Evaluation of the Impact of Eustenopus villosus on Centaurea solstitialis Seed Production in California
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Organisms
2.2. Study Sites
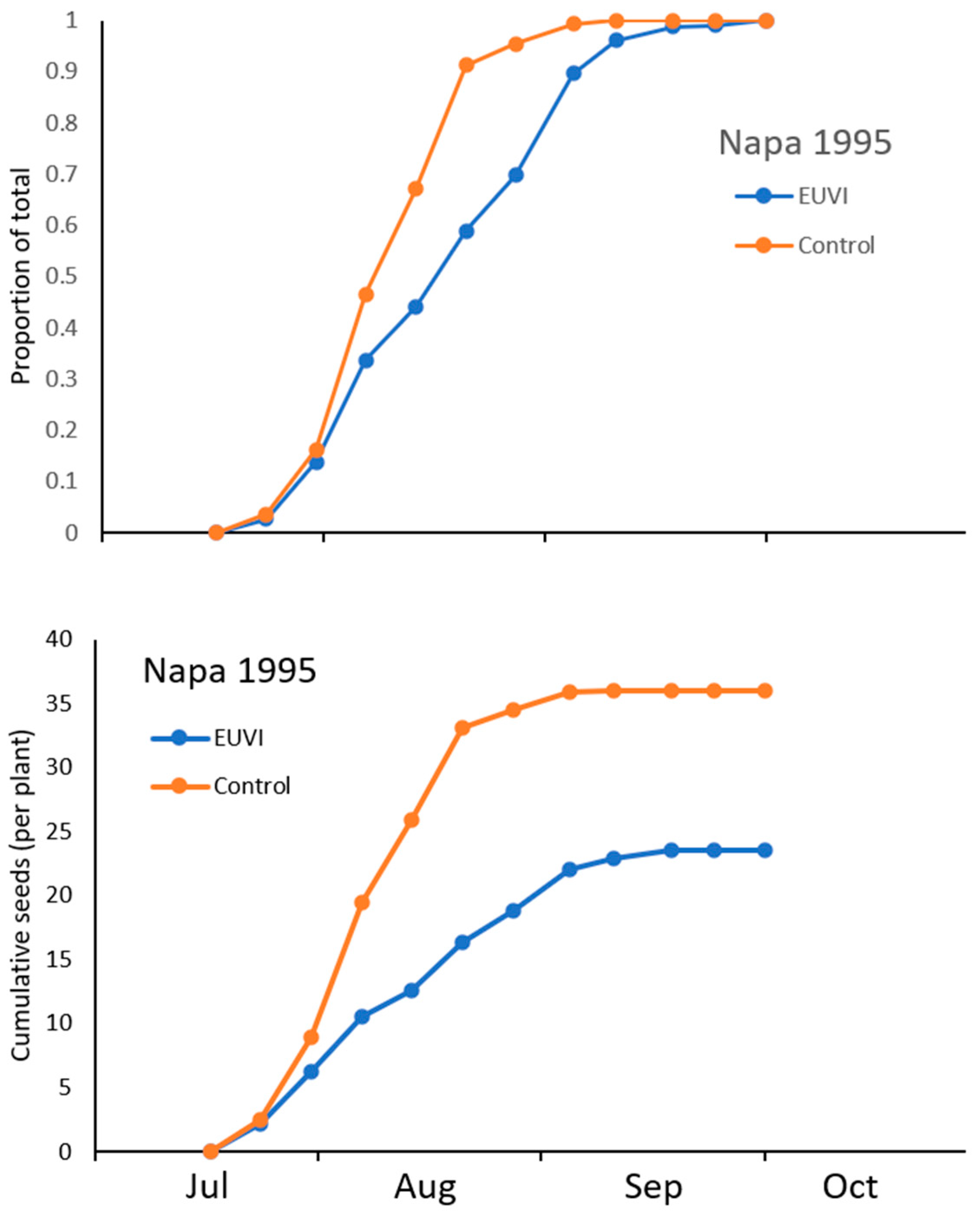
2.3. Phenology of Capitula Production and Seed Predation
2.4. Plant Density
2.5. Statistical Evaluations
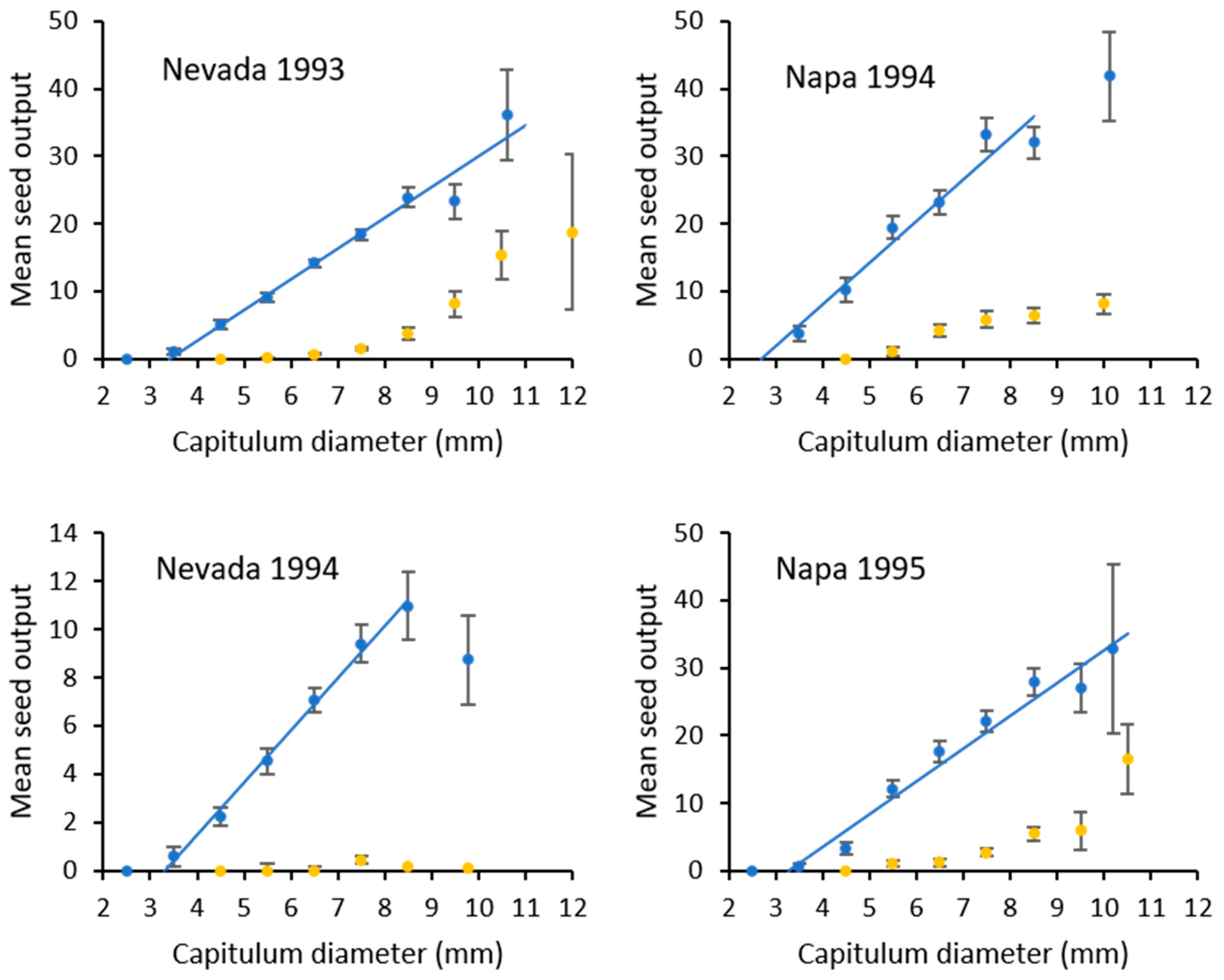
2.5.1. Seed Output
2.5.2. Seed Loss
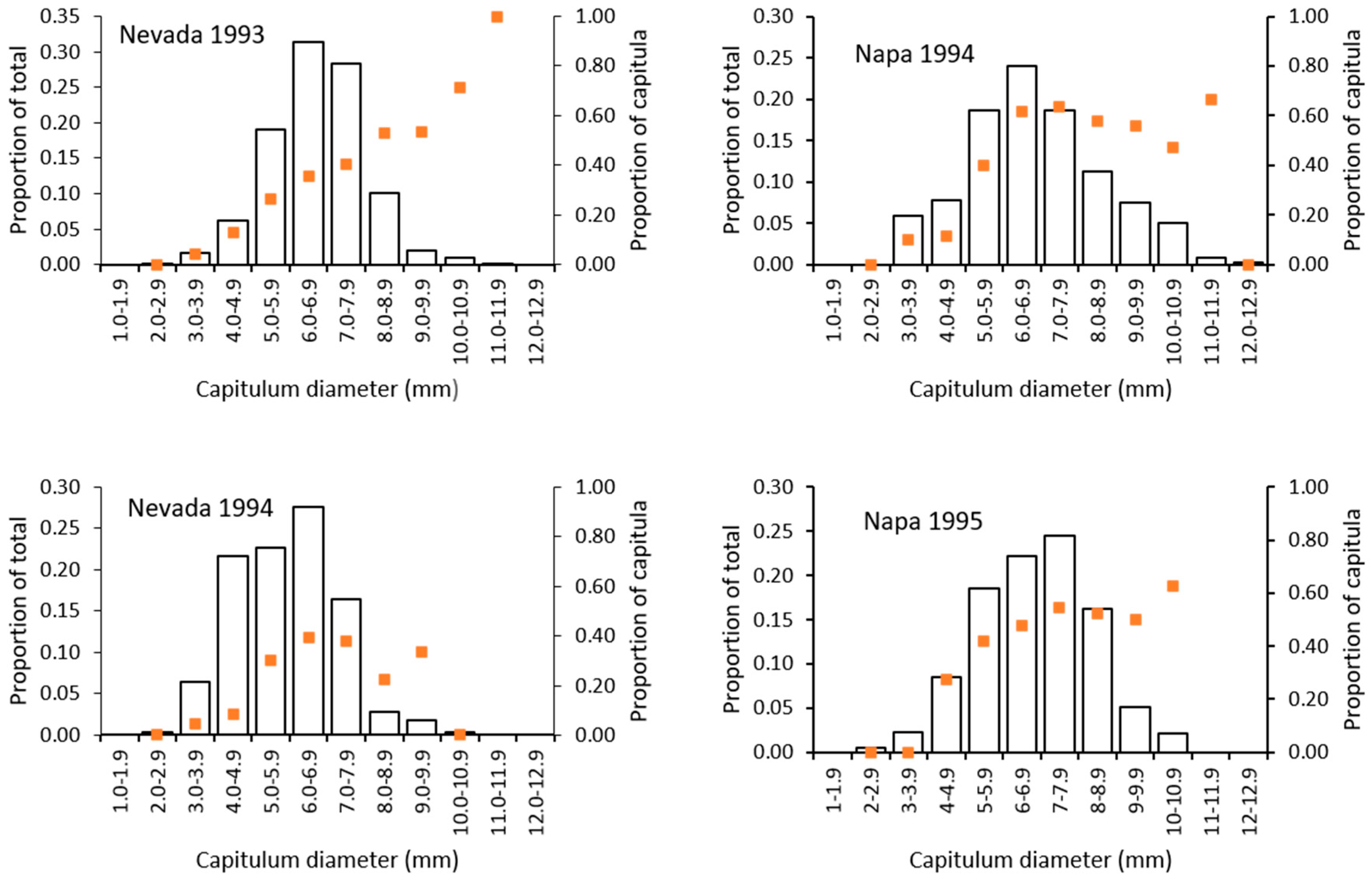
2.5.3. Risk of Attack to Capitula
3. Results
3.1. Plant Density and Size
3.2. Seed Viability and Production
3.3. Impact of Eustenopus Villosus
3.4. Risk of Capitula to Attack
3.5. Phenology of Capitula Production
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Tomaso, J.M. Yellow starthistle, Centaurea solstitialis L. In Invasive Plants of Range and Wildlands and Their Environmental, Economic, and Societal Impacts; Duncan, C.L., Clark, J.K., Eds.; Weed Science Society of America: Lawrence, KS, USA, 2005; pp. 36–50. [Google Scholar]
- Pitcairn, M.J.; Schoenig, S.; Yacoub, R.; Gendron, J. Yellow starthistle continues its spread in California. Calif. Agric. 2006, 60, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Cody, D.R. Centaurea species and equine nigropallidal encephalomalacia. In Effects of Poisonous Plants on Livestock; Keeler, R.F., Van Kampen, K.R., James, L.F., Eds.; Academic Press: Waltham, MA, USA, 1978. [Google Scholar]
- Panter, K.E. Neurotoxicity of the knapweeds (Centaurea spp.) in horses. In Noxious Range Weeds; James, L.F., Evans, J.O., Ralphs, M.H., Childs, R.D., Eds.; Westview Press: San Francisco, CA, USA, 1991. [Google Scholar]
- Eagle, A.J.; Eiswerth, M.E.; Johnson, W.S.; Schoenig, S.; Van Kooten, G.C. Costs and losses imposed on California ranchers by yellow starthistle. Rangel. Ecol. Manag. 2007, 50, 369–377. [Google Scholar] [CrossRef]
