Remarkable Species Diversity of the Leafhopper Genus Xestocephalus (Hemiptera: Cicadellidae: Aphrodinae) in Thailand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
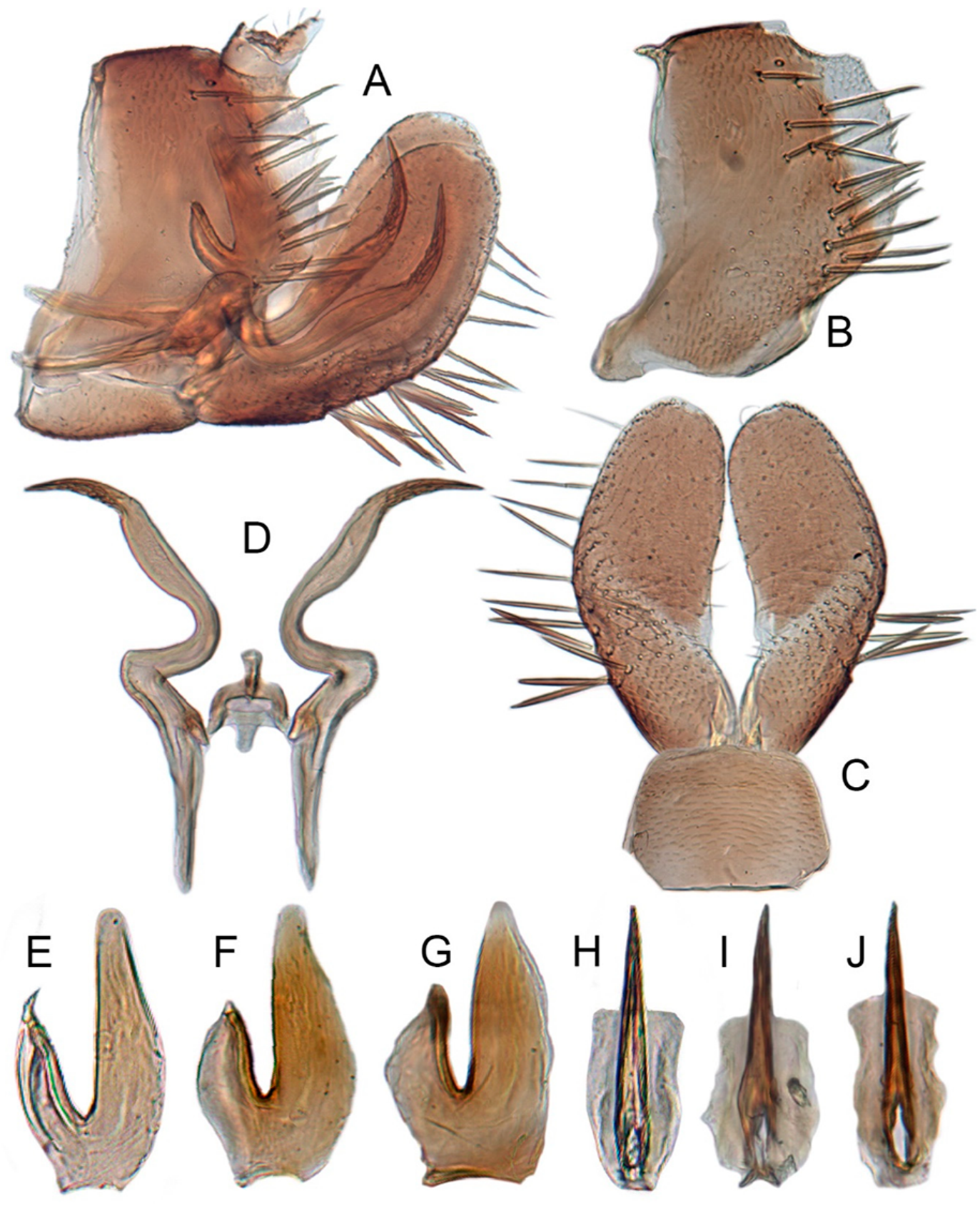
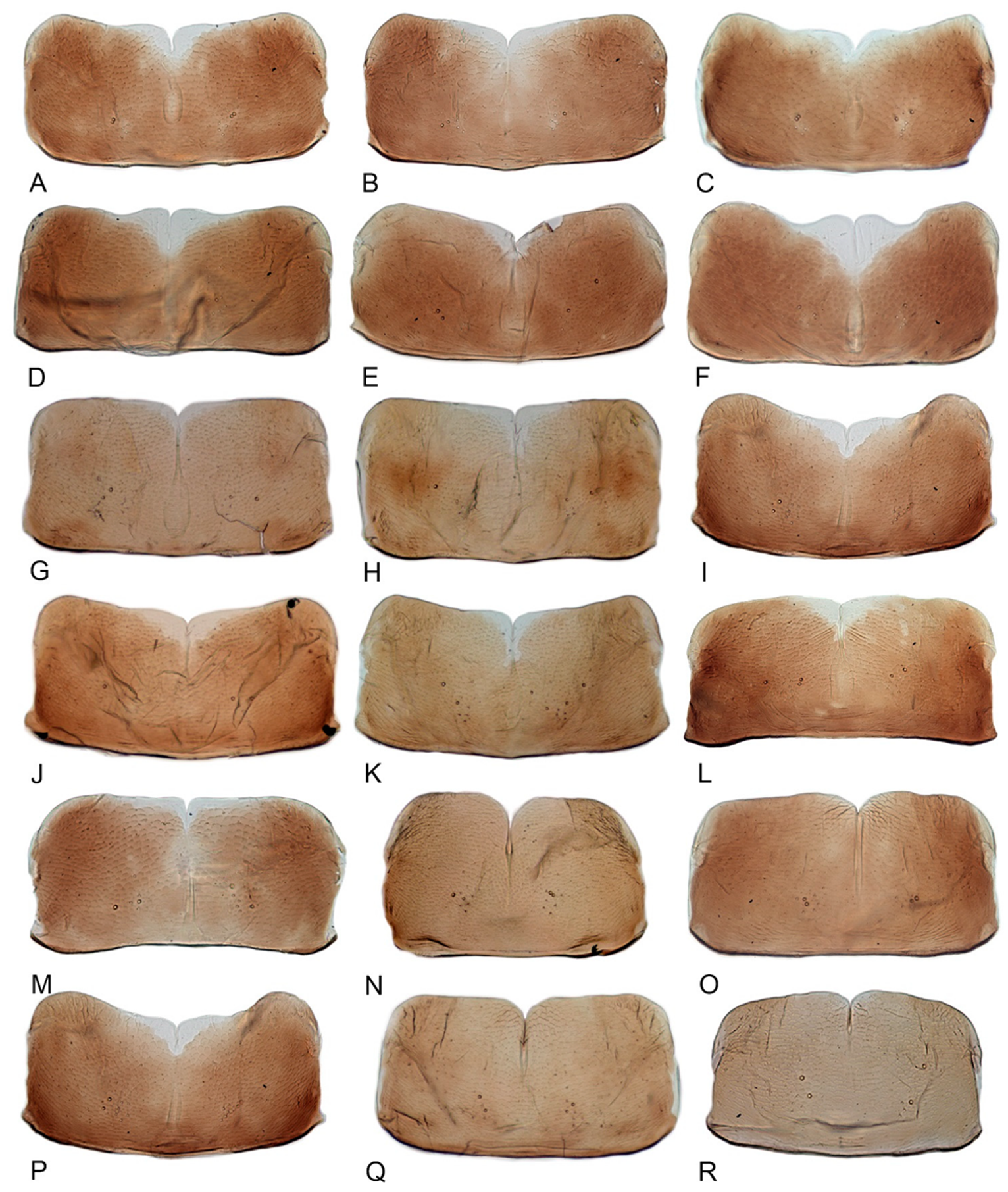
3.1. Generic Characters
3.2. Overview of Species Identification, Distribution
3.2.1. Checklist of the Genus Xestocephalus from Thailand
3.2.2. Key to Species of Xestocephalus from Thailand
- 1.
- Aedeagus without process.............................................................................................................................................................................................2
- -
- Aedeagus with pair of processes at apex or base..........................................................................................................................................................6
- 2.
