Biology of an Adventive Population of the Armored Scale Rhizaspidiotus donacis, a Biological Control Agent of Arundo donax in California
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
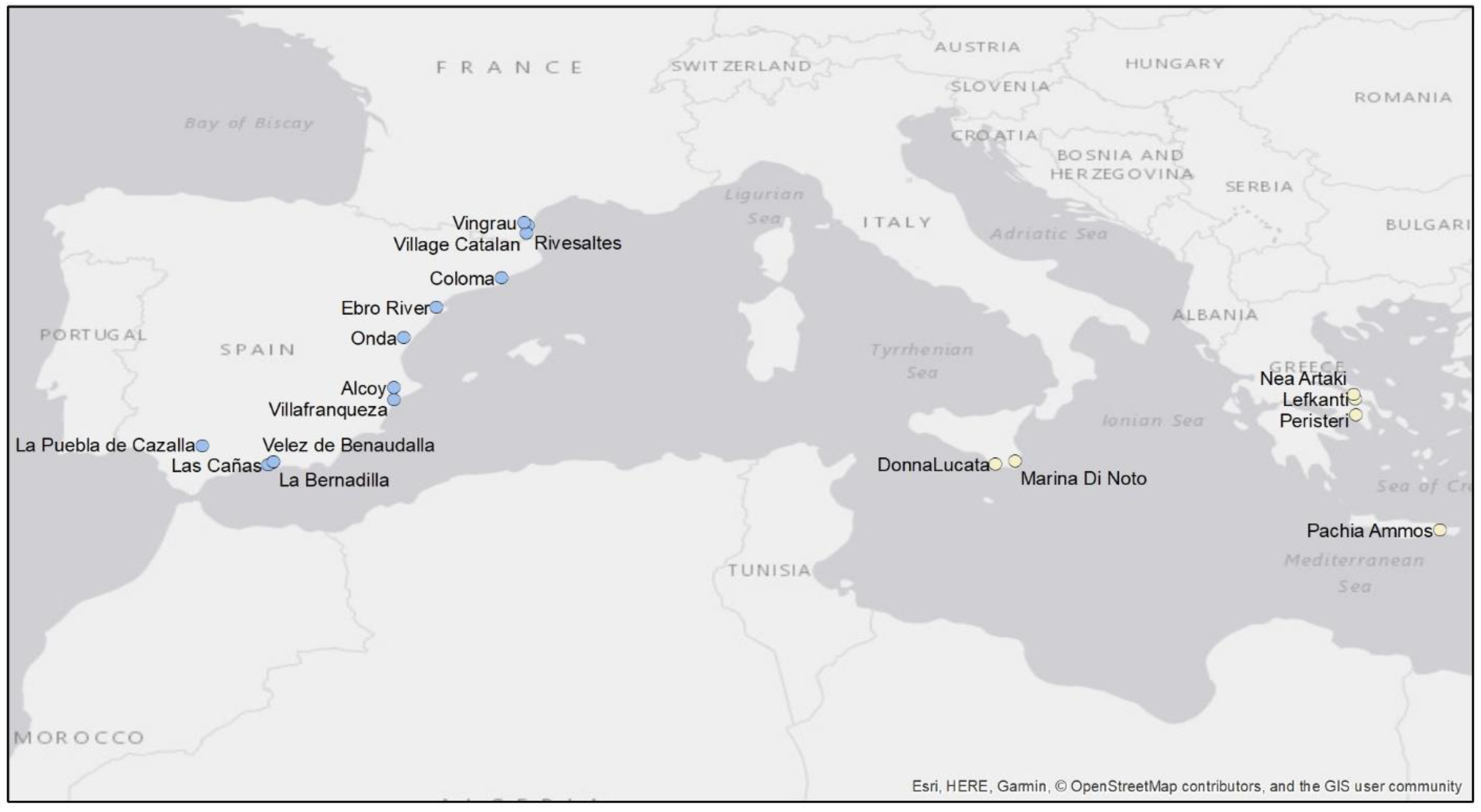
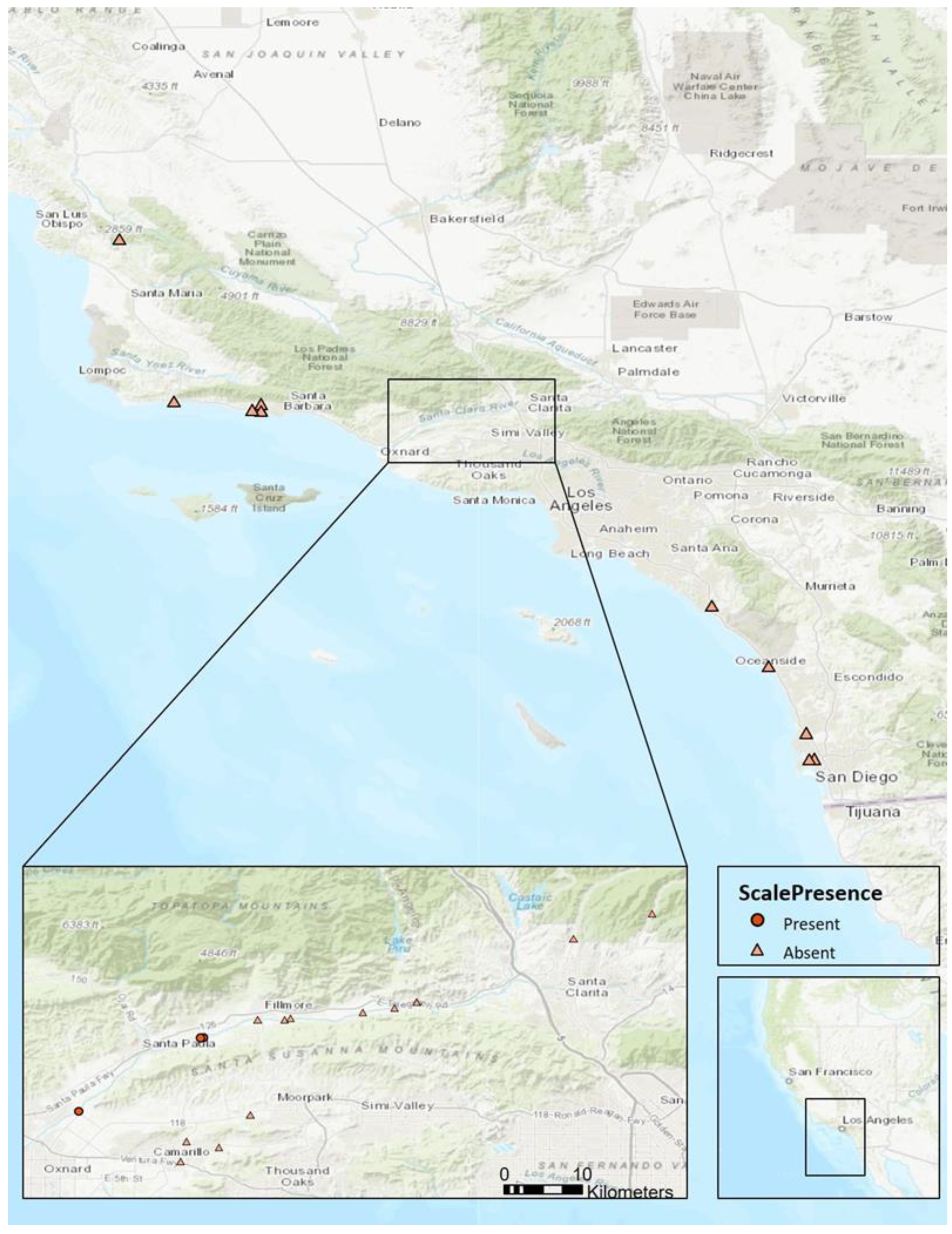
2.1. Distribution
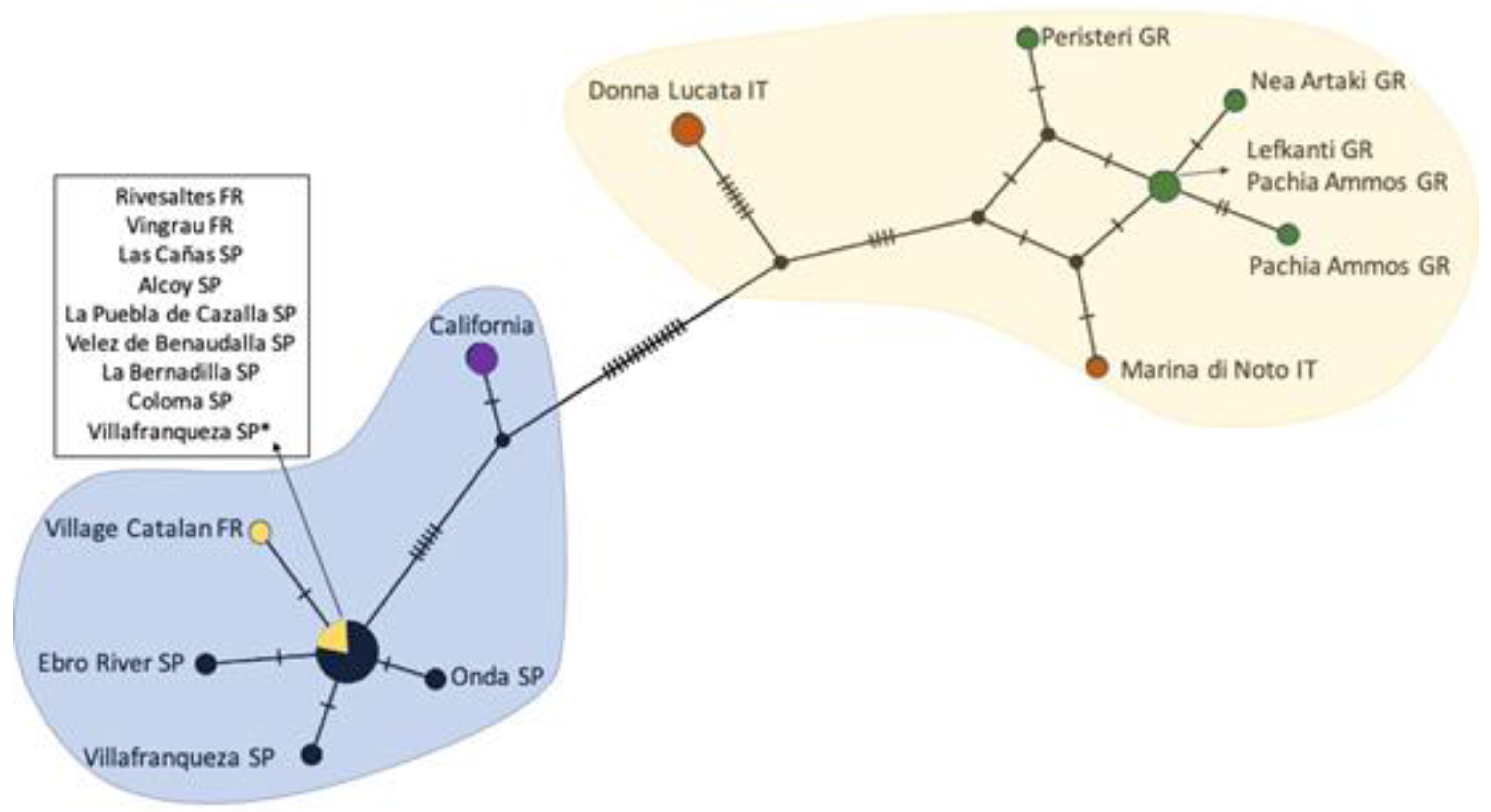
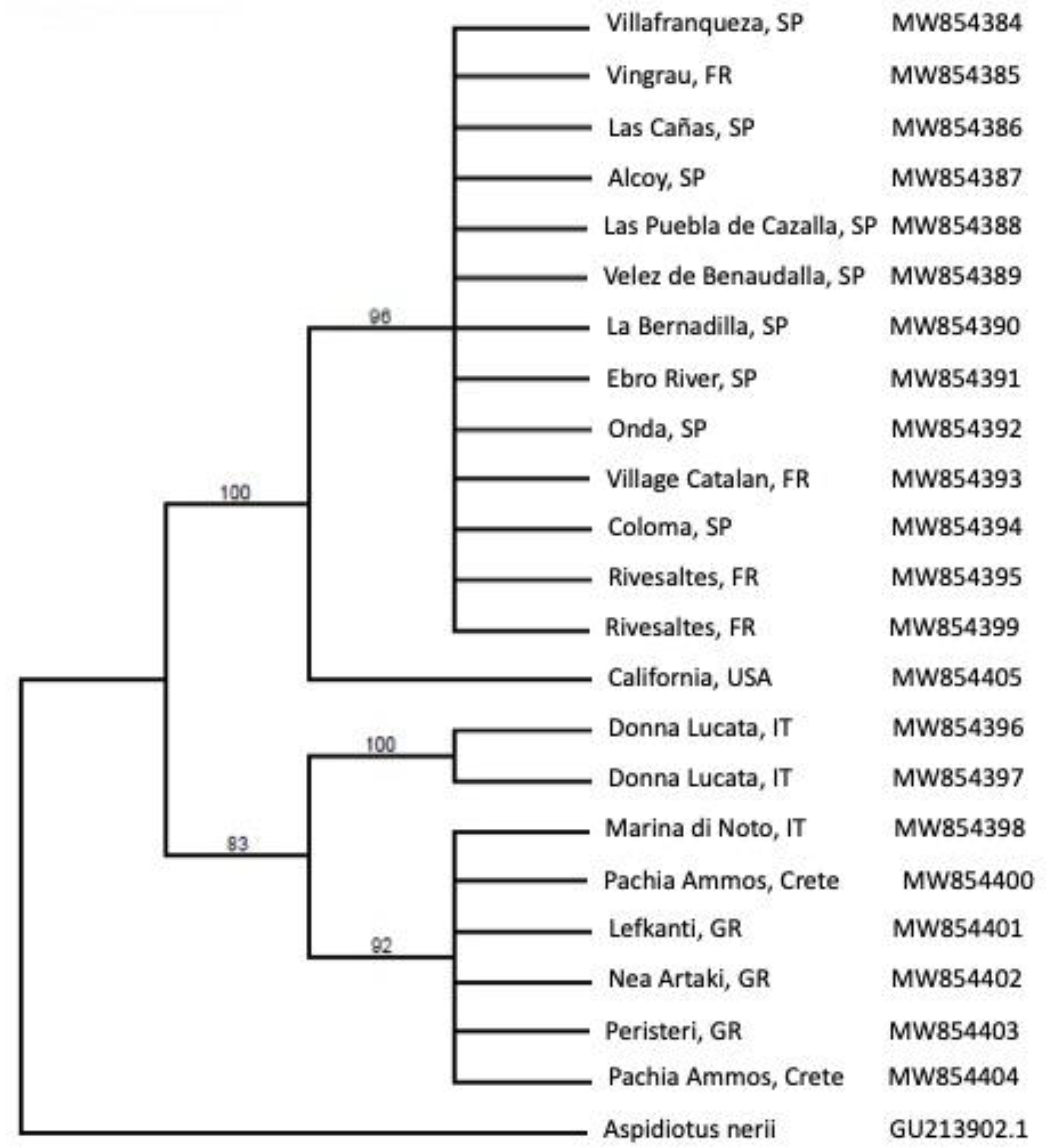
2.2. Rhizaspidiotis Donacis Genetics
2.3. Adventive Rhizaspidiotis Donacis Phenology
2.4. Rhizaspidiotis Donacis Host Range
2.5. Aphytis Melinus Parasitism
3. Results
3.1. Distribution
3.2. Rhizaspidiotis Donacis Genetics
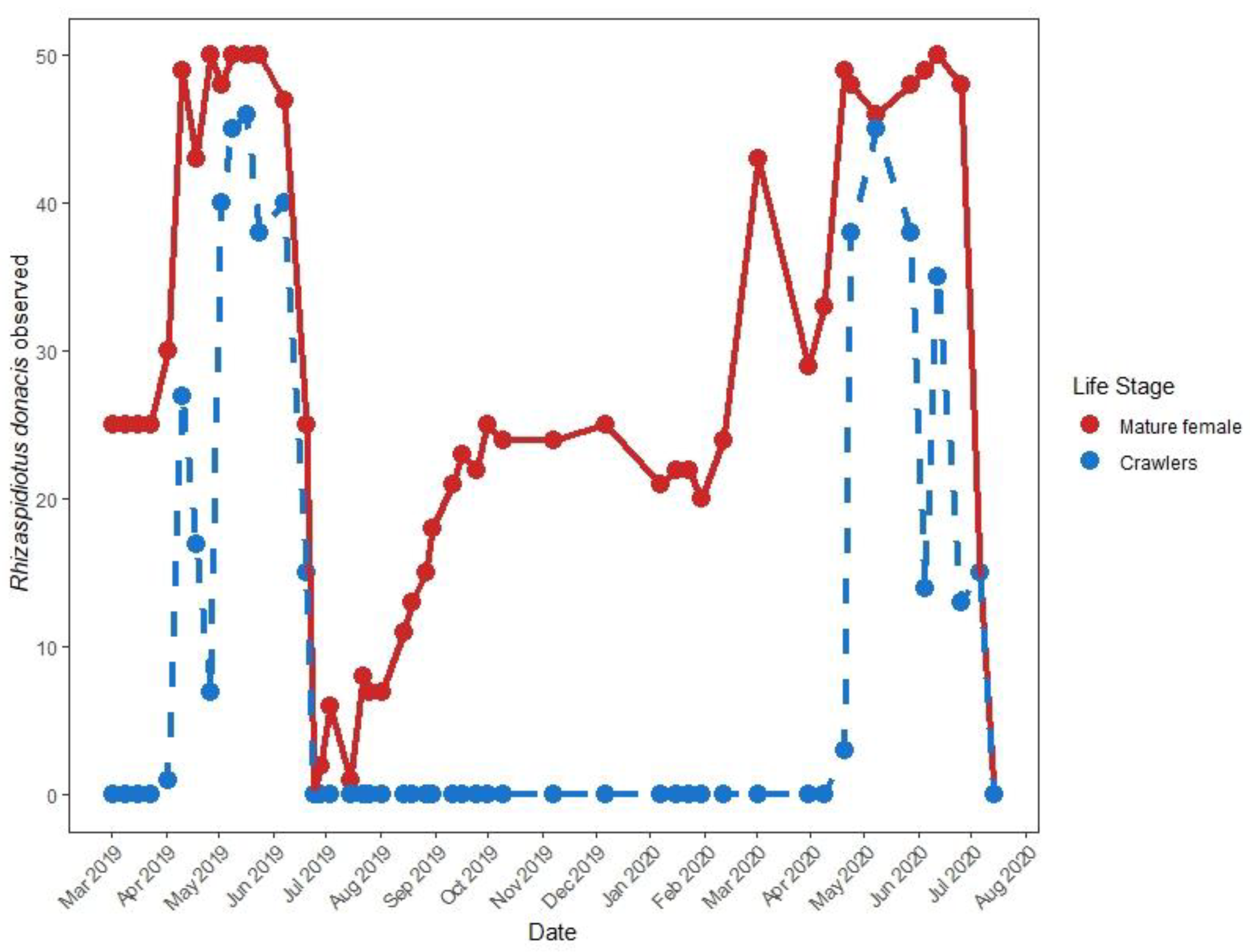
3.3. Adventive Scale Phenology
3.4. Host Range Trials
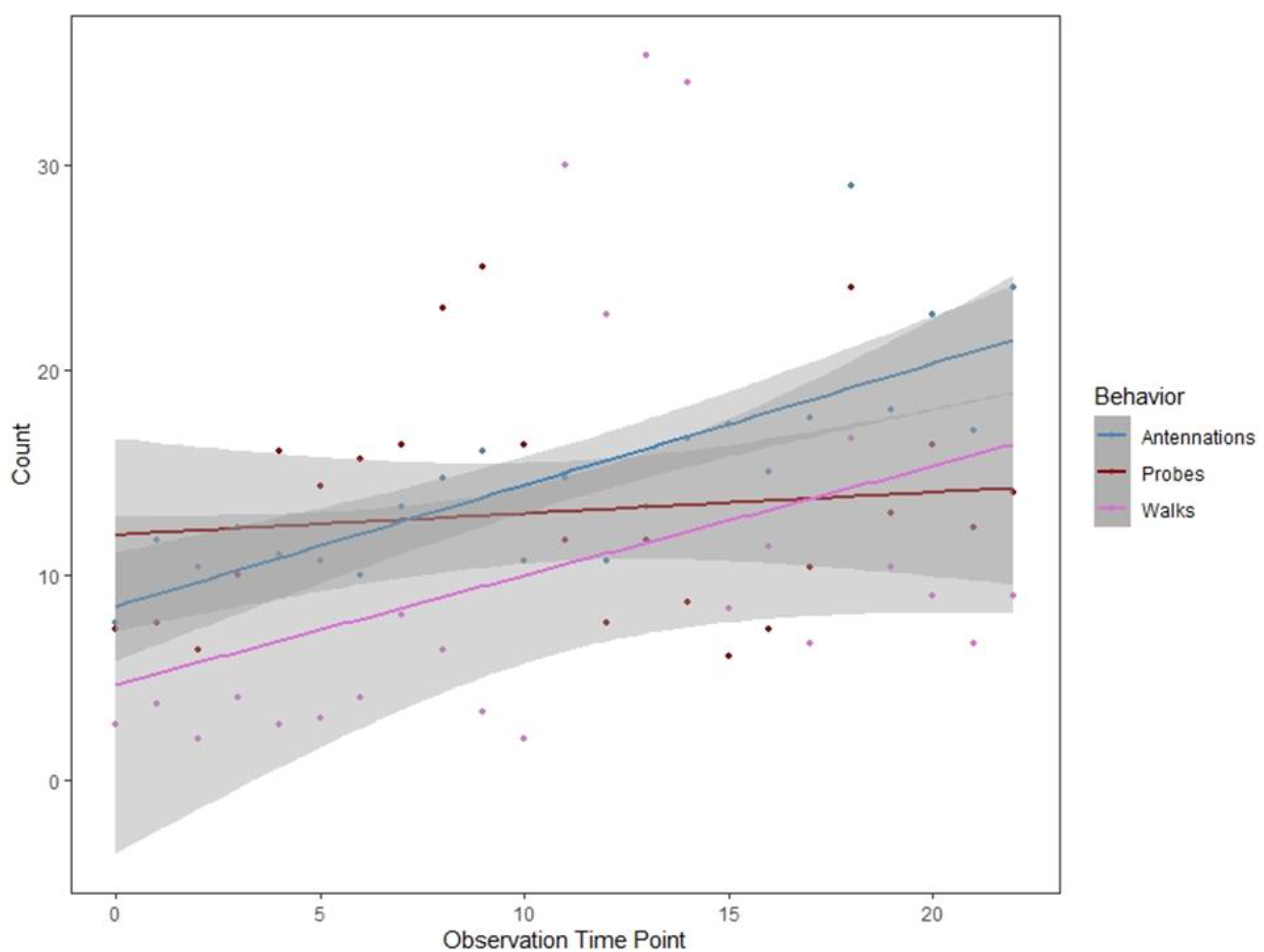
3.5. Aphytis Melinus Parasitism
4. Discussion
4.1. Distribution
4.2. Genetics
4.3. Phenology
4.4. Host Range
4.5. Aphytis Parasitoids
4.6. Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardion, L.; Verlaque, R.; Saltonstall, K.; Leriche, A.; Vila, B. Origin of the invasive Arundo donax (Poaceae): A trans-Asian expedition in herbaria. Ann. Bot. 2014, 114, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000; Volume 12. [Google Scholar]
- Dudley, T.L. Noxious wildland weeds of California: Arundo donax. In Invasive plants of California’s Wildlands; Bossard, C., Randall, J., Hoshovsky, M., Eds.; University of California Press: Berkeley, CA, USA, 2000. [Google Scholar]
- Lambert, A.M.; Dudley, T.L.; Saltonstall, K. Ecology and impacts of the large-statured invasive grasses Arundo donax and Phragmites australis in North America. Invasive Plant Sci. Manag. 2010, 3, 489–494. [Google Scholar] [CrossRef]
- Watts, D.A.; Moore, G.W. Water-use dynamics of an invasive reed, Arundo donax, from leaf to stand. Wetlands 2011, 31, 725–734. [Google Scholar] [CrossRef]
- Giessow, J.; Casanova, J.; Leclerc, R.; MacArthur, R.; Fleming, G.; Giessow, J. Arundo Donax (Giant Reed): Distribution and Impact Report. State Water Resources Control Board; California Invasive Plant Council (Cal-IPC): Richmond, CA, USA, 2011; p. 240. [Google Scholar]
- Coffman, G.; Ambrose, R.; Rundel, P. Wildfire promotes dominance of invasive giant reed (Arundo donax) in riparian ecosystems. Biol. Invasions 2010, 12, 2723–2734. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.M.; Dudley, T.; D’Antonio, C.M. Invasive species and fire in California. Fremontia 2010, 38, 29–36. [Google Scholar]
- Stover, J.E.; Keller, E.A.; Dudley, T.L.; Langendoen, E.J. Fluvial geomorphology, root distribution, and tensile strength of the invasive giant reed, Arundo donax and its role on stream bank stability in the Santa Clara River, Southern California. Geosciences 2018, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Maceda-Veiga, A.; Basas, H.; Lanzaco, G.; Sala, M.; de Sostoa, A.; Serra, A. Impacts of the invader giant reed (Arundo donax) on riparian habitats and ground arthropod communities. Biol. Invasions 2016, 18, 731–749. [Google Scholar] [CrossRef]