- Maddox, D.M. Introduction, Phenology and Density of Yellow Starthistle in Coastal, Intercoastal and Central Valley Situations in California; USDA-ARS Publication W-20; USDA-ARS: Oakland, CA, USA, 1981; 33p. [Google Scholar]
- Benefield, C.B.; Di Tomaso, J.M.; Kyser, G.B.; Tschohl, A. Reproductive biology of yellow starthistle: Maximizing late season control. Weed Sci. 2001, 49, 83–90. [Google Scholar] [CrossRef]
- Callihan, R.H.; Northam, F.E.; Johnson, J.B.; Michalson, E.L.; Prather, T.S. Yellow Starthistle Biology and Management in Pasture and Rangeland; Coop. Ext. Serv. CIS No. 634; University of Idaho: Moscow, ID, USA, 1989. [Google Scholar]
- Di Tomaso, J.M.; Kyser, G.B.; Pitcairn, M.J. Yellow Starthistle Management Guide; Cal-IPC Publication 2006-03; California Invasive Plant Council: Berkeley, CA, USA, 2006; 78p. [Google Scholar]
- Swope, S.M. Biocontrol attack increases pollen limitation under some circumstances in the invasive plant Centaurea solstitialis. Oecologia 2014, 174, 205–215. [Google Scholar] [CrossRef]
- Turner, C.E.; Johnson, J.B.; McCaffrey, J.P. Yellow Starthistle. In Biological Control in the Western United States; Nechols, J.R., Ed.; Publication 3361; University of California, Division of Agriculture and Natural Resources: Oakland, CA, USA, 1995; pp. 270–275. [Google Scholar]
- Callihan, R.H.; Prather, T.S.; Northam, F.E. Longevity of yellow starthistle (Centaurea solstitialis) achenes in soil. Weed Technol. 1993, 7, 33–35. [Google Scholar] [CrossRef]
- Joley, D.B.; Maddox, D.M.; Schoenig, S.E.; Mackey, B.E. Parameters affecting germinability and seed bank dynamics in dimorphic achenes of Centaurea solstitialis in California. Can. J. Bot. 2003, 81, 993–1007. [Google Scholar] [CrossRef]
- Garren, J.M.; Strauss, S.Y. Population-level compensation by an invasive thistle thwarts biological control from seed predators. Ecol. Appl. 2009, 19, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Swope, S.M.; Parker, I.M. Trait-mediated interactions and lifetime fitness of the invasive plant Centaurea solstitialis. Ecology 2010, 91, 2284–2293. [Google Scholar] [CrossRef]
