Molecular Evolution of Phototransduction Pathway Genes in Nocturnal and Diurnal Fireflies (Coleoptera: Lampyridae)
Abstract
Simple Summary
Abstract
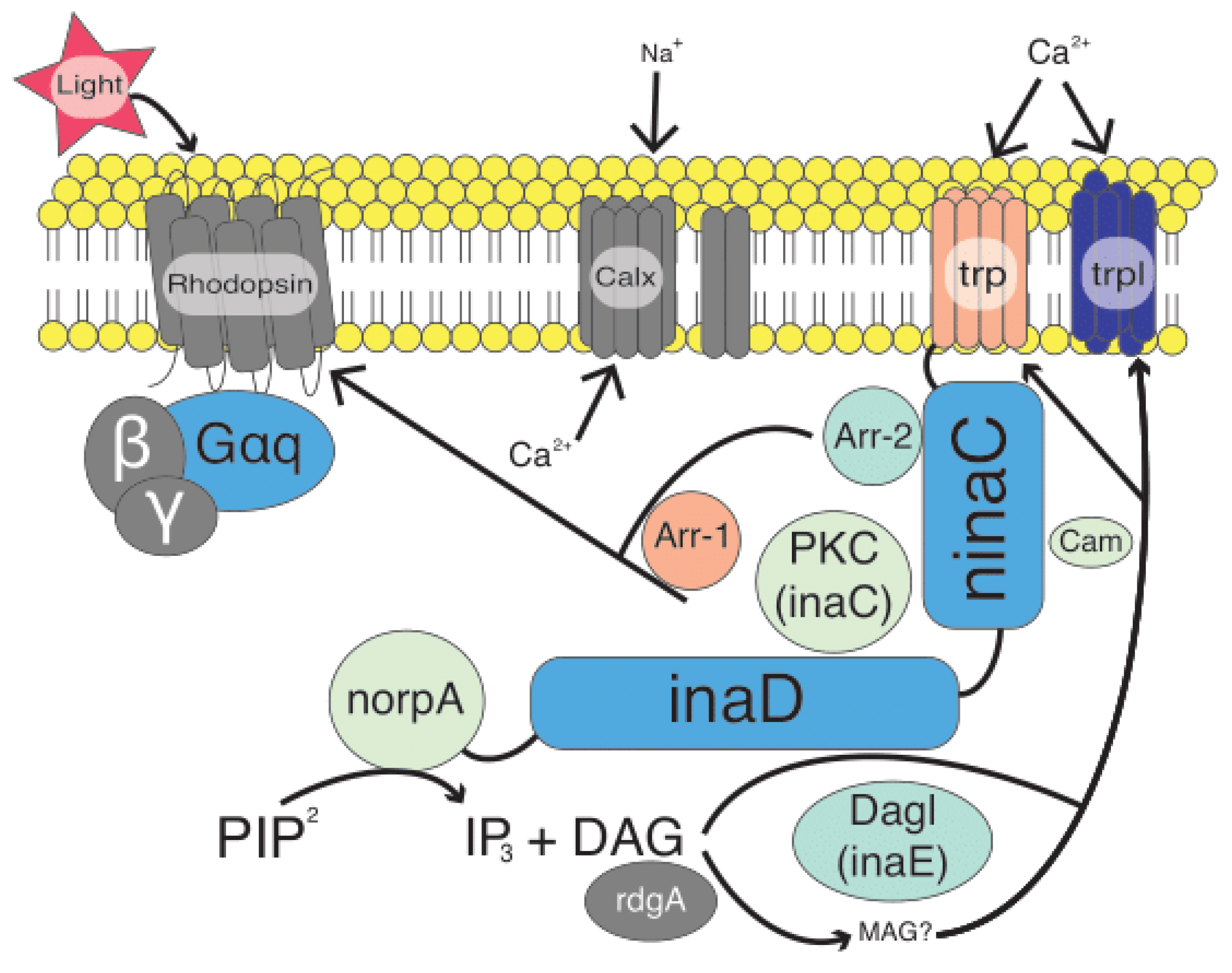
1. Introduction
2. Materials and Methods
2.1. Sampling and Sequencing
2.2. Transcriptome Assembly and Annotation
2.3. Analyses of Positive Selection
3. Results
3.1. Transcriptome Assemblies Recover Most Conserved Genes
3.2. Ortholog Search Strategy Identifies Phototransduction Genes
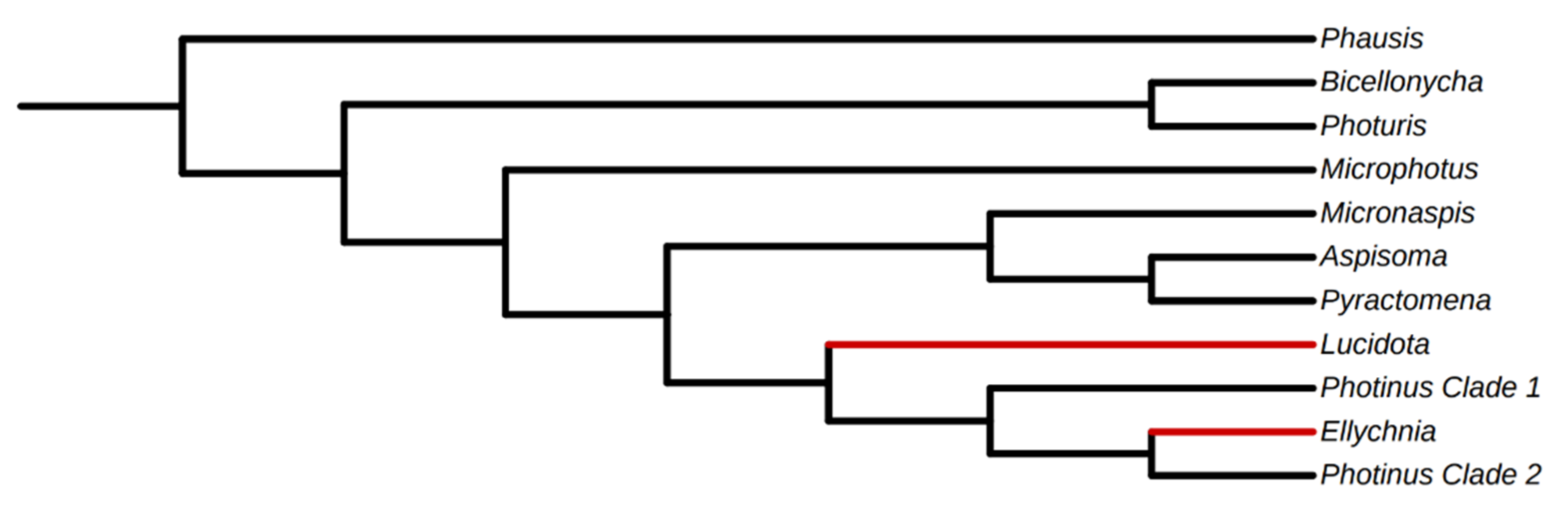
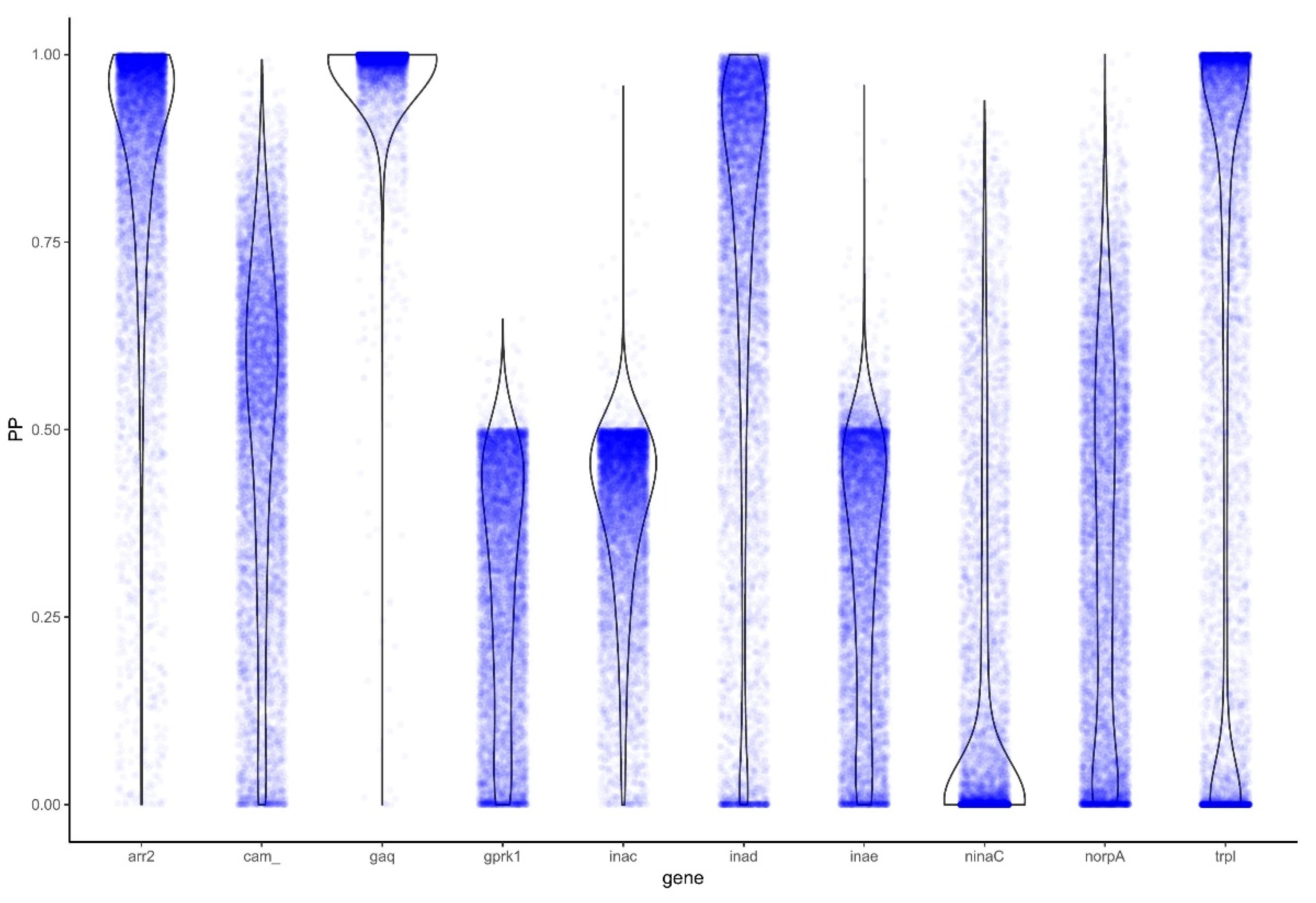
3.3. Analysis for Positive Selection Identifies Genes under Positive Selection
3.3.1. PAML
3.3.2. BAli-Phy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilad, Y.; Przeworski, M.; Lancet, D. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2004, 2, E5. [Google Scholar] [CrossRef]
- Bybee, S.; Córdoba-Aguilar, A.; Duryea, M.C.; Futahashi, R.; Hansson, B.; Lorenzo-Carballa, M.O.; Schilder, R.; Stoks, R.; Suvorov, A.; Svensson, E.I.; et al. Odonata (dragonflies and damselflies) as a bridge between ecology and evolutionary genomics. Front Zool. 2016, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Keesey, I.W.; Grabe, V.; Gruber, L.; Koerte, S.; Obiero, G.F.; Bolton, G.; Khallaf, M.A.; Kunert, G.; Lavista-Llanos, S.; Valenzano, D.R.; et al. Inverse resource allocation between vision and olfaction across the genus Drosophila. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Stanger-Hall, K.F.; Sander Lower, S.E.; Lindberg, L.; Hopkins, A.; Pallansch, J.; Hall, D.W. The evolution of sexual signal modes and associated sensor morphology in fireflies (Lampyridae, Coleoptera). Proc. R. Soc. B Biol. Sci. 2018, 285, 20172384. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.M.; Boston, E.S.M.; Finarelli, J.A.; Murphy, W.J.; Higgins, D.G.; Teeling, E.C. The Birth and Death of Olfactory Receptor Gene Families in Mammalian Niche Adaptation. Mol. Biol. Evol. 2018, 35, 1390–1406. [Google Scholar] [CrossRef]
- Montell, C. Drosophila visual transduction. Trends Neurosci. 2012, 35, 356–363. [Google Scholar] [CrossRef]
- Bao, R.; Friedrich, M. Molecular evolution of the Drosophila retinome: Exceptional gene gain in the higher Diptera. Mol. Biol. Evol. 2009, 26, 1273–1287. [Google Scholar] [CrossRef]
- Macias-Muñoz, A.; Rangel Olguin, A.G.; Briscoe, A.D. Evolution of Phototransduction Genes in Lepidoptera. Genome Biol. Evol. 2019, 11, 2107–2124. [Google Scholar] [CrossRef] [PubMed]