- Kisner, D.A. The Effect of Giant Reed (Arundo donax) on the Southern California Riparian Bird Community. Master’s Thesis, San Diego State University, San Diego, CA, USA, 2004. [Google Scholar]
- Hardesty-Moore, M.; Orr, D.; McCauley, D.J. Invasive plant Arundo donax alters habitat use by carnivores. Biol. Invasions 2020, 22, 1983–1985. [Google Scholar] [CrossRef]
- Bruno, D.; Zapata, V.; Guareschi, S.; Picazo, F.; Dettori, E.; Carbonell, J.A.; Millán, A.; Velasco, J.; Robledano, F. Short-term responses of aquatic and terrestrial biodiversity to riparian restoration measures designed to control the invasive Arundo donax L. Water 2019, 11, 2551. [Google Scholar] [CrossRef] [Green Version]
- DiTomaso, J.M.; Kyser, G.; Oneto, S.; Wilson, R.; Orloff, S.; Anderson, L.; Wright, S.; Roncoroni, J.; Miller, T.; Prather, T. Weed control in natural areas in the western United States. Weed Res. Inf. Cent. Univ. Calif. 2013, 544. [Google Scholar]
- Seawright, E.K.; Rister, M.E.; Lacewell, R.D.; McCorkle, D.A.; Sturdivant, A.W.; Yang, C.; Goolsby, J.A. Economic implications for the biological control of Arundo donax: Rio Grande Basin. Southwest. Entomol. 2009, 34, 377–394. [Google Scholar] [CrossRef]
- Tracy, J.L.; Deloach, C.J. Suitability of classical biological control of giant reed (Arundo donax) in the United States. In Proceedings of the Arundo and Saltcedar Workshop, Holtville, CA, USA, 17 June 1998; pp. 73–109. [Google Scholar]
- Goolsby, J.A.; Spencer, D.; Whitehand, L. Pre-release assessment of impact on Arundo donax by the candidate biological control agents Tetramesa romana (Hymenoptera: Eurytomidae) and Rhizaspidiotus donacis (Hemiptera: Diaspididae) under quarantine conditions. Southwest. Entomol. 2009, 34, 359–376. [Google Scholar] [CrossRef]
- Dudley, T.L.; Lambert, A.; Kirk, A. Augmentation biological control of Arundo donax. I. Citrus and Biological Control. Yesterday, Today, Tomorrow. In Proceedings of the CCBC V 2006, Riverside, CA, USA, 25–27 July 2006. [Google Scholar]
- Moran, P.J.; Vacek, A.T.; Racelis, A.E.; Pratt, P.D.; Goolsby, J.A. Impact of the arundo wasp, Tetramesa romana (Hymenoptera: Eurytomidae), on biomass of the invasive weed, Arundo donax (Poaceae: Arundinoideae), and on revegetation of riparian habitat along the Rio Grande in Texas. Biocontrol Sci. Technol. 2017, 27, 96–114. [Google Scholar] [CrossRef]
- Moran, P.J.; Goolsby, J.A. Biology of the galling wasp Tetramesa romana, a biological control agent of giant reed. Biol. Control 2009, 49, 169–179. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, J.; Hardion, L.; Del Monte, J.P.; Vila, B.; Santín-Montanyá, M.I. Monographs on invasive plants in Europe N°4: Arundo donax L. Bot. Lett. 2021, 161, 131–151. [Google Scholar] [CrossRef]