- Sobhian, R.; Zwolfer, H. Phytophagous insect species associated with flower heads of yellow starthistle (Centaurea solstitialis L.). Z. Angew. Entomol. 1985, 99, 301–321. [Google Scholar] [CrossRef]
- Fornasari, L.; Turner, C.E.; Andres, L.A. Eustenopus villosus (Coleoptera: Curculionidae) for biological control of yellow starthistle (Asteraceae: Cardueae) in North America. Environ. Entomol. 1991, 20, 1187–1194. [Google Scholar] [CrossRef]
- Coombs, E.M.; Clark, J.K.; Piper, G.L.; Cofrancesco, A.F., Jr. (Eds.) Biological Control of Invasive Plants in the United States; Oregon State University Press: Corvallis, OR, USA, 2004. [Google Scholar]
- Pitcairn, M.J.; Villegas, B.; Woods, D.M.; Yacoub, R.; Joley, D.B. Evaluating implementation success for seven seed head insects on Centaurea solstitialis in California, USA. In Proceedings of the 12th International Symposium on the Biological Control of Weeds, La Grande Motte, France, 22–27 April 2007; Julien, M.H., Sforza, R., Bon, M.C., Evans, H.C., Hatcher, P.E., Hinz, H.L., Rector, B.G., Eds.; CAB International: Wallingford, UK, 2008; pp. 610–616. [Google Scholar]
- Swope, S.M.; Satterthwaite, W.H. Variable effects of a generalist parasitoid on a biocontrol seed predator and its target weed. Ecol. Appl. 2012, 22, 20–34. [Google Scholar] [CrossRef]
- Connett, J.F.; Wilson, L.M.; McCaffrey, J.P.; Harmon, B.L. Phenological synchrony of Eustenopus villosus (Coleoptera: Curculionidae) with Centaurea solstitialis in Idaho. Environ. Entomol. 2001, 30, 439–442. [Google Scholar] [CrossRef]
- Roche, C.T.; Harmon, B.L.; Wilson, L.M.; McCaffrey, J.P. Eustenopus villosus (Coleoptera: Curculionidae) feeding of herbicide-resistant yellow starthistle (Centaurea solstitialis L.). Biol. Control 2001, 20, 279–286. [Google Scholar] [CrossRef]