- Tierney, S.M.; Friedrich, M.; Humphreys, W.F.; Jones, T.M.; Warrant, E.J.; Wcislo, W.T. Consequences of evolutionary transitions in changing photic environments. Austral Ento. 2017, 56, 23–46. [Google Scholar] [CrossRef]
- Hardie, R.C. Phototransduction in Drosophila melanogaster. J. Exp. Biol. 2001, 204 Pt 20, 3403–3409. [Google Scholar] [CrossRef]
- Katz, B.; Minke, B. Drosophila photoreceptors and signaling mechanisms. Front. Cell Neurosci. 2009, 3, 2. [Google Scholar] [CrossRef]
- Hardie, R.C.; Raghu, P. Visual transduction in Drosophila. Nature 2001, 413, 186–193. [Google Scholar] [CrossRef] [PubMed]
- White, R.H.; Xu, H.; Munch, T.A.; Bennett, R.R.; Grable, E.A. The retina of Manduca sexta: Rhodopsin expression, the mosaic of green-, blue- and UV-sensitive photoreceptors, and regional specialization. J. Exp. Biol. 2003, 206, 3337–3348. [Google Scholar] [CrossRef]
- Hughes, A.L. Gene duplication and the origin of novel proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 8791–8792. [Google Scholar] [CrossRef]
- Wakakuwa, M.; Kurasawa, M.; Giurfa, M.; Arikawa, K. Spectral heterogeneity of honeybee ommatidia. Naturwissenschaften 2005, 92, 464–467. [Google Scholar] [CrossRef]
- Conant, G.C.; Wolfe, K.H. Turning a hobby into a job: How duplicated genes find new functions. Nat. Rev. Genet. 2008, 9, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Plachetzki, D.C.; Fong, C.R.; Oakley, T.H. The evolution of phototransduction from an ancestral cyclic nucleotide gated pathway. Proc. R. Soc. B 2010, 277, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- South, A.; Lewis, S.M. Determinants of reproductive success across sequential episodes of sexual selection in a firefly. Proc. Biol. Sci. 2012, 279, 3201–3208. [Google Scholar] [CrossRef]
- Lloyd, J.E. Studies on the Flash Communication System in Photinus Fireflies. Misc. Publ. Mus. Zool. 1966, 130, 1–95. [Google Scholar]
- Bethune, C.J.S. A Luminous Larva. Can. Èntomol. 1868, 1, 2–3. [Google Scholar] [CrossRef]
- Branner, J.C. The luminosity of termites. Science 1910, 32, 342. [Google Scholar] [CrossRef]
- Allard, H.A. The synchronal flashing of fireflies. Science 1916, 44, 710. [Google Scholar] [CrossRef]
- Brown, B. A luminous spider. Science 1925, 62, 182. [Google Scholar] [CrossRef]
- South, A.; LeVan, K.; Leombruni, L.; Orians, C.M.; Lewis, S.M. Examining the Role of Cuticular Hydrocarbons in Firefly Species Recognition. Ethology 2008, 114, 916–924. [Google Scholar] [CrossRef]
- Cock, R.D.; De Cock, R.; Matthysen, E. Sexual communication by pheromones in a firefly, Phosphaenus hemipterus (Coleoptera: Lampyridae). Anim. Behav. 2005, 70, 807–818. [Google Scholar] [CrossRef]