- Goolsby, J.; Vacek, A.; Salinas, C.; Racelis, A.; Moran, P.; Kirk, A. Host range of the European leaf sheath mining midge, Lasioptera donacis Coutin (Diptera: Cecidomyiidae), a biological control of giant reed, Arundo donax L. Biocontrol Sci. Technol. 2017, 27, 781–795. [Google Scholar] [CrossRef]
- Escobar, Y.; Guermache, F.; Bon, M.-C.; Kerdellant, E.; Petoux, L.; Desurmont, G.A. Biology, ecology, and impact of Cryptonevra nigritarsis Duda, a potential biological control agent against the giant reed Arundo donax. Biol. Control 2020, 147, 104287. [Google Scholar] [CrossRef]
- Widmer, T.; Kirk, A. Nigrospora oryzae associated with shoot tip death of Arundo donax. In Proceedings of the 13th EWRS Symposium, Bari, Italy, 19–23 June 2005. [Google Scholar]
- Dudley, T.L.; Lambert, A.M.; Kirk, A.; Tamagawa, Y. Herbivores of Arundo donax in California. In Proceedings of the XII International Symposium on Biological Control of Weeds; Julien, M.H., Sforza, R., Bon, M.C., Evans, H.C., Hatcher, P.E., Hinz, H.L., Rector, B.G., Eds.; CAB International: Wallingford, UK, 2008; pp. 146–152. [Google Scholar]
- Undurraga, N.; Araya, J.E.; Zuazúa, F.; Alonso, M.F. Population dynamics of Melanaphis donacis (Hemiptera: Aphididae) and its Coccinellidae and Syrphidae predators on Arundo donax L. Int. J. Agric. Nat. Resour. 2020, 47, 117–125. [Google Scholar] [CrossRef]
- Racelis, A.E.; Goolsby, J.A.; Moran, P. Seasonality and movement of adventive populations of the Arundo wasp (Hymenoptera: Eurytomidae), a biological control agent of giant reed in the Lower Rio Grande Basin in South Texas. Southwest. Entomol. 2009, 34, 347–357. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Moran, P.; Falk, J.; Gilbert, L. Distribution and spread of an adventive population of the biological control agent Tetramesa romana in Austin, Texas. Southwest. Entomol. 2009, 34, 329–330. [Google Scholar] [CrossRef]
- Canavan, K.; Paterson, I.; Hill, M.; Dudley, T. Testing the Enemy Release Hypothesis on tall-statured grasses in South Africa, using Arundo donax, Phragmites australis, and Phragmites mauritianus as models. Bull. Entomol. Res. 2019, 109, 287–299. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Moran, P.J.; Racelis, A.E.; Summy, K.R.; Jimenez, M.M.; Lacewell, R.D.; Perez de Leon, A.; Kirk, A.A. Impact of the biological control agent Tetramesa romana (Hymenoptera: Eurytomidae) on Arundo donax (Poaceae: Arundinoideae) along the Rio Grande River in Texas. Biocontrol Sci. Technol. 2016, 26, 47–60. [Google Scholar] [CrossRef]
- Dudley, T.L.; Lambert, A.M. Biological Control of Invasive Giant Reed (Arundo donax); Report to the U.S. Fish & wildlife Service/Santa Clara River Trustee Council, 31 December 2007; Marine Science Institute: Redwood City, CA, USA; University of California: Santa Barbara, CA, USA, 2007. [Google Scholar]