- Style without conspicuous preapical teeth; aedeagus with gonopore subapical on caudal margin………..........................................................3
- -
- Style with conspicuous preapical teeth; aedeagus with gonopore near middle on caudal margin........................................................................5
- 3.
- Aedeagal shaft with longitudinal flange on either side of anterior margin, dorsal and ventral margins almost parallel (Figure 4F)...........................................................................................................................................................................................................................ishidae Matsumura
- -
- Aedeagal shaft without longitudinal flanges on anterior margin...............................................................................................................................4
- 4.
- Aedeagal shaft with anterior margin slightly curved anteriorly in lateral view (Figure 2F,H), tapering to apex in ventral view (Figure 2E,G)...............................................................................................................................................................................................................asper Linnavuori
- -
- Aedeagal shaft with anterior margin straight in lateral view (Figure 3F), broadened apically in ventral view (Figure 3E)....................................................................................................................................................................................................................................guttulatus (Motschulsky)
- 5.
- Pygofer without internal process (Figure 7B); aedeagal shaft compressed (Figure 7E–G) in posterior view, robust in lateral view (Figure 7H–J)...........................................................................................................................................................................................................nonattribus sp. nov.
- -
- 6.
- -
- Subgenital plates triangular; aedeagal with processes at apex.................................................................................................................................12
- 7.
- Aedeagus with two pairs of basal processes (Figure 17E,F)...............................................................................abyssinicus Heller and Linnavuori
- -
- 8.
- -
- 9.
- Pygofer with triangular posteroventral processes, internal processes triangular (Figure 23B); aedeagal shaft straight, with processes slightlybroadened at apex (Figure 23F).........................................................................................................................................................malleus sp. nov.
- -
- Pygofer with longer dorsal processes and ventro-posterior processes, internal processes hook-like (Figure 22B); aedeagal shaft slightly curvedanteriorly, with processes tapering to apex (Figure 22E,F)…………………….....................................................................dimiprocessus sp. nov.
- 10.
- Pygofer without processes on caudal margin (Figure 21B); style with prominent preapical heel, without teeth (Figure 21D); aedeagalapodeme swollen in lateral view (Figure 21F)........................................................................................................................limpidissimus sp. nov.
- -
- Pygofer with processes on caudal margin (Figure 20B); style with prominent preapical heel, with teeth (Figure 20D); aedeagal apodemesmooth in lateral view (Figure 20F)..............................................................................................................................................................................11
- 11.
- Pygofer with two large triangular processes at posteroventral margin (Figure 20B); style with several subapical tooth-like processes onlateral margin (Figure 20D)..............................................................................................................................................................recipinams sp. nov.
- -
- Pygofer with a long hook-like process at dorsoposterior margin (Figure 18B); style without tooth-like processes on lateral margin (Figure 18D)..........................................................................................................................................................................................................cowboyocreus sp. nov.
- 12.
- Aedeagus with a pair of apical processes (Figure 9E,F)..................................................................................................................binarius sp. nov.
- -
- Aedeagus with two pairs of apical processes (Figure 10E,F).....................................................................................................................................13
- 13.
- Aedeagal processes short, only 1/3 length of shaft (Figure 10E,F)..............................................................................................densprint sp. nov.
- -
- One pair of aedeagal processes equal to 1/2 length of shaft......................................................................................................................................14
- 14.
- -
- 15.
- Aedeagal processes slight curved anteriorly (Figure 12F), apical processes curved upper in caudal view (Figure 12E)...................................................................................................................................................................................................................................chrysanthemum sp. nov.
- -
- 16.
- Aedeagal shaft abruptly narrowed to distal third of shaft (Figure 15F), bases of two pairs of apical processes separated (Figure 15E,F)............................................................................................................................................................................................................exproiecturus sp. nov.