- Tonkel, K.C.; Piper, G.L. Patterns in resource partitioning by insect biological control agents of yellow starthistle (Centaurea solstitialis L.) in Washington. Northwest Sci. 2009, 83, 16–24. [Google Scholar] [CrossRef]
- Woodley, S.E.; Zamora, B.A.; Coffey, T. Percent infestation and seed consumption of Centaurea solstitialis L. (Asteraceae: Cardueae) by Eustenopus villosus and Larinus curtus (Coleoptera: Curculionidae) in Washington, USA. Biol. Control 2019, 134, 38–44. [Google Scholar] [CrossRef]
- Johnson, J.B.; McCaffrey, J.P.; Merickel, F.W. Endemic phytophagous insects associated with yellow starthistle in northern Idaho. Pan-Pacif. Entomol. 1992, 68, 169–173. [Google Scholar]
- Paynter, Q.; Buckley, Y.M.; Peterson, P.; Gourlay, A.H.; Fowler, S.V. Breaking and remaking a seed and seed predator interaction in the introduced range of Scotch broom (Cytisus scoparius) in New Zealand. J. Ecol. 2016, 104, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Ritland, K. Mating system of yellow starthistle (Centaurea solstitialis), a successful colonizer in North America. Heredity 1998, 80, 225–232. [Google Scholar] [CrossRef]
- Maddox, D.M.; Joley, D.B.; Supkoff, D.M.; Mayfield, A. Pollination biology of yellow starthistle (Centaurea solstitialis) in California. Can. J. Bot. 1996, 74, 262–267. [Google Scholar] [CrossRef]
- Barthell, J.F.; Randall, J.M.; Thorp, R.W.; Wenner, A.M. Promotion of seed set in yellow starthistle by honeybees: Evidence of an invasive mutualism. Ecol. Appl. 2001, 11, 1870–1883. [Google Scholar] [CrossRef]
- Clement, S.L.; Mimmocchi, T.; Sobhian, R.; Dunn, P.H. Host specificity of adult Eustenopus hirtus (Waltl) (Coleoptera: Curculionidae), a potential biological agent of yellow starthistle Centaurea solstitialis L. (Asteraceae, Cardueae). Proc. Entomol. Soc. Wash. 1988, 90, 501–507. [Google Scholar]
- Connett, J.F.; McCaffrey, J.P. Laboratory and field observations of the behavior of Eustenopus villosus while feeding and ovipositing on yellow starthistle. BioControl 2005, 50, 941–952. [Google Scholar] [CrossRef]