- Marshall, M.; Goolsby, J.; Vacek, A.; Moran, P.; Kirk, A.; Mendoza, E.C.; Cristofaro, M.; Bownes, A.; Mastoras, A.; Kashefi, J. Densities of the arundo wasp, Tetramesa romana (Hymenoptera: Eurytomidae) across its native range in Mediterranean Europe and introduced ranges in North America and Africa. Biocontrol Sci. Technol. 2018, 28, 772–785. [Google Scholar] [CrossRef]
- Rosen, D.; DeBach, P. Species of Aphytis of the World: Hymenoptera: Aphelinidae; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1979; Volume 17. [Google Scholar]
- Moran, P.; Goolsby, J.A. Biology of the armored scale Rhizaspidiotus donacis (Hemiptera: Diaspididae), a candidate agent for biological control of giant reed. Ann. Entomol. Soc. Am. 2010, 103, 252–263. [Google Scholar] [CrossRef]
- Goolsby, J.; Cortés Mendoza, E.; Moran, P.; Adamczyk, J.; García, M.Á.M.; Kirk, A. Evaluation of Spanish arundo scale Rhizaspidiotus donacis (Hemiptera; Diaspididae) survival and fecundity on three new world genotypes of Arundo donax (Poaceae; Arundinoideae). Biocontrol Sci. Technol. 2013, 23, 499–506. [Google Scholar] [CrossRef]
- Villarreal, J.M.; Goolsby, J.A.; Vacek, A.T.; de Lon, A.A.P.; Racelis, A.E. Horticultural technique for rearing and redistribution of the sessile biological control agent, Rhizaspidiotus donacis on its host plant, Arundo donax. Subtrop. Agric. Environ. 2016, 67, 19–23. [Google Scholar]
- Provencher, L.M.; Morse, G.E.; Weeks, A.R.; Normark, B.B. Parthenogenesis in the Aspidiotus nerii complex (Hemiptera:Diaspididae): A single origin of a worldwide, polyphagus lineage associated with Cardinium bacteria. Ann. Entomol. Soc. Am. 2005, 98, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Simon, C.; Frati, F.; Beckenback, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Swofford, D.L.; Sullivan, J. Phylogeny inference based on parsimony and other methods using PAUP*. In The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny Cáp; Cambridge University Press: Cambridge, UK, 2003; Volume 7, pp. 160–206. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saltonstall, K. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc. Natl. Acad. Sci. USA 2002, 99, 2445–2449. [Google Scholar] [CrossRef] [Green Version]
- Saltonstall, K.; Lambert, A.M.; Rice, N. What happens in Vegas, better stay in Vegas: Phragmites australis hybrids in the Las Vegas Wash. Biol. Invasions 2016, 18, 2463–2474. [Google Scholar] [CrossRef]
- Lambert, A.M.; Saltonstall, K.; Long, R.; Dudley, T.L. Biogeography of Phragmites australis lineages in the southwestern United States. Biol. Invasions 2016, 18, 2597–2617. [Google Scholar] [CrossRef]
