Simple Summary
In a framework of sustainable agriculture, strategies aimed at preserving and enhancing pest natural enemies are crucial. However, knowledge about the parasitoid complex associated with a particular pest is often fragmentary. Herein, we investigated the parasitoids associated with the European grapevine moth, one of the main vine pests in the Mediterranean area, in a natural context, where the moth lives on Daphne gnidium, deemed as its original host plant. We observed a heterogeneous and complex community, consisting of a few predominant parasitoid species, followed by satellite species, and occasional parasitoids. Parasitic wasps, such as Campoplex capitator and Trichogramma spp., can be also found in the vineyards, thus understanding their dynamics in the wild could be useful to improve biological control strategies for managing EGVM populations.
Abstract
The European grapevine moth (EGVM), Lobesia botrana (Lepidoptera: Tortricidae), is one of the major concerns for vineyard managers in the Mediterranean area. It is a polyphagous moth, which develops on a wide variety of host plants, among which the spurge flax, Daphne gnidium (Thymelaeaceae), very likely represents its originary wild host plant. In this study, we investigated the parasitoid complex of L. botrana feeding on D. gnidium during a three-year sampling in a natural reserve in Tuscany, Italy, where this plant is extremely abundant while the grapevine is absent. A total of 24 species of parasitoids were obtained from eggs, larvae, and pupae of EGVM, belonging to 6 families of Hymenoptera and a family of Diptera. The ichneumonid wasp Campoplex capitator was the most abundant larval parasitoid. Four species of the genus Trichogramma were obtained from parasitized eggs during the first year of sampling, with a peak in the parasitisation during the EGVM 3rd generation. Some of the main EGVM parasitoids on spurge flax were also observed in vineyards, although a certain degree of redundancy was observed in the wild, due to several less frequent “satellite” species exploiting the same host. Overall, this research sheds light on the parasitoid community and dynamics of this important moth pest in a grapevine-free natural ecosystem, discussing the possible role of natural areas as ecological reservoirs of pest natural enemies.
1. Introduction
Biodiversity conservation represents one of the main and attended objective of a sustainable agriculture [1,2]. Since the 1950s, when organochlorine insecticides were firstly introduced, concerns over side-effects of insecticides have grown, leading to reconsider insecticides as the lone control strategies against pests [3,4,5]. Consequences of insecticide overuse in agricultural settings include environmental pollution [6], toxicity towards non-target insects [7,8,9,10], and even risks for human health [11,12,13]. In this context, a strategy aimed at preserving pest natural enemies through environmental heterogeneity is encouraged. As predator and parasitoid insects rely on multiple resources for adult feeding and reproduction, enhancing the environmental heterogeneity can be a driver of their diversity at local scale, boosting species that forage on both semi-natural and crop habitats [14]. In addition, the presence of interconnected wild areas within an urban or rural landscape may promote species dispersal [15,16]. This concept applies well to the vineyard agroecosystem since the agricultural matrix can contribute to maintaining insect diversity by providing higher diversity of resources [17,18,19,20,21]. In Italy, the agricultural territory is characterised by a high fragmentation of the crops, with a landscape matrix where woods and Mediterranean scrubland habitats alternate with cultivated fields [14,22,23,24]. A good example is represented by the high-value vineyards characterizing Tuscany (Central Italy), a top producing wine region worldwide [4,14].
The European grapevine moth (EGVM), Lobesia botrana (Denis and Schiffermüller, 1775) (Lepidoptera: Tortricidae), is the main insect pest in most of the European wine-growing areas [25,26]. In recent years it has been introduced into the Americas, with first detections in Chile in 2008, California (USA) in 2009 and Argentina in 2010 [27,28]. EGVM is a polyphagous moth, which develops on a wide variety of host plants [29]. Among these, spurge flax, Daphne gnidium L. (Thymelaeaceae), very likely represents its originary wild host plant [30,31,32,33]. Several studies focused on the parasitoid complex of EGVM in the European vineyards [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59], but very little is still known about parasitoids developing on wild EGVM populations [32,59,60,61,62,63,64]. In this scenario, we investigated the community structure of EGVM parasitoids on D. gnidium in a wild area where grapevine is absent, during a three-year sampling. Moreover, a comparison with the parasitoid species complex associated with EGVM in the vineyards is provided.
2. Materials and Methods
2.1. Study Area
The study was carried out in the Regional Reserve of Migliarino, San Rossore, Massaciuccoli (Tuscany, Central Italy), a natural area that extends for 23,100 ha along the seacoast, from Viareggio to Pisa (Figure 1). This reserve is characterized by a sub-humid climate [65], with a mixed holm oak wood (Quercus ilex L.), partly replaced by anthropogenic coastal pine forests on the ancient dunes. The forest is characterized by a prevalence of stone pine (Pinus pinea L.) followed by oaks, alders (Alnus spp.), ashes (Fraxinus spp.), and common oaks (Quercus robur L.), while the understory consists of common myrtle (Myrtus communis L.), lentisk (Pistacea lentiscus L.), narrow-leaved mock privet (Phyllirea angustifolia L.), and blackberry (Rubus fruticosus L.). Moving towards the seashore, the dune develops for about 200 m, and it is characterized by the maritime pine (Pinus pinaster Aiton), planted as a windbreak to protect the artificial inland stone pine plantation, followed by a severely depleted coastal portion with Juniperus spp. [66,67,68]. D. gnidium largely occurs in all these habitats [67,68], whereas the grapevine is absent, and the nearest commercial vineyards are about 6 km away. D. gnidium is particularly widespread in the open spaces, where the sunlight can easily penetrate. Starting from March to October, the sprouts, flowers, or fruits of the bushes can host a diverse community of lepidopteran preimaginal stages, while EGVM eggs and larvae represent a suitable and abundant resource for a rich community of predators and parasitoids [59,62,63,69,70].
Figure 1.
Map of the shoreline north of Pisa, showing the Regional Reserve of Migliarino, San Rossore, Massaciuccoli (Tuscany, Central Italy); the study area is delimitated in red.