- Spencer, D.F.; Enloe, S.F.; Pitcairn, M.J.; Di Tomaso, J.M. Impacts of mowing and bud destruction on Centaurea solstitialis growth, flowering, root dynamics and soil moisture. Weed Res. 2013, 54, 140–150. [Google Scholar] [CrossRef]
- Fornasari, L.; Sobhian, R. Life history of Eustenopus villosus (Coleoptera: Curculionidae), a promising biological control agent for yellow starthistle. Environ. Entomol. 1993, 22, 684–692. [Google Scholar] [CrossRef]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley: New York, NY, USA, 1966. [Google Scholar]
- Krebs, C.J. Ecological Methodology; Harper Collins Publishers: New York, NY, USA, 1989. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry, 2nd ed.; W.H. Freeman & Company: New York, NY, USA, 1981. [Google Scholar]
- Swope, S.M.; Parker, I.M. Widespread seed limitation affects plant density but not population trajectory in the invasive plant Centaurea solstitialis. Oecologia 2005, 164, 117–128. [Google Scholar] [CrossRef] [PubMed]


| Species | Family | 1st Release | Result |
|---|---|---|---|
| Urophora jaculata Rondani | Diptera: Tephritidae | 1969 | Failed to Establish |
| Urophora sirunaseva (Hering) | Diptera: Tephritidae | 1984 | Established |
| Bangasternus orientalis (Capiomont) | Coleoptera: Curculionidae | 1985 | Established |
| Chaetorellia australis Hering | Diptera: Tephritidae | 1988 | Established |
| Eustenopus villosus (Boheman) | Coleoptera: Curculionidae | 1990 | Established |
| Larinus curtus Hochhut | Coleoptera: Curculionidae | 1992 | Established |
| Site | Plants m−2 | Capitula m−2 | Capitula Plant−1 |
|---|---|---|---|
| Nevada 1993 | 71.8 (62.1–85.2) * | 631.8 | 8.8 (±0.9) |
| Nevada 1994 | 166.0 (±17.0) | 377.2 (±48.3) | 2.4 (±0.3) |
| Napa 1994 | 202.0 (±28.9) | 289.2 (±34.3) | 1.7 (±0.2) |
| Napa 1995 | 208.8 (±22.0) | 357.2 (±35.3) | 1.8 (±0.1) |
| Site | Type | N | Not Viable | Viable Seed | PB/NPB Ratio | |