- Goolsby, J.; Moran, P.; Adamczyk, J.; Kirk, A.; Jones, W.; Marcos, M.; Cortés, E. Host range of the European, rhizome-stem feeding scale Rhizaspidiotus donacis (Hemiptera: Diaspididae), a candidate biological control agent for giant reed, Arundo donax (Poales: Poaceae) in North America. Biocontrol Sci. Technol. 2009, 19, 899–918. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Rosenheim, J.A.; Kattari, D. Adult feeding and lifetime reproductive success in the parasitoid Aphytis melinus. Entomol. Exp. Appl. 1997, 83, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Luck, R.; Podoler, H.; Kfir, R. Host selection and egg allocation behaviour by Aphytis melinus and A. lingnanensis: Comparison of two facultatively gregarious parasitoids. Ecol. Entomol. 1982, 7, 397–408. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Saltonstall, K.; Hauber, D. Notes on Phragmites australis (Poaceae: Arundinoideae) in North America. J. Bot. Res. Institue Tex. 2007, 1, 385–388. [Google Scholar]
- Goolsby, J.A.; Kirk, A.A.; Moran, P.J.; Racelis, A.E.; Adamczyk, J.J.; Cortés, E.; García, M.Á.M.; Jimenez, M.M.; Summy, K.R.; Ciomperlik, M.A. Establishment of the armored scale, Rhizaspidiotus donacis 1, a biological control agent of Arundo donax. Southwest. Entomol. 2011, 36, 373–374. [Google Scholar] [CrossRef]
- Goolsby, J.; Moran, P. Field impact of the arundo scale, Rhizaspidiotus donacis (Homoptera: Diaspididae) on Arundo donax on the Rio Grande. Subtrop. Agric. Environ. 2019, 70, 11–16. [Google Scholar]
- Goolsby, J.; Hathcock, C.; Vacek, A.; Kariyat, R.; Moran, P.; Martinez Jimenez, M. No evidence of non-target use of native or economic grasses and broadleaf plants by Arundo donax biological control agents. Biocontrol. Sci. Technol. 2020, 30, 795–805. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Racelis, A.E.; Goolsby, J.B.; Kirk, A.A.; Cristofaro, M.; Grusak, M.A.; de Leon, A.P. Evaluation of biogeographical factors in the native range to improve the success of biological control agents in the introduced range. Biocontrol Sci. Technol. 2013, 23, 1213–1230. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Asplen, M.K. A ‘Goldilocks’ hypothesis for dispersal of biological control agents. BioControl 2011, 56, 441–450. [Google Scholar] [CrossRef]
- Perdue, R.E. Arundo donax—source of musical reeds and industrial cellulose. Econ. Bot. 1958, 12, 368–404. [Google Scholar] [CrossRef]
- Szűcs, M.; Eigenbrode, S.D.; Schwarzländer, M.; Schaffner, U. Hybrid vigor in the biological control agent, Longitarsus jacobaeae. Evol. Appl. 2012, 5, 489–497. [Google Scholar] [CrossRef]
- Szűcs, M.; Salerno, P.E.; Teller, B.J.; Schaffner, U.; Littlefield, J.L.; Hufbauer, R.A. The effects of agent hybridization on the efficacy of biological control of tansy ragwort at high elevations. Evol. Appl. 2019, 12, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Cortes, E.; Goolsby, J.A.; Moran, P.J.; Marcos-Garcia, M.A. The effect of the armored scale, Rhizaspidiotus donacis (Hemiptera: Diaspididae), on shoot growth of the invasive plant Arundo donax (Poaceae: Arundinoideae). Biocontrol Sci. Technol. 2011, 21, 535–545. [Google Scholar] [CrossRef]