2.2. Sampling Design
Field observations were carried out inside the above-mentioned Natural Reserve in a selected rectangular-shaped area of about 150 ha, delimited by the following geographical points DD (43.733642° N, 10.277524° E; 43.712864° N, 10.279648° E; 43.732913° N, 10.292371° E; 43.720101° N, 10.293094° E). In this area, D. gnidium shows a spotted distribution, depending on the presence of open sunny spaces (Figure 2a). To get a homogeneous distribution of our samplings, a grid of 3 × 3 rectangles of 500 per 300 m side was superimposed on the selected area, being field observations on these nine sites carried out during 2014, 2015, and 2017. Each year, the sampling started at the end of April/beginning of May, with the appearance of the first visible nests of EGVM on D. gnidium sprouts, then lasting until the first week of October. Weekly, ten different plant per site were randomly selected and two infested sprouts for each plant were collected, totalizing 180 sprouts all over the selected area (Figure 2b). The sprouts were analysed under the stereomicroscope to isolate and identify EGVM preimaginal stages (1st instar larvae, 2nd–3rd instar larvae, 4th–5th instar larvae, and chrysalids). Preimaginal stages were stored in vials, supported with D. gnidium vegetal tissues as food, plugged with cotton to allow aeration, and maintained at natural environmental conditions of temperature and photoperiod until the insect emergence. Braconidae were identified by A. Loni and K. Samartsev (St. Petersburg, Russia) following Huddleston [71] and Loni et al. [62]; Chalcidoidea (excl. Trichogrammatidae) by Pier Luigi Scaramozzino, following Peck et al. [72]; Tachinidae by Filippo Di Giovanni, Pier Luigi Scaramozzino and Pierfilippo Cerretti (Rome, Italy) following Cerretti et al. [73]; Ichneumonidae have been identified by Filippo Di Giovanni and Pier Luigi Scaramozzino, following Aeschlimann [74], Kasparyan [75], Kasparyan [76], Gauld and Mitchell [77], Horstmann [78] and Tolkanitz [79]. In addition, from March to November 2015, the leaves and the tips of D. gnidium were examined to assess the presence of EGVM eggs parasitized by Trichogramma spp.; in each plot, the leaves of five buds on 20 shrubs were examined on a weekly basis, for a total of 100 shoots per plot. Eggs of EGVM were counted, distinguishing between healthy eggs and dark parasitized eggs. Leaves with parasitized eggs were stored in glass vials under controlled environmental conditions until parasitoid emergence. The emerged Trichogramma parasitoids were preserved in 96% (v:v) ethanol and identified at species level by Christoph Hoffmann (Siebeldingen, Germany) using the RFLP method and determination keys by Sumer et al. [80]; in one case (i.e., T. cacaeciae Marchal, 1927) ITS-2 DNA sequences were analysed at the Laboratory of Zoology and Integrated Production in Viticulture of the Julius Kühn-Institute (Quedlinburg, Germany) and compared to NCBI database. Results were partly published by Lucchi et al. [63]. All the insects were stored at the Department of Agriculture, Food and Environment, University of Pisa.

Figure 2.
(a) Daphne gnidium shrubs (arrows) in the Regional Reserve of Migliarino, San Rossore, Massaciuccoli (Tuscany, Central Italy). (b) Nest of Lobesia botrana on a D. gnidium sprout.
2.3. Host Parasitisation Rate and Statistical Analysis
For each year, the host parasitisation rate was calculated as the ratio between the number of emerged parasitoids and the total number of emerged specimens (hosts + parasitoids). The occupancy of the most represented species (i.e., species that have been collected in four out of nine of the selected sampling areas during at least one of the three years) was evaluated by considering the grid cells that contain at least one individual of the selected species on the nine sites of the grid [81,82]. Being Campoplex capitator Aubert, 1960 (Ichneumonidae, Campopleginae) the most abundant species of parasitoid emerged from infested sprouts of D. gnidium, the monthly emergence trend of this species during the three years was plotted and compared to that of EGVM using Kendall correlation coefficient (τ, significance threshold p < 0.05) as data were not normally distributed (test Shapiro-Wilk p < 0.05). The percentage of EGVM eggs parasitized by Trichogramma spp. in 2015 was counted, and a contingency analysis was carried out to test whether the number of parasitized eggs significantly varied over months. Finally, the community composition of parasitoids obtained from EGVM on D. gnidium was divided per guild, based on the attacked host stage. Then, parasitoids were further subdivided per guilds following Mills [83] and compared to the community composition of EGVM parasitoids as observed in vineyards or on spurge flax in different Italian regions [34,35,36,47,60,61,62,64].
3. Results
In our study, a total of 24 species of parasitoids were obtained from EGVM eggs, larvae, and pupae collected on D. gnidium during the three-year sampling in a grapevine-free natural environment (Table 1). From the total assemblage, 20 species of parasitoids belonging to 5 families of Hymenoptera (i.e., Braconidae, Eulophidae, Eupelmidae, Ichneumonidae, and Pteromalidae) and one family of Diptera (i.e., Tachinidae) were obtained from EGVM larvae and pupae collected during 2014, 2015, and 2017. In 2015, four species of parasitoids belonging to one family of Hymenoptera (i.e., Trichogrammatidae) were obtained from EGVM eggs collected on D. gnidium leaves. In 2014, 1223 adults of EGVM and 239 parasitoids were obtained, resulting in a host parasitisation rate of 16.35%. In 2015, 1070 adults of EGVM and 162 parasitoids were obtained, reaching a host parasitisation rate of 13.15%. During 2017, 1420 adults of EGVM and 163 parasitoids emerged, resulting in a host parasitisation rate of 10.30%. Overall, out of 3713 EGVM larvae or pupae, 564 parasitoids were obtained, with an average annual parasitisation rate of 13.27%. The family Ichneumonidae accounted for more than 80% of all the parasitoids, with a parasitisation rate ranging from about 9 to 12%. C. capitator was by far the most abundant species in our sampling, accounting for about 64% of all the emerged parasitoids, and with a parasitisation rate ranging from 7% to 9%.

Table 1.
Overall abundance of Lobesia botrana and its larval/pupal parasitoids (in bold) on Daphne gnidium over three years of field studies; family names and total number of parasitoids per family are in bold; for each parasitoid family as well as for Campoplex capitator, the host parasitisation rate (%) is given within brackets.