|---|---|---|---|---|---|---|
| Did Not Germ | Germinated | |||||
| Nevada 1993 | PB | 14,400 | 5.1% | 8.6% | 91.4% | |
| NPB | 4165 | 5.1% | 3.9% | 96.1% | 3.46 | |
| Nevada 1994 | PB | 2862 | 4.2% | 5.2% | 94.8% | |
| NPB | 758 | 4.6% | 5.0% | 95.0% | 3.78 | |
| Napa 1994 | PB | 7395 | 4.4% | 12.3% | 87.7% | |
| NPB | 3047 | 2.9% | 3.6% | 96.4% | 2.43 | |
| Napa 1995 | PB | 5993 | 5.6% | 6.3% | 93.7% | |
| NPB | 2707 | 5.7% | 8.9% | 91.1% | 2.21 | |
| Napa 1995 | PB | 4032 | 7.8% | 13.0% | 87.0% | |
| control | NPB | 1926 | 5.5% | 8.6% | 91.4% | 2.09 |
| Site | N | Dead Buds | Capitula | Total Buds | Success Rate |
|---|---|---|---|---|---|
| Nevada 1993 | 160 | 2500 | 1405 | 3905 | 36.0% |
| Nevada 1994 | 149 | 768 | 332 | 1100 | 30.2% |
| Napa 1994 | 157 | 189 | 336 | 525 | 64.0% |
| Napa 1995 | 154 | 620 | 388 | 1008 | 38.5% |
| Napa 1995 control | 156 | 34 | 288 | 322 | 89.4% |
| Site | N | Capitula per | Seeds per | Capitula with | Capitula with | Punctures per |
|---|---|---|---|---|---|---|
| Plant (±SE) | Plant (±SE) | Punctures | Larval Damage | Capitulum | ||
| Nevada 1993 | 160 | 8.8 (±0.9) | 84.2 (±11.7) | 91.7% | 35.6% | 1.25 (±0.01) |
| Nevada 1994 | 149 | 2.2 (±0.2) | 6.80 (±0.9) | 78.9% | 27.2% | 1.58 (±0.05) |
| Napa 1994 | 157 | 1.7 (±0.2) | 26.0 (±3.2) | 68.7% | 49.3% | 1.21 (±0.03) |
| Napa 1995 | 154 | 1.8 (±0.1) | 23.4 (±3.0) | 80.3% | 46.4% | 1.12 (±0.02) |
| Napa 1995 control | 156 | 1.8 (±0.2) | 35.8 (±5.4) | 0.0% | 0.0% | 0 |
| Site | y = 0 | Slope (±SE) | Intercept (±SE) | Upper Range |
|---|---|---|---|---|
| Nevada 1993 | <3.42 mm | 4.58 (±0.37) a | 15.69 (±2.76) | y = 36.15 for values >10.0 mm |
| Nevada 1994 | <3.32 mm | 2.17 (±0.08) b | 7.20 (±0.48) | y = 8.72 for values >9.0 mm |
| Napa 1994 | <2.63 mm | 5.93 (±0.66) a | 15.58 (±4.12) | y = 41.80 for values >9.0 mm |
| Napa 1995 | <3.16 mm | 4.72 (±0.38) a | 15.12 (±2.81) | y = 32.80 for values >10.0 mm |