- -
3.3. Species Descriptions
3.3.1. Xestocephalus asper Linnavuori, 1969, n. rec.
3.3.2. Xestocephalus guttulatus (Motschulsky, 1859)
3.3.3. Xestocephalus ishidae Matsumura, 1914, n. rec.
3.3.4. Xestocephalus gracilus sp. nov.
3.3.5. Xestocephalus nonattribus sp. nov.
3.3.6. Xestocephalus binarius sp. nov.
3.3.7. Xestocephalus densprint sp. nov.
3.3.8. Xestocephalus toroensis Matsumura, 1914, n. rec.
3.3.9. Xestocephalus chrysanthemum sp. nov.
3.3.10. Xestocephalus tenusis sp. nov.
3.3.11. Xestocephalus exproiecturus sp. nov.
3.3.12. Xestocephalus abyssinicus Heller and Linnavuori, 1968, n. rec.
3.3.13. Xestocephalus cowboyocreus sp. nov.
3.3.14. Xestocephalus recipinams sp. nov.
3.3.15. Xestocephalus limpidissimus sp. nov.
3.3.16. Xestocephalus dimiprocessus sp. nov.
3.3.17. Xestocephalus malleus sp. nov.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Worldwide Checklist of the Genus Xestocephalus
References
- Ashton, P.S. Thailand: Biodiversity center for the tropics of Indo-Burma. J. Sci. Soc. Thail. 1990, 16, 107–116. [Google Scholar] [CrossRef]
- Sharkey, M.; Clutts, S. TIGER: Thailand Inventory Group for Entomological Research Newsletter. Available online: http://sharkeylab.org/tiger/docs/TIGER_newsletter_1.pdf (accessed on 5 February 2021).
- Dai, W.; Dietrich, C.H. Review of the old world leafhopper genus Scaphoidella Vilbaste (Hemiptera: Cicadellidae: Deltocephalinae), with description of ten new species from Thailand and Vietnam. Ann. Soc. Entomol. Fr. 2010, 47, 457–473. [Google Scholar] [CrossRef]
- Dai, W.; Dietrich, C.H. Review of the oriental leafhopper genus Lampridius Distant (Hemiptera: Cicadellidae: Deltocephalinae), with description of a related new genus. Zool. Sci. 2011, 28, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Dietrich, C.H.; Zhang, Y.L. A review of the leafhopper tribe Hyalojassini (Hemiptera: Cicadellidae: Iassinae) with description of new taxa. Zootaxa 2015, 3911, 1–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dietrich, C.H. A remarkable new genus of Nirvanini (Hemiptera: Cicadellidae: Evacanthinae) from Southeast Asia. Zootaxa 2011, 2970, 63–67. [Google Scholar] [CrossRef]
- Dietrich, C.H. Two new genera of Dikraneurini (Hemiptera, Cicadellidae, Typhlocybinae) from Thailand with unusual hind wing venation. Entomotaxonomia 2013, 35, 138–145. [Google Scholar]
- Duan, Y.; Dietrich, C.H.; Webb, M.D.; Zhang, Y.L. New taxa and new records of Deltocephalini leafhoppers from Thailand (Hemiptera: Cicadellidae: Deltocephalinae). Zootaxa 2017, 4350, 363–373. [Google Scholar] [CrossRef]
- Dmitriev, D.A. 3i World Auchenorrhyncha Database. Available online: http://dmitriev.speciesfile.org (accessed on 19 May 2021).