- Ming, Q.-L.; Lewis, S.M. Mate Recognition and Sex Differences in Cuticular Hydrocarbons of the Diurnal Firefly Ellychnia corrusca (Coleoptera: Lampyridae). Ann. Èntomol. Soc. Am. 2010, 103, 128–133. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Emlen, D.J. Competition among body parts in the development and evolution of insect morphology. Proc. Natl. Acad. Sci. USA 1998, 95, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.J.; Lord, N.P.; Branham, M.A.; Bybee, S.M. Review of the firefly visual system (Coleoptera: Lampyridae) and evolution of the opsin genes underlying color vision. Org. Divers. Evol. 2015, 15, 513–526. [Google Scholar] [CrossRef]
- Sander, S.E.; Hall, D.W. Variation in opsin genes correlates with signalling ecology in North American fireflies. Mol. Ecol. 2015, 24, 4679–4696. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, C.R.; Fujimoto, M.S.; Lord, N.P.; Shin, S.; McKenna, D.D.; Suvorov, A.; Martin, G.J.; Bybee, S.M. Overcoming the loss of blue sensitivity through opsin duplication in the largest animal group, beetles. Sci. Rep. 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Kainuma, T. Diel changes in the expression of long wavelength-sensitive and ultraviolet-sensitive opsin genes in the Japanese firefly, Luciola cruciata. Gene 2009, 436, 66–70. [Google Scholar] [CrossRef]
- Smith, W.C.; Ayers, D.M.; Popp, M.P.; Hargrave, P.A. Short wavelength-sensitive opsins from the Saharan silver and carpenter ants. Invert. Neurosci. 1997, 3, 49–56. [Google Scholar] [CrossRef]
- Briscoe, A.D.; Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef]
- Feuda, R.; Marlétaz, F.; Bentley, M.A.; Holland, P.W.H. Conservation, Duplication, and Divergence of Five Opsin Genes in Insect Evolution. Genome Biol. Evol. 2016, 8, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, A.D.; Bybee, S.M.; Bernard, G.D.; Yuan, F.; Sison-Mangus, M.P.; Reed, R.D.; Warren, A.D.; Llorente-Bousquets, J.; Chiao, C.-C. Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl. Acad. Sci. USA 2010, 107, 3628–3633. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A.; Jensen, N.O.; Sharkey, C.R.; Fujimoto, M.S.; Bodily, P.; Wightman, H.M.C.; Ogden, T.H.; Clement, M.J.; Bybee, S.M. Opsins have evolved under the permanent heterozygote model: Insights from phylotranscriptomics of Odonata. Mol. Ecol. 2016, 26, 1306–1322. [Google Scholar] [CrossRef] [PubMed]