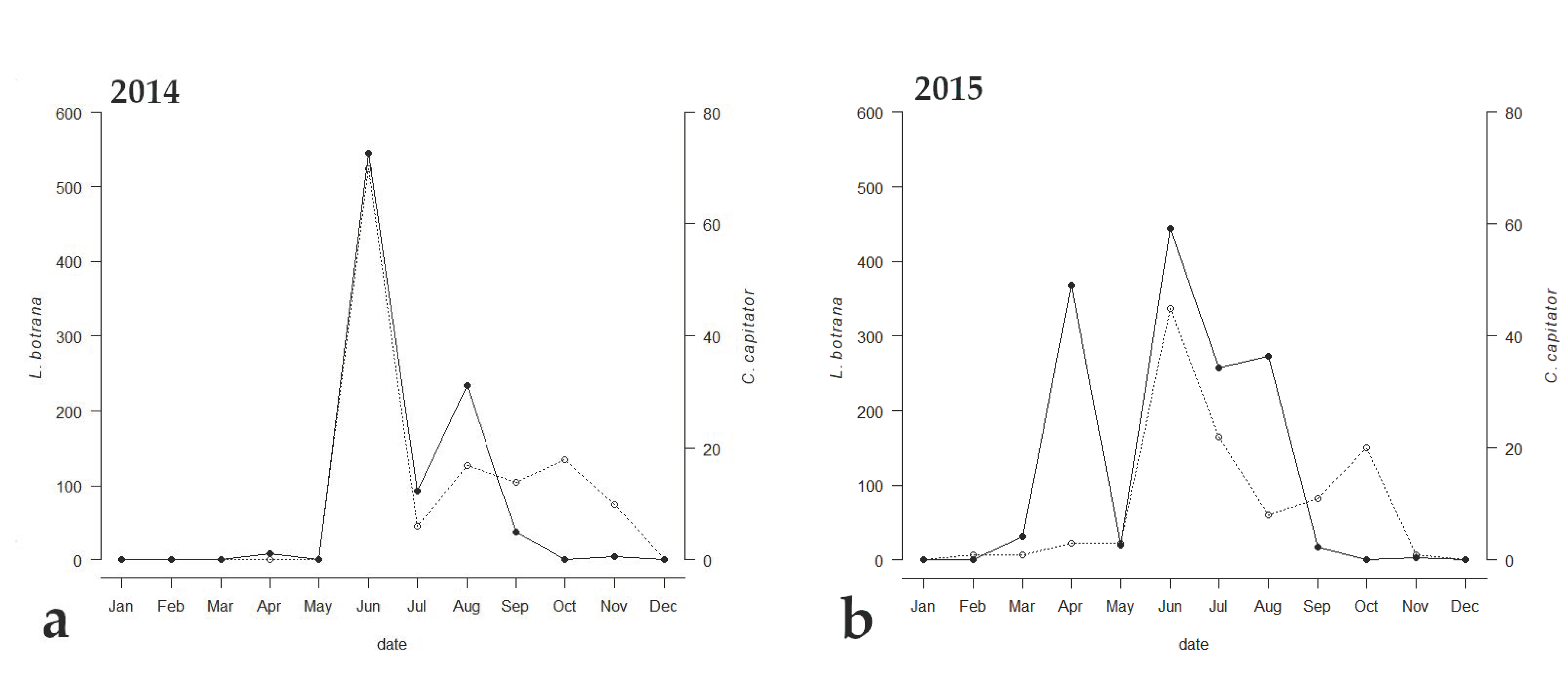
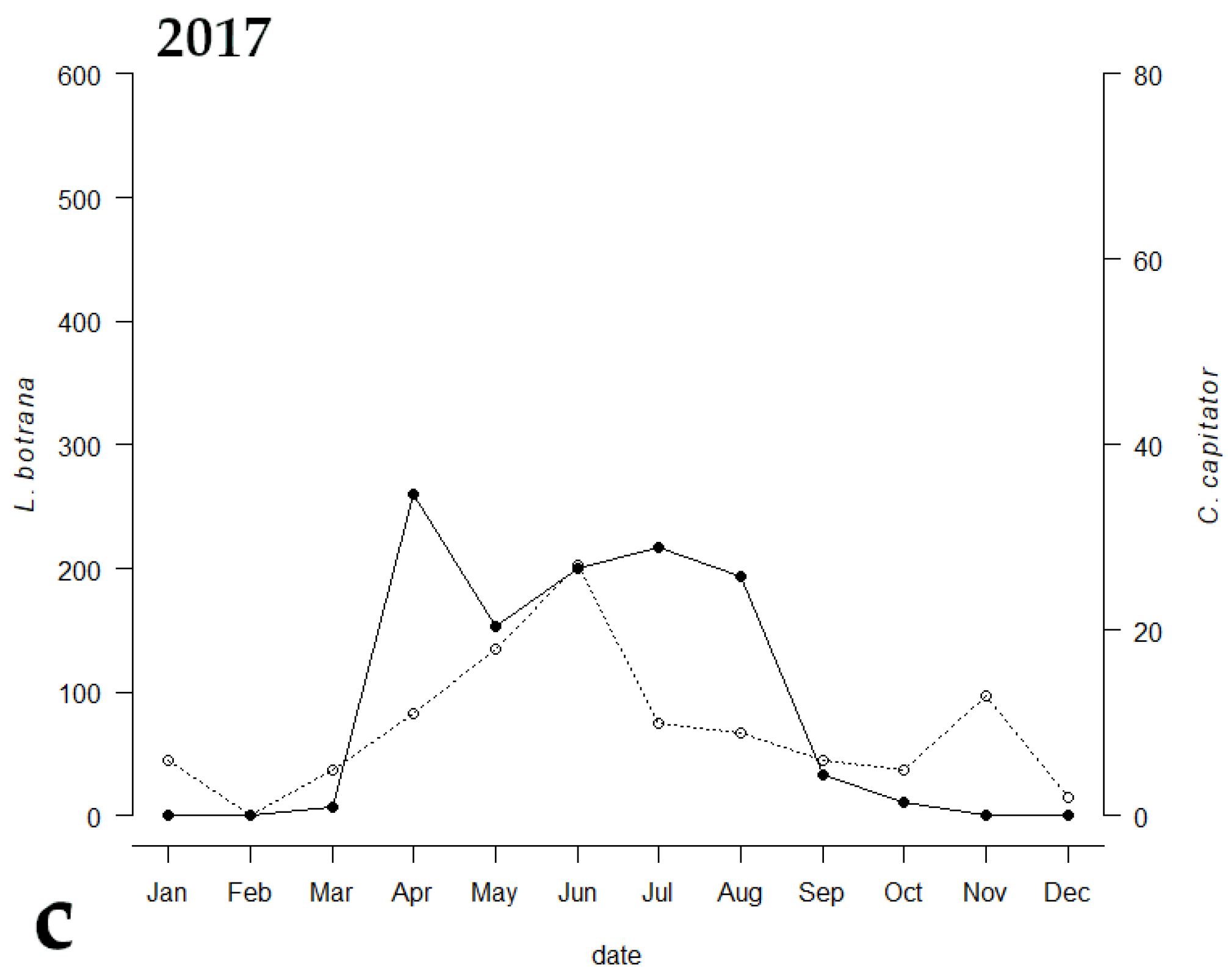
Among the emerged parasitoids, only 8 species reached an occupancy threshold of at least 44%, (Table 2). Only two species (i.e., C. capitator and Triclistus pallipes Holmgren, 1873 (Ichneumonidae, Metopiinae)) reached an occupancy percentage value of at least 44% all the three years, with C. capitator having an occupancy of 100% during all the sampling periods. Comparing the emergence trend of C. capitator with that of EGVM, a strong correlation between the two curves was observed (Figure 3). The Kendall correlation coefficient was strong in 2014, when the trend of the graphs of the two species was almost identical (τ = 0.588, p = 0.0179), and less but still strong in 2015 and 2017 (τ = 0.484, p = 0.0355 and τ = 0.437, p = 0.0526 respectively).

Table 2.