- Dietrich, C.H.; Vega, F.E. Leafhoppers (Homoptera: Cicadellidae) from Dominican Amber. Ann. Entomol. Soc. Am. 1995, 88, 263–270. [Google Scholar] [CrossRef]
- Beirne, B.P. Leafhoppers (Homoptera: Cicadellidae) of Canada and Alaska. Can. Entomol. 1956, 88, 1–180. [Google Scholar] [CrossRef]
- Cwikla, P.S.; Blocker, H.D. An annotated list of the leafhoppers (Homoptera: Cicadellidae) from tallgrass prairie of Kansas and Oklahoma. Trans. Kans. Acad. Sci. 1981, 84, 89–97. [Google Scholar] [CrossRef]
- Oman, P.W. The Nearctic leafhoppers (Homoptera: Cicadellidae). A generic classification and check list. Wash. Ent. Soc. Mem. 1949, 3, 1–253. [Google Scholar]
- Rakitov, R.A. Nymphal biology and anointing behaviors of Xestocephalus desertorum (Berg) (Hemiptera: Cicadellidae), a leafhopper feeding on grass roots. J. N. Y. Entomol. Soc. 2000, 108, 171–180. [Google Scholar] [CrossRef]
- Baker, W.L. Transmission by leafhoppers of the virus causing phloem necrosis of American elm. Science 1948, 108, 307–308. [Google Scholar] [CrossRef]
- Nielson, M.W. Leafhopper systematics. In The Leafhoppers and Planthoppers; John Wiley & Sons: New York, NY, USA, 1985; pp. 11–39. [Google Scholar]
- Rosenberger, D.A.; Jones, A.L. Leafhopper vectors of peach X-disease pathogen and its seasonal transmission from chokecherry. Phytopathology 1978, 68, 782–790. [Google Scholar] [CrossRef]
- Cwikla, P.S. Classification of the genus Xestocephalus (Homoptera: Cicadellidae) for North and Central America including the West Indies. Brenesia 1985, 24, 175–272. [Google Scholar]
- Linnavuori, R. Revision of the Neotropical Deltocephalinae and some related subfamilies (Homoptera). Ann. Zool. Soc. 1959, 20, 1–370. [Google Scholar]
- DeLong, D.M.; Linnavuori, R. New tropical Xestocephalus (Homoptera: Cicadellidae) and illustrations of little known species. J. Kans. Entomol. Soc. 1978, 51, 35–41. [Google Scholar]
- DeLong, D.M.; Wolda, H.; Estribi, M. The Xestocephaline leafhoppers (Homoptera: Cicadellidae) known to occur in Panama. Brenesia 1980, 17, 251–280. [Google Scholar]
- DeLong, D.M. New species of Xestocephalinae (Homoptera: Cicadellidae) from Mexico, Panama, Peru and Brazil. Proc. Entomol. Soc. Wash. 1982, 84, 391–396. [Google Scholar]
- Freytag, P.H. A review of the species of the genus Xestocephalus found in Colombia (Hemiptera: Cicadellidae: Xestocephalinae), with the description of 40 new species. Trans. Am. Entomol. Soc. 2020, 146, 265–303. [Google Scholar] [CrossRef]
- Linnavuori, R. Revision of the African Cicadellidae (Homoptera Auchenorrhyncha). Part II. Rev. Zool. Bot. Afr. 1979, 93, 929–1010. [Google Scholar]
- Evans, J.W. Les Cicadellidae de Madagascar (Homoptères). Mémoires Inst. Sci. Madag. 1953, 4, 87–137. [Google Scholar]
- Evans, J.W. The leafhoppers and froghoppers of Australia and New Zealand (Homoptera: Cicadelloidea and Cercopoidea). Aust. Mus. Mem. 1966, 12, 1–347. [Google Scholar] [CrossRef]
- Knight, W.J. Leafhoppers of New Zealand: Subfamilies Aphrodinae, Jassinae, Xestocephalinae, Idiocerinae, and Macropsinae (Homoptera:Cicadellidae). N. Z. J. Zool. 1974, 1, 475–493. [Google Scholar] [CrossRef]
- Distant, W.L. The phynchota-homoptera. In The Fauna of British India Including Ceylon and Burma; Bingham, C.T., Ed.; The Secretary of State for India in Council: London, UK, 1908; Volume 4, pp. 1–501. [Google Scholar]