- Fallon, T.R.; Lower, S.E.; Chang, C.-H.; Bessho-Uehara, M.; Martin, G.J.; Bewick, A.J.; Behringer, M.; Debat, H.J.; Wong, I.; Day, J.C.; et al. Firefly genomes illuminate parallel origins of bioluminescence in beetles. Elife 2018, 7. [Google Scholar] [CrossRef]
- Martin, G.J.; Stanger-Hall, K.F.; Branham, M.A.; Da Silveira, L.F.L.; Lower, S.E.; Hall, D.W.; Li, X.-Y.; Lemmon, A.R.; Lemmon, E.M.; Bybee, S.M. Higher-level phylogeny and reclassification of Lampyridae (Coleoptera: Elateroidea). Insect Syst. Divers. 2019, 3, 11. [Google Scholar] [CrossRef]
- Kofler, R.; Orozco-Terwengel, P.; De Maio, N.; Pandey, R.V.; Nolte, V.; Futschik, A.; Kosiol, C.; Schlötterer, C. PoPoolation: A toolbox for population genetic analysis of next generation sequencing data from pooled individuals. PLoS ONE 2011, 6, e15925. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Waterhouse, R.M.; Tegenfeldt, F.; Li, J.; Zdobnov, E.M.; Kriventseva, E.V. OrthoDB: A hierarchical catalog of animal, fungal and bacterial orthologs. Nucleic Acids Res. 2013, 41, D358–D365. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7, improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Speiser, D.I.; Pankey, M.S.; Zaharoff, A.K.; Battelle, B.A.; Bracken-Grissom, H.D.; Breinholt, J.W.; Bybee, S.M.; Cronin, T.W.; Garm, A.; Lindgren, A.R.; et al. Using phylogenetically-informed annotation (PIA) to search for light-interacting genes in transcriptomes from non-model organisms. BMC Bioinform. 2014, 15, 350. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4, Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Redelings, B. Erasing Errors due to Alignment Ambiguity When Estimating Positive Selection. Mol. Biol. Evol. 2014, 31, 1979–1993. [Google Scholar] [CrossRef]
- Suchard, M.A.; Redelings, B.D. BAli-Phy: Simultaneous Bayesian inference of alignment and phylogeny. Bioinformatics 2006, 22, 2047–2048. [Google Scholar] [CrossRef]
- Kass, R.E.; Raftery, A.E. Bayes Factors. J. Am. Stat. Assoc. 1995, 90, 773. [Google Scholar] [CrossRef]
- Yang, Z.; Nielsen, R. Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol. Biol. Evol. 2002, 19, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.S.; Pankey, M.S.; Plachetzki, D.C.; Villacorta, C.; Syme, A.E.; Serb, J.M.; Omilian, A.R.; Oakley, T.H. Gene duplication and the origins of morphological complexity in pancrustacean eyes, a genomic approach. BMC Evol. Biol. 2010, 10, 123. [Google Scholar] [CrossRef]
- Chevesich, J.; Kreuz, A.J.; Montell, C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron 1997, 18, 95–105. [Google Scholar] [CrossRef]