- González-Zamora, J.E.; Castillo, M.L.; Avilla, C. Assessment of life history parameters of Aspidiotus nerii (Hemiptera: Diaspididae) to improve the mass rearing of Aphytis melinus (Hymenoptera: Aphelinidae). Biocontrol Sci. Technol. 2012, 22, 791–801. [Google Scholar] [CrossRef]
- Baffoe, K.O.; Dalin, P.; Nordlander, G.; Stenberg, J.A. Importance of temperature for the performance and biocontrol efficiency of the parasitoid Perilitus brevicollis (Hymenoptera: Braconidae) on Salix. BioControl 2012, 57, 611–618. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Lambert, A.M.; Saltonstall, K. A tale of three lineages: Expansion of common reed (Phragmites australis) in the U.S. Southwest and gulf coast. Invasive Plant Sci. Manag. 2010, 3, 515–520. [Google Scholar] [CrossRef]
- Blossey, B.; Endriss, S.B.; Casagrande, R.; Häfliger, P.; Hinz, H.; Dávalos, A.; Brown-Lima, C.; Tewksbury, L.; Bourchier, R.S. When misconceptions impede best practices: Evidence supports biological control of invasive Phragmites. Biol. Invasions 2020, 22, 873–883. [Google Scholar] [CrossRef] [Green Version]
- Suckling, D.M.; Sforza, R.F.H. What magnitude are observed non-target impacts from weed biocontrol? PLoS ONE 2014, 9, e84847. [Google Scholar] [CrossRef] [Green Version]
- Hinz, H.L.; Winston, R.L.; Schwarzländer, M. A global review of target impact and direct nontarget effects of classical weed biological control. Curr. Opin. Insect Sci. 2020, 38, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.G.; Zhou, Q.S.; Yu, F.; Wang, X.B.; Wei, J.F.; Zhu, C.D.; Zhang, Y.Z.; Vogler, A.P. Host specificity of parasitoids (Encyrtidae) toward armored scale insects (Diaspididae): Untangling the effect of cryptic species on quantitative food webs. Ecol. Evol. 2018, 8, 7879–7893. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Mills, N.J. Single vs. multiple introduction in biological control: The roles of parasitoid efficiency, antagonism and niche overlap. J. Appl. Ecol. 2004, 41, 973–984. [Google Scholar] [CrossRef]
- Milbrath, L.R.; Nechols, J.R. Plant-mediated interactions: Considerations for agent selection in weed biological control programs. Biol. Control 2014, 72, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Malecki, R.A.; Blossey, B.; Hight, S.D.; Schroeder, D.; Kok, L.T.; Coulson, J.R. Biological control of purple loosestrife. BioScience 1993, 43, 680–686. [Google Scholar] [CrossRef]
- Barratt, B.; Moran, V.; Bigler, F.; Van Lenteren, J. The status of biological control and recommendations for improving uptake for the future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Frick, K.; Chandler, J. Augmenting the moth (Bactra verutana) in field plots for early-season suppression of purple nutsedge (Cyperus rotundus). Weed Sci. 1978, 703–710. [Google Scholar] [CrossRef]