- Distant, W.L. Rhynchota-homoptera: Appendix. Heteroptera: Addenda. In The Fauna of British India, Including Ceylon and Burma; Shipley, A.E., Marshall, A.K.G., Eds.; The Secretary of State for India in Council: London, UK, 1918; Volume 7, pp. 1–210. [Google Scholar]
- Ishihara, T. The family Xestocephalidae of Japan (Hemiptera). Trans. Shikoku Ent. Soc. 1961, 7, 19–25. [Google Scholar]
- Kamitani, S. A revision of the genus Xestocephalus Van Duzee (Homoptera, Cicadellidae) of Japan, Part 1. Jpn. J. Ent. 1996, 64, 602–613. [Google Scholar]
- Kamitani, S. A revision of the genus Xestocephalus Van Duzee (Auchenorrhyncha, Cicadellidae, Xestocephalinae) of Japan, part 2. Jpn. J. System. Entomol. 2005, 11, 39–54. [Google Scholar]
- Kamitani, S. Taxonomic study on two Taiwanese species of the genus Xestocephalus (Auchenorrhyncha, Cicadellidae). Esakia 2008, 48, 41–46. [Google Scholar]
- Li, Z.Z.; Dai, R.H. Description of one new species of Xestocephalus from China. Entomotaxonomia 2005, 27, 263–265. [Google Scholar]
- Li, Z.Z.; Zhang, B. Description of one new species of Xestocephalus from Guizhou. Acta Zootaxonomica Sin. 2006, 31, 401–402. [Google Scholar]
- Kamitani, S.; Ubaidillah, R.; Kahono, S.; Ghani, I.A. Taxonomic study on four Southeast Asian species of the genus Xestocephalus (Auchenorrhyncha, Cicadellidae). Esakia 2009, 49, 95–101. [Google Scholar]
- Ishihara, T. Homoptera of Southeast Asia collected by the Osaka City University Biological Expedition to Southeast Asia 1957–1958. Kyungpook J. Biol. Sci. 1961, 1, 225–257. [Google Scholar]
- Rakitov, R.A. On differentiation of cicadellid leg chaetotaxy (Homoptera: Auchenorrhyncha: Membracoidea). Russ. Entomol. J. 1998, 6, 7–27. [Google Scholar]
- Dietrich, C.H. Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Fla. Entomol. 2005, 88, 502–517. [Google Scholar] [CrossRef]
- Van Duzee, E.P. A synoptical arrangement of the genera of the North American Jassidae, with descriptions of some new species. Trans. Am. Entomol. Soc. 1892, 19, 295–307. [Google Scholar]
- Van Duzee, E.P. Descriptions of some new North American homopterous insects. Bull. Buffalo Soc. Nat. Sci. 1894, 5, 205–216. [Google Scholar]
- Lindberg, H. Die Cicadinen der Kanarischen Inseln. Commentat. Biol. 1936, 4, 1–19. [Google Scholar]
- Holdhaus, K. Kritisches Verzeichnis der bisher von den Samoainseln bekannten Orthopteren. Denkschr. Kais. Akad. Wiss. 1909, 84, 537–562. (In German) [Google Scholar]
- Metcalf, Z.P. New names in the Homoptera. J. Wash. Acad. Sci. 1952, 42, 226–231. [Google Scholar]
- Kirkaldy, G.W. Leafhoppers supplement (Hemiptera). In Report of Work of the Experiment Station of the Hawaiian Sugar Planters’ Association; Hawaiian Sugar Planters’ Association: Honolulu, HI, USA, 1907; Volume 3, pp. 1–186. [Google Scholar]
- Oman, P.W. A generic revision of the Nearctic Cicadellidae. Bull. George Wash. Univ. 1943, 43, 15–17. [Google Scholar]
- Evans, J.W. A natural classification of leafhoppers (Jassoidea, Homoptera). Part 3. Trans. Entomol. Soc. Lond. 1947, 98, 105–271. [Google Scholar] [CrossRef]
- Linnavuori, R. Neotropical Homoptera of the Hungarian National Museum and some other European Museums. Ann. Entomol. Fenn. 1956, 22, 5–35. [Google Scholar]
- Linnavuori, R. Insects of Micronesia. Homoptera: Cicadellidae. Bishop Mus. 1960, 6, 231–344. [Google Scholar]
- Linnavuori, R. Cicadelliae (Homoptera, Auchenorrhyncha) of Fiji. Acta Entomol. Fenn. 1960, 15, 1–71. [Google Scholar]