Occupancy (%) for parasitoid species of Lobesia botrana that reached a value of at least 44% (i.e., 4 out of 9 sampled areas) during the three-year field study in Tuscany, Italy.
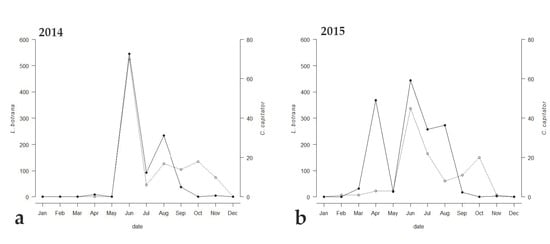
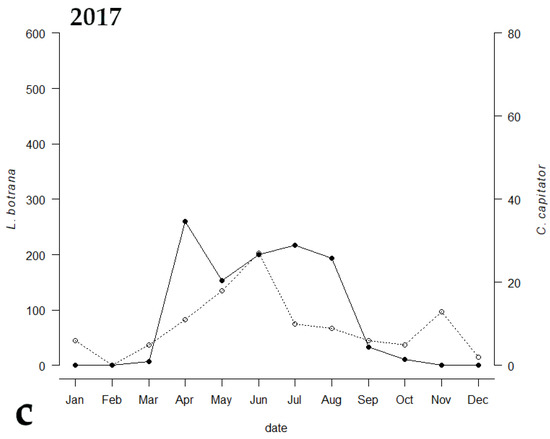
Figure 3.
Emergence trends of Lobesia botrana (solid line) and its parasitoid, Campoplex capitator (dotted line), in (a) 2014, (b) 2015, and (c) 2017 in Tuscany, Central Italy.
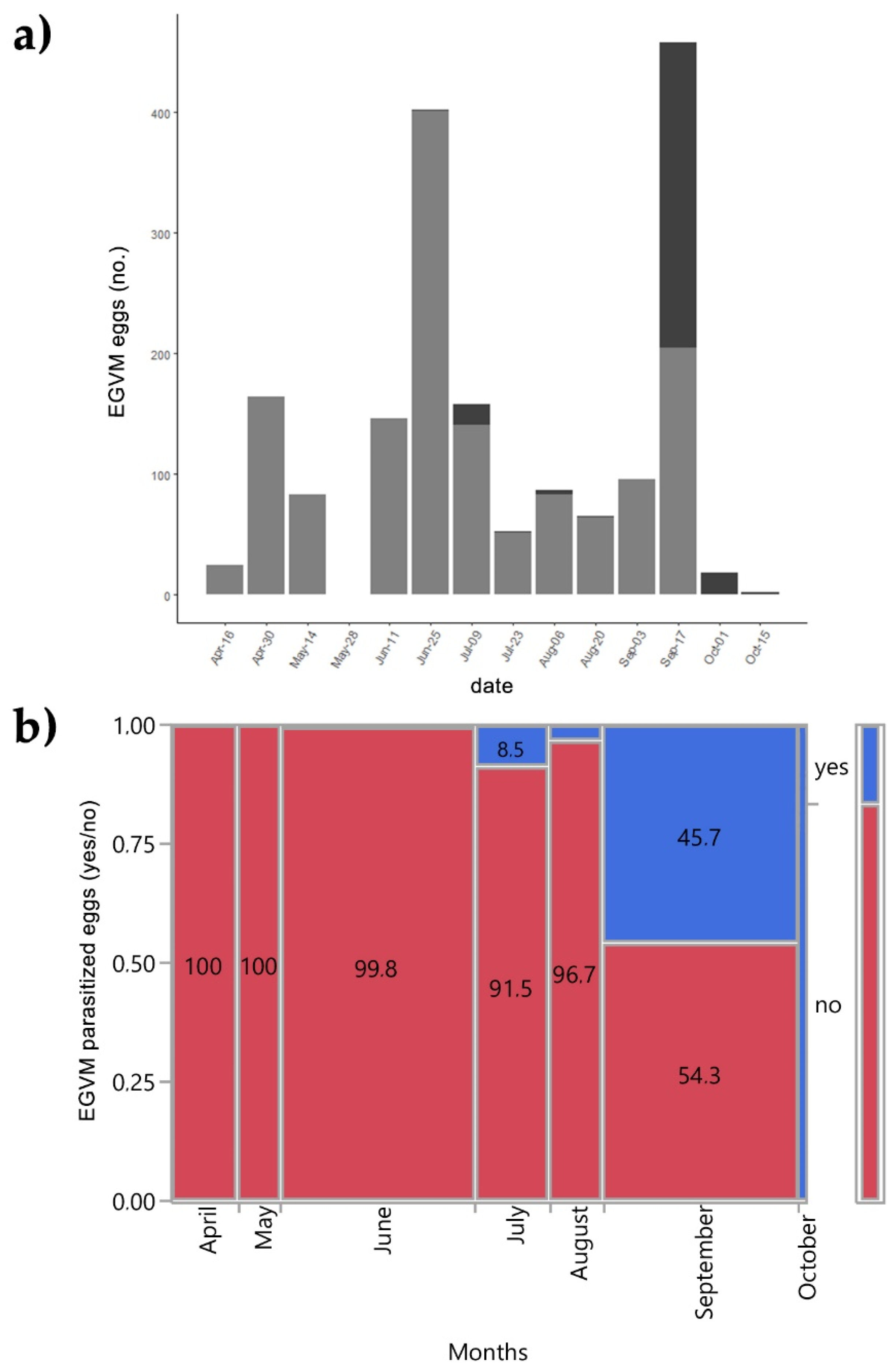
In 2015 the leaves and the tips of D. gnidium were also examined to assess the presence of EGVM eggs. Out of 1794 EGVM eggs found on the underside of leaves of D. gnidium shoots, 293 were parasitized by 4 Trichogramma species: T. cordubense Vargas and Cabello, 1985 (58.3%), T. evanescens Westwood, 1833 (35.4%), T. euproctidis (Girault, 1911) (4.2%) and T. cacaeciae Marchal, 1927 (2.1%) (see also Lucchi et al. [63]). The overall egg parasitisation rate was 16.33%. The number of parasitized eggs varies greatly over the months (χ2 = 665.42; p < 0.001). The first parasitized eggs were found at the end of June, during the second EGVM flight. No parasitized eggs were found between the end of August and the beginning of September. In mid-September, there was a strong resumption of Trichogramma spp. activity, with 271 out of 475 eggs parasitized, resulting in a parasitisation rate of 57% (Figure 4).
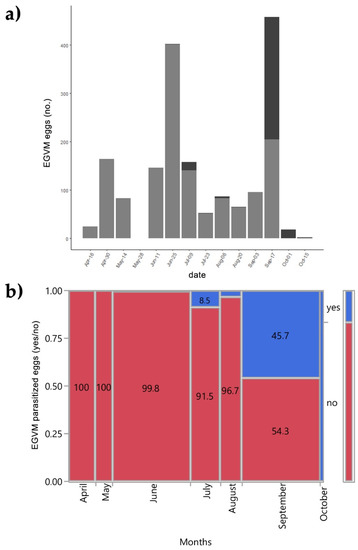
Figure 4.