- Orr, D. Biological control and integrated pest management. In Integrated Pest Management: Innovation-Development Process; Springer: Berlin/Heidelberg, Germany, 2009; pp. 207–239. [Google Scholar]
- Sun, Y.; Ding, J.; Siemann, E.; Keller, S.R. Biocontrol of invasive weeds under climate change: Progress, challenges and management implications. Curr. Opin. Insect Sci. 2020, 38, 72–78. [Google Scholar] [CrossRef]
- Swope, S.M.; Parker, I.M. Complex interactions among biocontrol agents, pollinators, and an invasive weed: A structural equation modeling approach. Ecol. Appl. 2012, 22, 2122–2134. [Google Scholar] [CrossRef] [Green Version]
- Caesar, A. Synergistic interaction of soilborne plant pathogens and root-attacking insects in classical biological control of an exotic rangeland weed. Biol. Control 2003, 28, 144–153. [Google Scholar] [CrossRef]
- Buccellato, L.; Byrne, M.J.; Fisher, J.T.; Witkowski, E.T. Post-release evaluation of a combination of biocontrol agents on Crofton weed: Testing extrapolation of greenhouse results to field conditions. BioControl 2019, 64, 457–468. [Google Scholar] [CrossRef]

| Location | City | Latitude | Longitude |
|---|---|---|---|
| Little Falls Creek | San Luis Obispo | 35.24578 | −120.48773 |
| Bouquet Canyon | Valencia | 34.51580 | −118.44873 |
| Whispering Pines | Valencia | 34.48612 | −118.54285 |
| Refugio Creek | Goleta | 34.47301 | −120.06892 |
| Gaviota Beach | Gaviota | 34.47134 | −120.22722 |
| San Jose Creek | Santa Barbara | 34.45812 | −119.80940 |
| Santa Barbara Airport | Santa Barbara | 34.42988 | −119.85166 |
| Lower Maria Ygnacio Creek | Santa Barbara | 34.42648 | −119.80915 |
| Atascadero | Santa Barbara | 34.42525 | −119.81018 |
| Santa Clara River, Route 126 | Piru | 34.41005 | −118.73131 |
| Newhall Orchards | Newhall | 34.41000 | −118.73174 |
| Rancho Camulos | Piru | 34.40282 | −118.75805 |
| Santa Clara River | Piru | 34.39702 | −118.79628 |
| Sespe Cienega Site 2 | Fillmore | 34.39008 | −118.88324 |
| School Farm | Fillmore | 34.38875 | −118.92273 |
| Sespe Cienega | Fillmore | 34.38854 | −118.89019 |
| Balcom | Santa Paula | 34.36631 | −118.98730 |
| Taylor property | Santa Paula | 34.36560 | −118.99095 |
| Saticoy | Ventura | 34.27727 | −119.13735 |
| Calleguas Creek 3 | Somis | 34.27365 | −118.93174 |
| Calleguas Creek 2 | Somis | 34.24199 | −119.00829 |
| Arroyo Conejo | Camarillo | 34.23498 | −118.96936 |
| Calleguas Creek | Camarillo | 34.21831 | −119.01564 |
| San Juan Creek | San Juan Capistrano | 33.49504 | −117.65840 |
| Oceanside | Oceanside | 33.20625 | −117.38623 |
| Carroll Canyon | San Diego | 32.88784 | −117.20662 |
| Mission Valley | San Diego | 32.76488 | −117.16893 |
| Friars Road | San Diego | 32.76246 | −117.19450 |