- Hamilton, K.G.A. A review of the Northern Hemisphere Aphrodina (Rhynchota: Homoptera: Cicadellidae), with special reference to the Nearctic fauna. Can. Entomol. 1975, 107, 1009–1027. [Google Scholar] [CrossRef]
- Hamilton, K.G.A. Review of the tribal classification of the leafhopper subfamily Aphrodinae (Deltocephalinae of authors) of the Holarctic region (Rhynchota: Homoptera: Cicadellidae). Can. Entomol. 1975, 107, 477–498. [Google Scholar] [CrossRef]
- Motschulsky, V.I. Homoptères. In Insectes des Indes Orientales, et de Contrées Analogues; Motschulsky, V.I., Ed.; Etudes Entomologiques: Paris, France, 1859; Volume 8, pp. 25–118. [Google Scholar]
- Berg, C. Hemiptera Argentina. Ann. Soc. Cient. Argent. 1879, 8, 241–272. [Google Scholar]
- Provancher, L. Additions et corrections. In Petite Faune Entomologique du Canada, Précédée d’Un Traité Élémentaire d’Entomologiev; C. Darveau: Sainte-Sophie, QC, Canada, 1890; Volume 3, pp. 335–340. [Google Scholar]
- Peters, H.T. The genus Xestocephalus (Homoptera, Cicadellidae). J. Kansas Entomol. Soc. 1933, 6, 73–80. [Google Scholar]
- DeLong, D.M. New South American Xestocephaline leafhoppers (Homoptera: Cicadellidae). Ent. News 1980, 91, 79–84. [Google Scholar]
- Cwikla, P.S. Description of last nymphal instar of Xestocephalus ancorifer (Homoptera: Cicadellidae). Ent. News 1984, 95, 40–42. [Google Scholar]
- Matsumura, S. Die Jassinen und einige neue Acocephalinen Japans. J. Sapporo Agric. Coll. 1914, 5, 165–240. [Google Scholar]
- Matsumura, S. Homopterous insects at Kotosho, Formosa, by Mr. Tadao Kano. Ins. Mats. 1940, 15, 34–51. [Google Scholar]
- Capco, S.R. Philippine species of Xestocephalus Van Duzee (Cicadellidae, Homoptera) in the Baker collection, United States National Museum. Philipp. J. Sci. 1960, 89, 41–46. [Google Scholar]
- Melichar, L. Homopteren von Java, Gesammelt von Herrn Edw. Notes Leyden Mus. 1914, 36, 91–146. [Google Scholar]
- Merino, G. Philippine Cicadellidae (Homoptera). Philipp. J. Sci. 1936, 61, 307–400. [Google Scholar]
- Linnavuori, R. Contribution à la faune du Congo (Brazzaville). Mission, A. Villiers et A. Descarpentries XCIII. Hemipteres Hylicidae et Cicadellidae. Bull. Inst. Fondam. Afr. Noire Ser. A 1969, 31, 1129–1185. [Google Scholar]
- Motschulsky, V.I. Essai d’un catalogues des insectes de l’île Ceylan. Mém. Soc. Imp. Amis Sci. Nat. 1863, 36, 1–153. [Google Scholar]
- Matsumura, S. Monographie der Jassinen Japans. Term. Fuzet. 1902, 15, 353–404. [Google Scholar]
- Melichar, L. Homopteren-Fauna von Ceylon; Verlag von Felix L. Dames: Berlin, Germany, 1903; Volume i–iv, pp. 1–248. [Google Scholar]
- Melichar, L. Beitrag zur Kenntnis der Homopterenfauna Deutsch-Ost-Afrikas. Wien. Entomol. Ztg. 1905, 24, 279–304. [Google Scholar]
- Oshanin, V.T. Verzeichnis der Palaearktischen Hemipteren, mit Besonderer Berücksichtigung Ihrer Verteilung im Russischen Reiche. II. Band. Homoptera; Lieferung, I., der Buchdr, K., Eds.; Akademie der Wissenschaften: St. Petersburg, Russia, 1906; Volume 11, pp. 1–192. [Google Scholar]
- Oshanin, V.T. Verzeichnis der Palaearktischen Hemipteren mit Besonder Berücksichtigung Ihrer Verteilung im Russischen Reiche. III. Band. Nachträge und Verbesserungen zum I und II. Bande; der Buchdr, K., Ed.; Akademie der Wissenschaften: St. Petersburg, Russia, 1910; Volume 15, pp. 1–218. [Google Scholar]