- Shieh, B.H.; Zhu, M.Y. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron 1996, 16, 991–998. [Google Scholar] [CrossRef][Green Version]
- Wes, P.D.; Xu, X.Z.; Li, H.S.; Chien, F.; Doberstein, S.K.; Montell, C. Termination of phototransduction requires binding of the NINAC myosin III and the PDZ protein INAD. Nat. Neurosci. 1999, 2, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Z.S.; Wes, P.; Chen, H.; Li, H.-S.; Yu, M.; Morgan, S.; Liu, Y.; Montell, C. Retinal targets for calmodulin include proteins implicated in synaptic transmission. J. Biol. Chem. 1998, 273, 31297–31307. [Google Scholar] [CrossRef]
- Huber, A.; Sander, P.; Gobert, A.; Bähner, M.; Hermann, R.; Paulsen, R. The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J. 1996, 15, 7036–7045. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, S.; Sierralta, J.; Sun, Y.; Bodner, R.; Suzuki, E.; Becker, A.; Socolich, M.; Zuker, C.S. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature 1997, 388, 243–249. [Google Scholar] [CrossRef]
- Laughlin, S.B.; Weckstrom, M. Fast and slow photoreceptors—a comparative-study of the functional diversity of coding and conductances in the Diptera. J. Comp. Physiol. A 1993, 172, 593–609. [Google Scholar] [CrossRef]
- Mishra, P.; Socolich, M.; Wall, M.A.; Graves, J.; Wang, Z.; Ranganathan, R. Dynamic scaffolding in a G protein-coupled signaling system. Cell 2007, 131, 80–92. [Google Scholar] [CrossRef]
- Li, H.S.; Montell, C. TRP and the PDZ protein, INAD, form the core complex required for retention of the signalplex in Drosophila photoreceptor cells. J. Cell Biol. 2000, 150, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, S.; Sun, Y.; Suzuki, E.; Zuker, C. Independent anchoring and assembly mechanisms of INAD signaling complexes in Drosophila photoreceptors. J. Neurosci. 2001, 21, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Shah, S.; Suzuki, E.; Zars, T.; O’Day, P.M.; Hyde, D.R. The drosophila dgq gene encodes a Gα protein that mediates phototransduction. Neuron 1994, 13, 1143–1157. [Google Scholar] [CrossRef]
- Leung, H.-T.; Tseng-Crank, J.; Kim, E.; Mahapatra, C.; Shino, S.; Zhou, Y.; An, L.; Doerge, R.W.; Pak, W.L. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 2008, 58, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Rubin, G.M. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- French, A.S.; Meisner, S.; Liu, H.; Weckström, M.; Torkkeli, P.H. Transcriptome analysis and RNA interference of cockroach phototransduction indicate three opsins and suggest a major role for TRPL channels. Front. Physiol. 2015, 6, 207. [Google Scholar] [CrossRef]
