 8.3 ± 0.7 e | |
| 6 wks | 15.2 ± 1.1 a | 13.8 ± 1.0 a | 13.4 ± 1.0 a | 12.5 ± 1.0 b | 11.6 ± 1.1 c | |
| 12 wks | 21.2 ± 2.1 a | 20.4 ± 1.5 b | 19.2 ± 1.9 c | 17.8 ± 1.8 d | 16.6 ± 1.7 e | |
| BW (g) | Initial | 2.9 ± 0.5 a | 2.9 ± 0.5 a | 3.0 ± 0.5 a | 2.8 ± 0.4 a | 3.1 ± 0.5 a |
| 3 wks | 11.8 ± 2.2 a | 9.0 ± 2.2 c | 10.0 ± 2.0 b | 7.7 ± 1.8 d | 6.4 ± 1.7 e | |
| 6 wks | 47.5 ± 10.3 a | 34.1 ± 8.2 a | 31.7 ± 7.7 a | 24.6 ± 6.5 b | 19.8 ± 6.0 c | |
| 12 wks | 132.8 ± 41.2 a | 116.9 ± 27.4 b | 98.3 ± 31.2 c | 73.9 ± 25.3 d | 59.0 ± 20.9 e | |
| Specific growth rate (% day−1) | 4.39 | 4.24 | 4.03 | 3.75 | 3.40 | |
| Feed conversion ratio | 0.99 | 0.96 | 1.00 | 1.23 | 1.14 | |
| Control | Control+L | 10PD | 20PD | 10CD | 20CD | ||
|---|---|---|---|---|---|---|---|
| N | Initial | 60 | 60 | 60 | 60 | 60 | 60 |
| 2.5 wks | 57 | 60 | 58 | 58 | 60 | 59 | |
| 5 wks | 52 | 59 | 58 | 57 | 59 | 59 | |
| FL (cm) | Initial | 7.6 ± 0.4 a | 7.7 ± 0.5 a | 7.8 ± 0.5 a | 7.7 ± 0.5 a | 7.8 ± 0.5 a | 7.8 ± 0.5 a |
| 2.5 wks | 11.4 ± 0.7 a, b | 11.6 ± 0.8 a | 11.2 ± 0.8 a, b | 10.7 ± 0.8 c | 11.1 ± 0.8 b, c | 11.2 ± 0.7 b | |
| 5 wks | 15.6 ± 0.9 a | 15.7 ± 0.9 a | 14.8 ± 1.1 b | 13.8 ± 1.1 c | 14.8 ± 1.0 b | 14.6 ± 0.9 b | |
| BW (g) | Initial | 5.0 ± 1.0 a | 5.4 ± 1.0 a | 5.4 ± 1.2 a | 5.2 ± 1.2 a | 5.4 ± 1.2 a | 5.4 ± 1.2 a |
| 2.5 wks | 18.2 ± 3.5 a, b | 18.7 ± 4.1 a | 16.8 ± 3.9 a, b | 14.6 ± 3.7 c | 16.7 ± 3.5 b, c | 17.0 ± 3.7 a,b | |
| 5 wks | 50.8 ± 9.8 a | 49.6 ± 10.1 a | 41.8 ± 10.6 b | 32.9 ± 8.3 c | 41.9 ± 9.8 b | 39.6 ± 8.8 b | |
| Specific growth rate (% day−1) | 6.61 | 6.32 | 5.85 | 5.25 | 5.85 | 5.69 | |
| Feed conversion ratio | 1.06 | 1.15 | 1.07 | 0.97 | 1.10 | 1.09 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ido, A.; Ali, M.-F.-Z.; Takahashi, T.; Miura, C.; Miura, T. Growth of Yellowtail (Seriola quinqueradiata) Fed on a Diet Including Partially or Completely Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal. Insects 2021, 12, 722. https://doi.org/10.3390/insects12080722
Ido A, Ali M-F-Z, Takahashi T, Miura C, Miura T. Growth of Yellowtail (Seriola quinqueradiata) Fed on a Diet Including Partially or Completely Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal. Insects. 2021; 12(8):722. https://doi.org/10.3390/insects12080722
Chicago/Turabian StyleIdo, Atsushi, Muhammad-Fariz-Zahir Ali, Takayuki Takahashi, Chiemi Miura, and Takeshi Miura. 2021. "Growth of Yellowtail (Seriola quinqueradiata) Fed on a Diet Including Partially or Completely Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal" Insects 12, no. 8: 722. https://doi.org/10.3390/insects12080722
APA StyleIdo, A., Ali, M.-F.-Z., Takahashi, T., Miura, C., & Miura, T. (2021). Growth of Yellowtail (Seriola quinqueradiata) Fed on a Diet Including Partially or Completely Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal. Insects, 12(8), 722. https://doi.org/10.3390/insects12080722