- Oshanin, V.T. Katalog der Paläarktischen Hemipteren (Heteroptera, Homoptera-Auchenorhyncha und Psylloideae); R. Friedländer & Sohn: Berlin, Germany, 1912; Volume I–XVI, pp. 1–187. [Google Scholar]
- Nawa, U. Investigation of insects injurious to rice plants. Insect World 1914, 18, 189–194. [Google Scholar]
- Matsumura, S. Neue Cicadinen Koreas. Trans. Sapporo Nat. Hist. Soc. 1915, 5, 154–184. [Google Scholar]
- Schumacher, F. Der gegenwärtige Stand unserer Kenntnis von der Homopteren-Fauna der Insel Formosa unter besonderer Berücksichtigung von Sauter’schem material. Mitt. Zool. Mus. 1915, 8, 73–134. [Google Scholar]
- Schumacher, F. Homoptera in H. Sauter’s Formosa Ausbeute Suppl. Entomol. 1915, 4, 108–142. [Google Scholar]
- China, W.E. The terrestrial Hemiptera of the German Limnological Sunda-Expedition. Trop. Binn. 1935, 6, 295–307. [Google Scholar]
- Zachvatkin, A.A. Note on the Homoptera-Cicadina of Jemen. Otdiel Estestv. Istor. 1935, 4, 106–115. [Google Scholar]
- Ishihara, T. A tentative list of the superfamily Cicadelloidae of Japan (Homoptera). Sci. Rept. Matsuyama Agric. Coll. 1953, 11, 1–72. [Google Scholar]
- Esaki, T.; Ito, S. A tentative catalogue of Jassoidea of Japan, and her adjacent territories. Jpn. Soc. Promot. Sci. 1954, 1–315. [Google Scholar]
- Metcalf, Z.P. Fascicle VI. Cicadelloidea. Part 10. Euscelidae. Section III. In General Catalogue of the Homoptera; United States Department of Agriculture (USDA): Washington, DC, USA, 1967; pp. 2075–2695. [Google Scholar]
- Nast, J. Palaearctic Auchenorrhyncha (Homoptera). An Annotated Check List; Polish Scientific Publishers: Warszawa, Poland, 1972; pp. 1–550. [Google Scholar]
- Anufriev, G.A.; Emeljanov, A.F. Suborder Cicadinea (Auchenorrhyncha). In Keys to the Insects of the Far East of the USSR; Ler, P.A., Ed.; Nauka: Saint Petersburg, Russia, 1988; Volume 2, pp. 12–495. [Google Scholar]
- Carvalho, A.N.; Cavichioli, R.R. Xestocephalus van Duzee: Descriptions of six new species (Hemiptera, Auchenorrhyncha, Cicadellidae, Xestocephalinae). Rev. Bras. Zool. 2001, 18, 869–881. [Google Scholar] [CrossRef]
- Cai, P.; He, J.H.; Gu, X.L. Homoptera: Cicadellidae. In Insects of Tianmushan National Nature Reserve; Wu, H., Pan, C.W., Eds.; Science Press: Beijing, China, 2001; pp. 185–218. [Google Scholar]
- Heller, F.; Linnavuori, R. Cicadelliden aus Äthiopien. Stuttg. Beitr. Naturkd. 1968, 186, 1–42. [Google Scholar]

























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Dietrich, C.H.; Dai, W. Remarkable Species Diversity of the Leafhopper Genus Xestocephalus (Hemiptera: Cicadellidae: Aphrodinae) in Thailand. Insects 2021, 12, 514. https://doi.org/10.3390/insects12060514
Liang Z, Dietrich CH, Dai W. Remarkable Species Diversity of the Leafhopper Genus Xestocephalus (Hemiptera: Cicadellidae: Aphrodinae) in Thailand. Insects. 2021; 12(6):514. https://doi.org/10.3390/insects12060514
Chicago/Turabian StyleLiang, Zonglei, Christopher H. Dietrich, and Wu Dai. 2021. "Remarkable Species Diversity of the Leafhopper Genus Xestocephalus (Hemiptera: Cicadellidae: Aphrodinae) in Thailand" Insects 12, no. 6: 514. https://doi.org/10.3390/insects12060514
APA StyleLiang, Z., Dietrich, C. H., & Dai, W. (2021). Remarkable Species Diversity of the Leafhopper Genus Xestocephalus (Hemiptera: Cicadellidae: Aphrodinae) in Thailand. Insects, 12(6), 514. https://doi.org/10.3390/insects12060514