- Liu, C.-H.; Satoh, A.K.; Postma, M.; Huang, J.; Ready, D.F.; Hardie, R.C. Ca2+-dependent metarhodopsin inactivation mediated by calmodulin and NINAC myosin III. Neuron 2008, 59, 778–789. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Wasserman, D.; Wang, X.; Li, R.; Mills, E.; Elsaesser, R.; Li, H.-S.; Montell, C. Dependence on a retinophilin/myosin complex for stability of PKC and INAD and termination of phototransduction. J. Neurosci. 2010, 30, 11337–11345. [Google Scholar] [CrossRef]
- Lee, S.-J.; Montell, C. Light-dependent translocation of visual arrestin regulated by the NINAC myosin III. Neuron 2004, 43, 95–103. [Google Scholar] [CrossRef]
- Satoh, A.K.; Ready, D.F. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr. Biol. 2005, 15, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Dolph, P.J.; Ranganathan, R.; Colley, N.J.; Hardy, R.W.; Socolich, M.; Zuker, C.S. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science 1993, 260, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Kühn, H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry 1978, 17, 4389–4395. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Chabre, M.; Plouet, J.; Tuyen, V.V.; De Kozak, Y.; Faure, J.P.; Kuhn, H. Retinal S antigen identified as the 48K protein regulating light-dependent phosphodiesterase in rods. Science 1985, 228, 891–893. [Google Scholar] [CrossRef]
- Gurevich, V.V.; Gurevich, E.V. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol. Ther. 2006, 110, 465–502. [Google Scholar] [CrossRef]
- Smith, D.P.; Shieh, B.H.; Zuker, C.S. Isolation and structure of an arrestin gene from Drosophila. Proc. Natl. Acad. Sci. USA 1990, 87, 1003–1007. [Google Scholar] [CrossRef]
- Hyde, D.R.; Mecklenburg, K.L.; Pollock, J.A.; Vihtelic, T.S.; Benzer, S. Twenty Drosophila visual system cDNA clones: One is a homolog of human arrestin. Proc. Natl. Acad. Sci. USA 1990, 87, 1008–1012. [Google Scholar] [CrossRef]
- LeVine, H.; Smith, D.P.; Whitney, M.; Malicki, D.M.; Dolph, P.J.; Smith, G.F.; Burkhart, W.; Zuker, C.S. Isolation of a novel visual-system-specific arrestin: An in vivo substrate for light-dependent phosphorylation. Mech. Dev. 1990, 33, 19–25. [Google Scholar] [CrossRef]
- Yamada, T.; Takeuchi, Y.; Komori, N.; Kobayashi, H.; Sakai, Y.; Hotta, Y.; Matsumoto, H. A 49-kilodalton phosphoprotein in the Drosophila photoreceptor is an arrestin homolog. Science 1990, 248, 483–486. [Google Scholar] [CrossRef]