| Species/Type | Lineage/Haplotype * | # of Plants Tested | # Infested 11 Weeks | Scale Density 11 Weeks | # Infested 19 Weeks | Avg Adult Scales/Plant 19 Weeks |
|---|---|---|---|---|---|---|
| Phragmites australis | Asian/P | 3 | 2 | Medium to High | 0 | 0 |
| P. australis subsp. berlandieri | Gulf Coast/I | 3 | 2 | Low | 0 | 0 |
| P. australis | native X introduced hybrid | 5 | 4 | Low to Medium | 3 | 1 |
| P. australis | Introduced/M | 11 | 10 | Low to High | 1 | 12 |
| P. australis subsp. americanus | Native/B, E, H, S | 22 | 18 | Low to High | 10 | 5.2 |
| Arundo donax | 2 | 2 | Medium to high | 2 | 104.5 | |
| A. formosana | 2 | 0 | None | 2 | 6.5 |
| Species/Type | Lineage/Haplotype | # of Plants Tested | # Infested with Adult Females after 26 Weeks | Total Adults Produced | Avg Adult Female Density per Plant 1 | # From Which Females Produced Crawlers | Total Crawlers Produced | Avg Crawler Production per Plant 1 |
|---|---|---|---|---|---|---|---|---|
| P. australis subsp. americanus | Native/B, E, H, S | 6 | 2 | 6 | 1 | 1 | 10 | 1.7 |
| P. australis subsp. americanus | Native/B, E, H, S | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| P. australis subsp. berlandieri | Gulf Coast/I | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| P. australis | Introduced/M | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arundo donax | 8 | 8 | 221 | 27.6 | 5 | 1010 | 126.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braman, C.A.; Lambert, A.M.; Özsoy, A.Z.; Hollstien, E.N.; Sheehy, K.A.; McKinnon, T.; Moran, P.; Gaskin, J.F.; Goolsby, J.A.; Dudley, T.L. Biology of an Adventive Population of the Armored Scale Rhizaspidiotus donacis, a Biological Control Agent of Arundo donax in California. Insects 2021, 12, 588. https://doi.org/10.3390/insects12070588
Braman CA, Lambert AM, Özsoy AZ, Hollstien EN, Sheehy KA, McKinnon T, Moran P, Gaskin JF, Goolsby JA, Dudley TL. Biology of an Adventive Population of the Armored Scale Rhizaspidiotus donacis, a Biological Control Agent of Arundo donax in California. Insects. 2021; 12(7):588. https://doi.org/10.3390/insects12070588
Chicago/Turabian StyleBraman, Charles A., Adam M. Lambert, A. Zeynep Özsoy, Ellen N. Hollstien, Kirsten A. Sheehy, Tara McKinnon, Patrick Moran, John F. Gaskin, John A. Goolsby, and Thomas L. Dudley. 2021. "Biology of an Adventive Population of the Armored Scale Rhizaspidiotus donacis, a Biological Control Agent of Arundo donax in California" Insects 12, no. 7: 588. https://doi.org/10.3390/insects12070588
APA StyleBraman, C. A., Lambert, A. M., Özsoy, A. Z., Hollstien, E. N., Sheehy, K. A., McKinnon, T., Moran, P., Gaskin, J. F., Goolsby, J. A., & Dudley, T. L. (2021). Biology of an Adventive Population of the Armored Scale Rhizaspidiotus donacis, a Biological Control Agent of Arundo donax in California. Insects, 12(7), 588. https://doi.org/10.3390/insects12070588