(a) Field parasitisation of Lobesia botrana eggs by Trichogramma spp. during 2015 in Tuscany, Italy; light grey: eggs of L. botrana; dark grey: eggs of L. botrana parasitized by Trichogramma spp. (b) Contingency analysis between L. botrana eggs parasitized by Trichogramma spp. and months during 2015. Values within tiles are percentages.
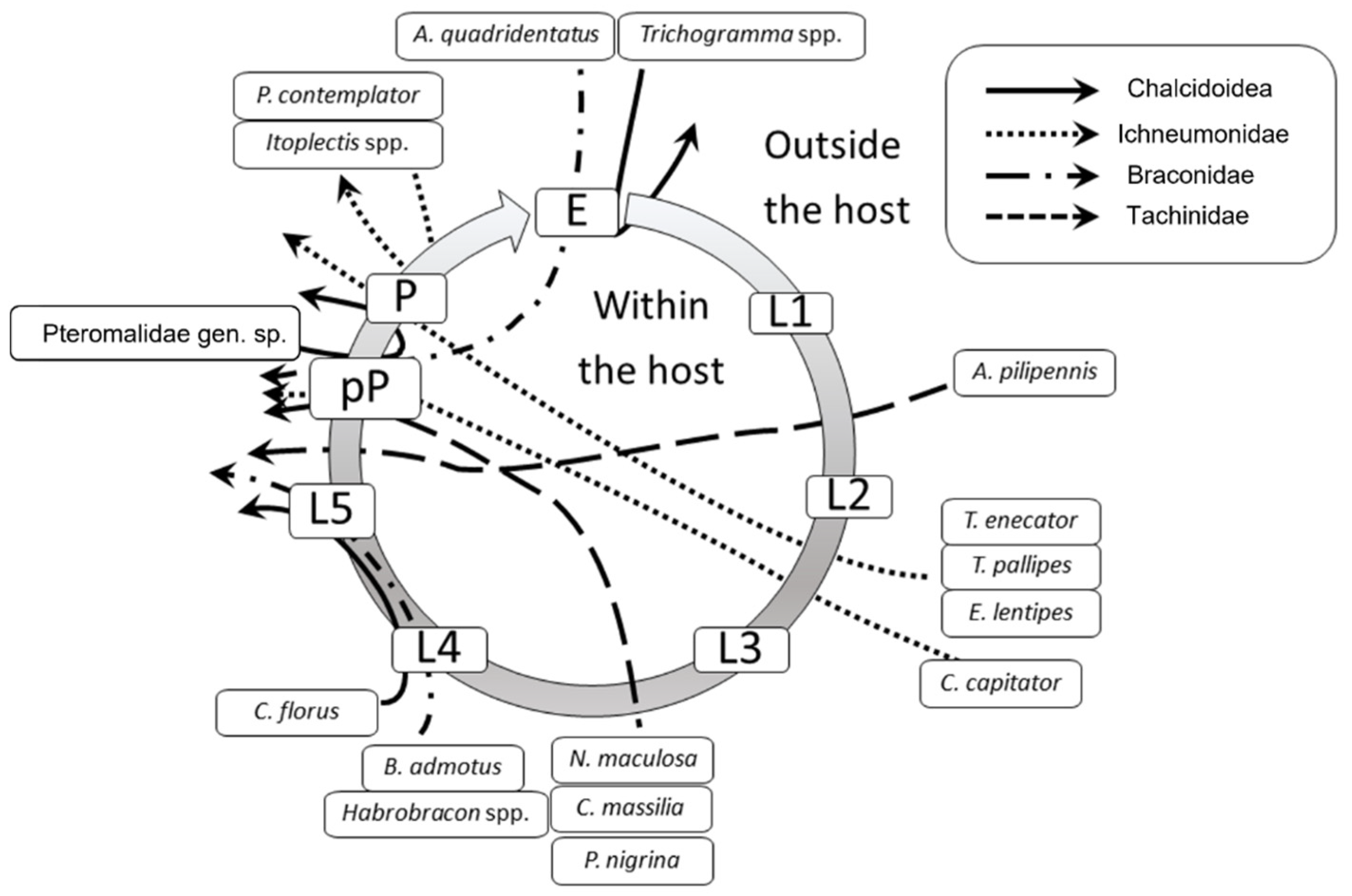
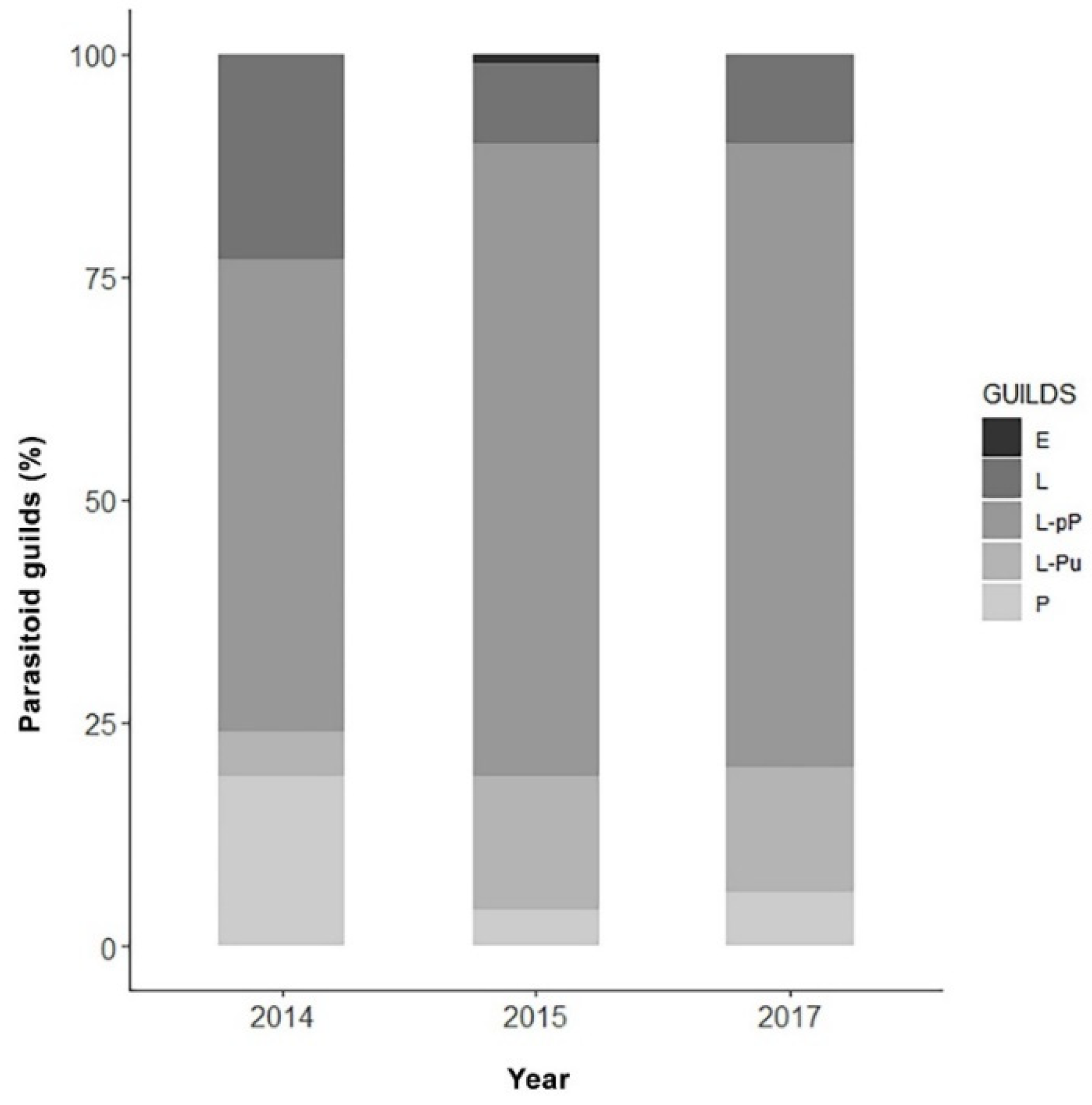
Figure 5 shows the parasitoid community associated with EGVM on D. gnidium divided per guilds, based on the host stage attacked and the host stage killed by the parasitoid. In 2015, the analysis of parasitized eggs revealed that Trichogramma spp. and the braconid Ascogaster quadridentata Wesmael, 1835 were the only parasitoids attacking EGVM eggs, with the former completing development in the moth’s egg, and the latter emerging at the prepupal stage. Most of the parasitoids observed attack the early (such as Actia pilipennis (Fallén, 1810), T. pallipes or C. capitator) or late (such as Habrobracon spp. or Colpoclypeus florus (Walker, 1839)) larval stages of the moth, emerging from the last stages of host development. Observing the parasitoid community over the years, the larvo-prepupal guild (L-pP) was the relatively richest in terms of number of species, accounting for more than 50% in 2014, and about 70% in 2015 and 2017, followed by the larvo-pupal and pupal guilds (L-Pu and P) and by the larval parasitoids (L) (Figure 6).
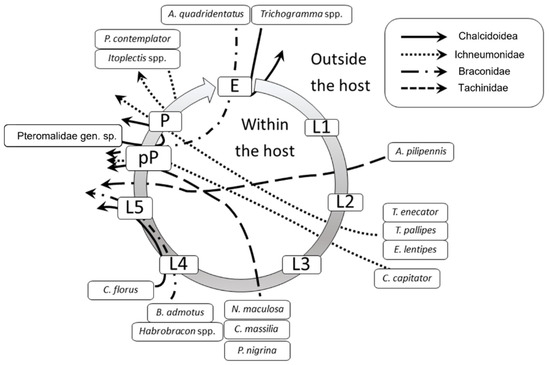
Figure 5.
Parasitoids attacking different Lobesia botrana (EGVM) instars on Daphne gnidium sprouts in Tuscany, Central Italy. The grey circle represents the EGVM development. E: egg; L1-L5: I–V larval stages; pP: prepupa; P: pupa. Arrows connect the host stage attacked to the host stage killed by the parasitoid. Arrows passing through the host circle indicate an endoparasitic development, whereas those remaining outside the circle indicate an ectoparasitic development.
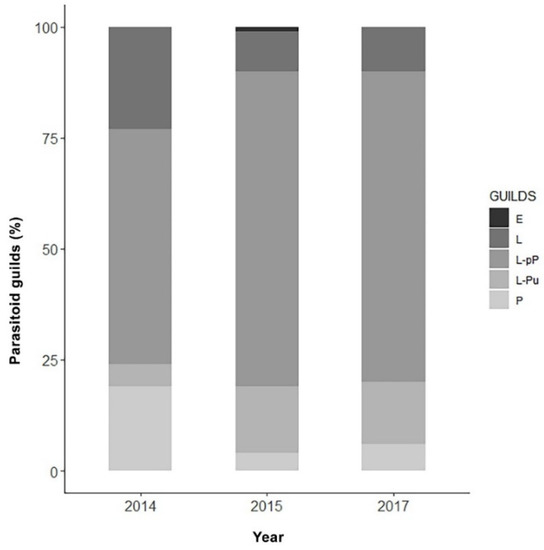
Figure 6.
Parasitoid guilds (%) obtained across the three-year sampling in Tuscany, Central Italy, on the base of the host stage attacked. E: parasitoids attacking eggs (excluding Trichogramma spp.); L: larval parasitoids; L-pP: larvo-prepupal parasitoids; L-Pu: larvo-pupal parasitoids; P: pupal parasitoids.