- Lloyd, J.E. Bioluminescent Communication in Insects. Annu. Rev. Èntomol. 1971, 16, 97–122. [Google Scholar] [CrossRef]
- Ohba, N. Flash Communication Systems of Japanese Fireflies. Integr. Comp. Biol. 2004, 44, 225–233. [Google Scholar] [CrossRef]
- Stanger-Hall, K.F.; Lloyd, J.E.; Hillis, D.M. Phylogeny of North American fireflies (Coleoptera: Lampyridae): Implications for the evolution of light signals. Mol. Phylogenetics Evol. 2007, 45, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y. False-positive results obtained from the branch-site test of positive selection. Genes Genet. Syst. 2008, 83, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; He, J.; Dong, Z.; Liu, G.; Yin, Y.; Zhang, X.; Li, Q.; Ren, Y.; Yang, Y.; Liu, W.; et al. Genomic and experimental data provide new insights into luciferin biosynthesis and bioluminescence evolution in fireflies. Sci. Rep. 2020, 10, 1–19. [Google Scholar]
- Friedrich, M. Evolution of visual performance-associated genes in Drosophila. eLS 2010. [Google Scholar] [CrossRef]
| Species | arr1 | arr2 | Cam | Gq | Gprk1 | inaC | inaC Copy | inaD | inaE | ninaC | norpA | trp | trpl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspisoma sp. | |||||||||||||
| Bicellonycha wickershamorum | |||||||||||||
| Ellychnia sp. | |||||||||||||
| Lucidota atra | |||||||||||||
| Micronaspis floridana | |||||||||||||
| Microphotus sp. | |||||||||||||
| Phausis reticulata | |||||||||||||
| Photinus australis | |||||||||||||
| Photinus carolinus | * | ||||||||||||
| Photinus macdermotti | |||||||||||||
| Photinus marginellus | |||||||||||||
| Photinus pyralis | |||||||||||||
| Photinus scintillans | |||||||||||||
| Photuris “A” | |||||||||||||
| Photuris frontalis | |||||||||||||
| Photuris sp. | |||||||||||||
| Photuris sp. 1 | |||||||||||||
| Photuris sp. 2 larva | |||||||||||||
| Pyractomena borealis | |||||||||||||
| Pyractomena dispersa |
| Summary of Positive Selection Analyses | PAML | BAli-Phy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAFFT Alignment | Bali-Phy Alignment | |||||||||||||||
| Model | lnl | lrt | np | df | p-Value | Omega | Model | lnl | lrt | np | df | p-Value | Omega | Bayes Factor | Level of Support | |
| arr2 | null | −3504.00804 | 43.58565 | 19 | 1 | <0.00001 | 0.0545 | null | −3447.79062 | 56.286878 | 19 | 1 | <0.00001 | 0.0459 | 9.502073 | Positive |
| alt | −3482.21521 | 20 | alt | −3419.64718 | 20 | |||||||||||
| Gq | null | −6134.51482 | 0.050698 | 35 | 1 | 0.82202 | 0.1139 | null | −5227.9707 | 5.017252 | 34 | 1 | 0.0251 | 0.0305 | 82.27678 | Strong |