| Napa 1995 control | 5.59 (±0.51) a | 20.87 (±3.44) |
| Site | Stage | N | Viable Seed | % Loss | |
|---|---|---|---|---|---|
| Predicted | Observed | ||||
| Nevada 1993 | Pupa, Adult | 201 | 3418 | 347 | 89.8 |
| Died as larva | 319 | 5320 | 1265 | 76.2 | |
| Nevada 1994 | Pupa, Adult | 32 | 218 | 11 | 95.0 |
| Died as larva | 56 | 372 | 53 | 85.8 | |
| Napa 1994 | Pupa, Adult | 111 | 3088 | 647 | 79.0 |
| Died as larva | 55 | 1517 | 297 | 80.4 | |
| Napa 1995 | Pupa, Adult | 146 | 279 | 1496 | 82.2 |
| Died as larva | 34 | 601 | 139 | 76.9 | |
| Site | N | Total Seed Production | % Loss to Insects | % Capitula | Efficiency | |
|---|---|---|---|---|---|---|
| Observed | Predicted | with Larval Damage | ||||
| Nevada 1993 | 160 | 13,453 | 20,579 | 34.6 | 35.6 | 0.97 |
| Nevada 1994 | 149 | 1027 | 1553 | 33.9 | 27.2 | 1.25 |
| Napa 1994 | 157 | 4155 | 7816 | 46.8 | 49.3 | 0.95 |
| Napa 1995 | 154 | 3623 | 6380 | 43.2 | 46.4 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitcairn, M.J.; Woods, D.M.; Joley, D.B.; Turner, C.E. Evaluation of the Impact of Eustenopus villosus on Centaurea solstitialis Seed Production in California. Insects 2021, 12, 606. https://doi.org/10.3390/insects12070606
Pitcairn MJ, Woods DM, Joley DB, Turner CE. Evaluation of the Impact of Eustenopus villosus on Centaurea solstitialis Seed Production in California. Insects. 2021; 12(7):606. https://doi.org/10.3390/insects12070606
Chicago/Turabian StylePitcairn, Michael J., Dale M. Woods, Donald B. Joley, and Charles E. Turner. 2021. "Evaluation of the Impact of Eustenopus villosus on Centaurea solstitialis Seed Production in California" Insects 12, no. 7: 606. https://doi.org/10.3390/insects12070606
APA StylePitcairn, M. J., Woods, D. M., Joley, D. B., & Turner, C. E. (2021). Evaluation of the Impact of Eustenopus villosus on Centaurea solstitialis Seed Production in California. Insects, 12(7), 606. https://doi.org/10.3390/insects12070606