4. Discussion
The list of parasitoids associated with EGVM is extremely long, and several authors have attempted to provide a complete list of species parasitizing this lepidopteran [84,85,86,87]. Not all these records should be considered valid, mainly due to inaccuracies in rearing, errors in identifications, and difficulties in interpreting names given by some authors in the past, as the taxonomic meaning of a name often changes over time or according to the author [69,70,88]. Most of the data comes from the investigation of the EGVM parasitoids in vineyards, as from the beginning this moth has been associated with the grapevine, showing its potential as a pest since the second half of 19th [69]. On the other hand, despite being considered the most important host plant of the EGVM and suggested as its original host [31,32], few Italian studies tried to highlight the population dynamics of this moth and its parasitoids on the spurge flax, D. gnidium [62,64]. In Italy, studies have been conducted in Tuscany [32,59,62,63,64], Apulia [60], and Sardinia [61], for a total of 28 (morpho)-species of parasitoids associated with EGVM developing on D. gnidium (Table 3). One of the major difficulties in identifying the correct host-parasitoid associations in a natural ecosystem is the coexistence of several lepidopteran species in the same microhabitat, which can be attacked in turn by unspecific parasitoids that exploit the habitat rather than the host [89]. An earlier investigation by Scaramozzino et al. [64] in the same area showed that the nests on sprouts of D. gnidium can be formed also by Cacoecimorpha pronubana (Hübner), a tortricid moth whose young larval stages can be confused with those of the EGVM. In addition, sprouts of D. gnidium often host a large community of moth larvae, belonging to several different species (e.g., leaf rollers such as Anchinia cristalis (Scopoli)), inquiline species such as Cryptoblabes gnidiella (Millère) or Gymnoscelis rufifasciata (Haworth), leaf-miners such as Phyllobrostis fregenella Hartig [64]. It follows that associating a parasitoid with its right host can be a difficult and time-consuming task [64,89].