| alt | −6134.48947 | 36 | alt | −5225.46207 | 35 | |||||||||||
| Gprk1 | null | −9493.07143 | 1.05478 | 45 | 1 | 0.30443 | 0.0087 | null | −9526.73339 | 0.411348 | 41 | 1 | 0.52131 | 0.0081 | 0.623359 | None |
| alt | −9492.54404 | 46 | alt | −9526.52772 | 42 | |||||||||||
| inaC | null | −17053.9096 | 64.3459 | 61 | 1 | <0.00001 | 0.0186 | null | −16617.8413 | 0.06558 | 58 | 1 | 0.79788 | 0.013 | 0.778239 | None |
| alt | −17021.7366 | 62 | alt | −16617.8085 | 59 | |||||||||||
| inaD | null | −19140.0313 | 28.04327 | 45 | 1 | <0.00001 | 0.1139 | null | −18588.4152 | 22.296904 | 43 | 1 | <0.00001 | 0.0921 | 5.128159 | Positive |
| alt | −19126.0097 | 46 | alt | −18577.2668 | 44 | |||||||||||
| inaE | null | −21162.9887 | 0.938762 | 45 | 1 | 0.33261 | 0.0576 | null | −20557.5219 | 0.925638 | 42 | 1 | 0.33601 | 0.0429 | 0.673093 | None |
| alt | −21162.5193 | 46 | alt | −20557.0591 | 43 | |||||||||||
| ninaC | null | −17058.6637 | 43.75643 | 15 | 1 | <0.00001 | 0.1104 | null | −16930.1126 | 45.098156 | 15 | 1 | <0.00001 | 0.1092 | 0.990066 | None |
| alt | −17036.7855 | 16 | alt | −16907.5635 | 16 | |||||||||||
| norpA | null | −19292.2825 | 0.01304 | 43 | 1 | 0.90909 | 0.0226 | null | −18587.9541 | 3.361894 | 42 | 1 | 0.06673 | 0.0145 | 1.041881 | None |
| alt | −19292.276 | 44 | alt | −18586.2732 | 43 | |||||||||||
| trpl | null | −23948.4017 | 16.06823 | 33 | 1 | 0.000061 | 0.2298 | null | −27362.9713 | 114.4442 | 33 | 1 | <0.00001 | 0.0821 | 8.59116 | Positive |
| alt | −23940.3676 | 34 | alt | −27305.7492 | 34 | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, G.J.; Lower, S.E.; Suvorov, A.; Bybee, S.M. Molecular Evolution of Phototransduction Pathway Genes in Nocturnal and Diurnal Fireflies (Coleoptera: Lampyridae). Insects 2021, 12, 561. https://doi.org/10.3390/insects12060561
Martin GJ, Lower SE, Suvorov A, Bybee SM. Molecular Evolution of Phototransduction Pathway Genes in Nocturnal and Diurnal Fireflies (Coleoptera: Lampyridae). Insects. 2021; 12(6):561. https://doi.org/10.3390/insects12060561
Chicago/Turabian StyleMartin, Gavin J., Sarah E. Lower, Anton Suvorov, and Seth M. Bybee. 2021. "Molecular Evolution of Phototransduction Pathway Genes in Nocturnal and Diurnal Fireflies (Coleoptera: Lampyridae)" Insects 12, no. 6: 561. https://doi.org/10.3390/insects12060561
APA StyleMartin, G. J., Lower, S. E., Suvorov, A., & Bybee, S. M. (2021). Molecular Evolution of Phototransduction Pathway Genes in Nocturnal and Diurnal Fireflies (Coleoptera: Lampyridae). Insects, 12(6), 561. https://doi.org/10.3390/insects12060561