Table 3.
Parasitoids of preimaginal stages of the European grapevine moth, Lobesia botrana, found on Daphne gnidium (D.g.) and Vitis vinifera (V.v.) in different Italian regions, based on main previous studies on the topic (references given in square brackets; black squares indicate presence). Species are divided into guilds as proposed by Mills [83]. Lifestyles: I, idiobiont; K, koinobiont; G, gregarious, H, hyperparasitoid; FH, facultative hyperparasitoid.
The species diversity of parasitoids emerged from nests of EGVM on D. gnidium is slightly higher, although comparable, to that of the parasitoid community known from the few studies of EGVM parasitoids carried out in Tuscan vineyards [47], while the high prevalence of this plant in the Regional Reserve of Migliarino, San Rossore, Massaciuccoli made it possible to obtain much more information than in previous studies carried out on D. gnidium in Apulia and Sardinia [60,61]. Although a strict comparison between natural environment and vineyard is not possible, as a general trend, the parasitoid complex of EGVM in Tuscan vineyards is quite similar to what observed on D. gnidium, with many species in common between the two ecosystems, e.g., C. capitator Aubert, 1960 (Ichneumonidae), T. pallipes Holmgren, 1873 (Ichneumonidae), A. quadridentata Wesmael, 1835 (Braconidae), Habrobracon pillerianae Fisher, 1980 (Braconidae), T. evanescens Westwood, 1833 (Trichogrammatidae) and Phytomyptera nigrina (Meigen, 1824) (Tachinidae). In addition, some sporadic species have been recorded both in vineyards and on D. gnidium, such as species of the genus Exochus (Ichneumonidae) or representatives of the genera Itoplectis (Ichneumonidae). Of note, few relevant species obtained in Tuscan vineyards [47] were not recorded in our study, i.e., Elachertus affinis Masi, 1911 (Eulophidae), Dibrachys affinis Masi 1907 (Pteromalidae) and Dicaelotus inflexus Thomson, 1891 (Ichneumonidae). This is probably due to an underestimation of the pupal parasitoid guild in our study; this component is usually sampled in vineyards, as the overwintering cocoons of EGVM can be easily found under the bark of vines or collected using rag-traps on vine stumps [42,44]. During the first two generations of the moth, the number of EGVM chrysalids on fronds of D. gnidium was quite low, while we miss to find overwintering pupae of the third generation on the plant, likely since the EGVM full-grown larvae leave the plant and pupate in the soil.
In our sampling, C. capitator resulted the most abundant parasitoid in all the three years, covering the 53%, 72% and 86% of the parasitoid community in 2014, 2015, and 2017, respectively. It was also the only parasitoid with a 100% occupancy in the selected area, proving to be a structural member of the community [81]. C. capitator is a Mediterranean species [87,88], widespread in most of the southern European wine-growing areas, where it is one of the main if not the most efficient parasitoid of EGVM [46,47,48,50,52,70,99,100]. Beyond EGVM, its host range seems restricted to a few other tortricid species feeding on grapevine, such as Eupoecilia ambiguella (Hübner, 1796) and occasionally Sparganothis pilleriana (Denis and Schiffermüller, 1775) [89]. Indeed, C. capitator belongs to a group of sibling species, which are difficult to separate on a morphological basis but that have probably differentiated based on host specialisation [88]. Our data strongly support the strict association of C. capitator with EGVM, as the trend of emergency of this parasitoid during the three-year sampling clearly followed that of the EGVM and gives credit to its possible use as a biological control agent against this pest as soon as the difficulties in its mass rearing can be overcome [101].
Apart from C. capitator, a plethora of “satellite species” appear in our sample, which although not reaching the parasitisation rates of the former, seem to be stably associated with EGVM. The ichneumonid T. pallipes Holmgren was the only species, together with C. capitator, to occur every year. This parasitoid species attacks the larval stages and emerges from the pupa and, although in relatively low numbers, showed a prevalent activity during the third generation of the moth. The tachinid fly P. nigrina (Meigen) has proven to be a constant presence, being found both on D. gnidium and in the vineyards in several studies [34,40,43,47,49,59,60,61,69]. This species seems to play an important role in the natural control of summer population of EGVM, sometimes reaching high parasitisation rates on 1st or 2nd generations of the moth [34,47,49,60,61]. However, the presence of this parasitoid is restricted to the Mediterranean area, so it can only be considered a suitable candidate for biological control of EGVM in southern European vineyards [52].
So far, 12 species of Trichogramma have been reported as parasitoids of EGVM eggs [98], some of which have been employed in inundative biological control against EGVM and E. ambiguella but with contrasting results [25,102]. Interestingly, T. evanescens was the only species of the genus collected in Italian vineyards [47]; the species is active during the 1st generation of the moth, when it can reach a parasitisation rate of about 25% [47]. In France, Hommay et al. [103] observed that T. evanescens is an efficient parasitoid of EGVM eggs during the 1st generation, while T. cacoeciae becomes more common during the 2nd generation. In Turkey, two species of Trichogramma were obtained from parasitized eggs of EGVM, with T. euproctidis being the most common one [56]. Interestingly, T. cordubense was by far the most abundant species of Trichogramma in our sampling to attack EGVM eggs on D. gnidium. In addition, this species did not attack the first generation of the moth, reaching the maximum parasitisation rate at the end of the third generation of the moth in September, when the parasitoid prolonged activity leads to the attack of all remaining laid eggs.
In general, what we observed in the wild is a complex community, with the impact of the different guilds that fluctuate over seasons and change over years, according to the different environmental conditions. Typically, the percentage of egg and larval parasitism is higher during the first two EGVM generations and decreases during the ovewintering generation, which is mainly affected by larvo-pupal and pupal parasitoids [25,47]. In our research, the larval endophagous guild exploited the great majority of EGVM larvae, mainly dragged by the high prevalence of C. capitator. However, when the parasitisation rate of this guild was lower (as in 2014), the incidence of the larval ectophagous and pupal parasitoids increased. This is consistent with the hypothesis that the members of these guilds would play a role as redundant species [104,105]. Therefore, this study highlights an extremely complex and dynamic parasitoid community; in this context, distinguishing parasitoid species that are closely associated with a host from occasional parasitoids and understand how this community varies across regions and seasons are two crucial aspects for the implementation of biological control strategies.
5. Conclusions
Eggs, larvae, and pupae of EGVM represent an abundant resource for a rich parasitoid community both in vineyards and in the wild. Despite a lack in sampling pupal parasitoids in our study, the community of parasitoids of EGVM on D. gnidium appeared highly diverse if compared to what observed in the vineyards. Some genera, as for Trichogramma or Habrobracon, were present with several species, showing off a certain degree of redundancy, with similar species exploiting the same resource and with one species that may replace another in the case of temporary or local extinctions [106]. In both ecosystems, C. capitator represents the main parasitoid of EGVM, confirming the importance of this species in the control of EGVM populations and its possible use as a biocontrol agent in vineyards [70,107,108]. Several other parasitoid species have been recorded in smaller numbers but appear to be stably associated with EGVM, suggesting that they may exert an important control on EGVM populations under local conditions.
This issue, coupled with the high number of EGVM parasitoid species in natural contexts, highlights the ecological value of wild areas as possible reservoirs from which even not common species with specific ecological requirements can move to reach surrounding cultivated areas, thus contributing to the control of economically important pests in the crops.
Author Contributions
Conceptualization, F.D.G., A.L. (Augusto Loni), R.R., A.L. (Andrea Lucchi); methodology, F.D.G., A.L. (Augusto Loni), P.L.S., R.R., A.L. (Andrea Lucchi); validation, F.D.G., R.R., G.B., A.L. (Andrea Lucchi); formal analysis, F.D.G., A.L. (Augusto Loni), P.L.S., R.R., G.B., A.L. (Andrea Lucchi); investigation, F.D.G., A.L. (Augusto Loni), P.L.S., R.R., A.L. (Andrea Lucchi); data curation, F.D.G., R.R., G.B.; writing—original draft preparation, F.D.G., A.L. (Augusto Loni), R.R., G.B., A.L. (Andrea Lucchi); writing—review and editing, F.D.G., A.L. (Augusto Loni), P.L.S., R.R., G.B., A.L. (Andrea Lucchi); visualization, F.D.G., A.L. (Augusto Loni), P.L.S., R.R., G.B., A.L. (Andrea Lucchi); supervision, G.B., A.L. (Andrea Lucchi); project administration, A.L. (Andrea Lucchi); funding acquisition, G.B., A.L. (Andrea Lucchi). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Acknowledgments
We want to thank the anonymous referees for their helpful comments on an early version of this manuscript. We are also grateful to the editors for her kind support throughout the editorial process.
Conflicts of Interest
The authors declare no competing interests.
References
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Vandermeer, J.; Whitbread, A. Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Gavin, M.C.; McCarter, J.; Berkes, F.; Mead, A.T.P.; Sterling, E.J.; Tang, R.; Turner, N.J. Effective biodiversity conservation requires dynamic, pluralistic, partnership-based approaches. Sustainability 2018, 10, 1846. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, A.; Benelli, G. Towards pesticide-free farming? Sharing needs and knowledge promotes Integrated Pest Management. Environ. Sci. Pollut. Res. 2018, 25, 13439–13445. [Google Scholar] [CrossRef] [PubMed]
- Dent, D.; Binks, R.H. Insect Pest Management, 3rd ed.; CABI: Wallingford, UK, 2020; ISBN 978-1-78924-105-1. [Google Scholar]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Brühl, C.A.; Zaller, J.G. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Mancini, F.; Woodcock, B.A.; Isaac, N.J.B. Agrochemicals in the wild: Identifying links between pesticide use and declines of nontarget organisms. Curr. Opin. Environ. Sci. Health 2019, 11, 53–58. [Google Scholar] [CrossRef]
- Palma-Onetto, V.; Oliva, D.; González-Teuber, M. Lethal and oxidative stress side effects of organic and synthetic pesticides on the insect scale predator Rhyzobius lophanthae. Entomol. Gen. 2021, 345–355. [Google Scholar] [CrossRef]
- European Food Safety Authority; Medina-Pastor, P.; Triacchini, G. The 2018 European Union report on pesticide residues in food. EFSA J. 2020, 18, e06057. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef] [PubMed]
- Corcos, D.; Inclán, D.J.; Cerretti, P.; Mei, M.; Di Giovanni, F.; Birtele, D.; Rosa, P.; De Biase, A.; Audisio, P.; Marini, L. Environmental heterogeneity effects on predator and parasitoid insects vary across spatial scales and seasons: A multi-taxon approach. Insect Conserv. Divers. 2017, 10, 462–471. [Google Scholar] [CrossRef]
- Rudd, H.; Vala, J.; Schaefer, V. Importance of backyard habitat in a comprehensive biodiversity conservation strategy: Aconnectivity analysis of urban green spaces. Restor. Ecol. 2002, 10, 368–375. [Google Scholar] [CrossRef]
- Tscharntke, T.; Rand, T.A.; Bianchi, F.J. The landscape context of trophic interactions: Insect spillover across the crop—Noncrop interface. Ann. Zool. Fenn. 2005, 42, 421–432. [Google Scholar]
- Weibull, A.-C.; Bengtsson, J.; Nohlgren, E. Diversity of butterflies in the agricultural landscape: The role of farming system and landscape heterogeneity. Ecography 2000, 23, 743–750. [Google Scholar] [CrossRef]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.-L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Miyashita, T.; Chishiki, Y.; Takagi, S.R. Landscape heterogeneity at multiple spatial scales enhances spider species richness in an agricultural landscape. Popul. Ecol. 2012, 54, 573–581. [Google Scholar] [CrossRef]
- Bertrand, C.; Burel, F.; Baudry, J. Spatial and temporal heterogeneity of the crop mosaic influences carabid beetles in agricultural landscapes. Landsc. Ecol. 2016, 31, 451–466. [Google Scholar] [CrossRef]
- Martin, E.A.; Seo, B.; Park, C.-R.; Reineking, B.; Steffan-Dewenter, I. Scale-dependent effects of landscape composition and configuration on natural enemy diversity, crop herbivory, and yields. Ecol. Appl. 2016, 26, 448–462. [Google Scholar] [CrossRef]
- Romano, B.; Zullo, F. Landscape fragmentation in Italy dices implementation to support territorial policies. In Planning Support Tools: Policy Analysis, Implementation and Evaluation; Campagna, M., De Montis, A., Isola, F., Lai, S., Pira, C., Zoppi, C., Eds.; Franco Angeli: Milano, Italy, 2012; pp. 399–414. [Google Scholar]
- Godone, D.; Garbarino, M.; Sibona, E.; Garnero, G.; Godone, F. Progressive fragmentation of a traditional Mediterranean landscape by hazelnut plantations: The impact of CAP over time in the Langhe region (NW Italy). Land Use Policy 2014, 36, 259–266. [Google Scholar] [CrossRef]
- Pili, S.; Serra, P.; Salvati, L. Landscape and the city: Agro-forest systems, land fragmentation and the ecological network in Rome, Italy. Urban. For. Urban. Green. 2019, 41, 230–237. [Google Scholar] [CrossRef]
- Ioriatti, C.; Anfora, G.; Tasin, M.; De Cristofaro, A.; Witzgall, P.; Lucchi, A. Chemical ecology and management of Lobesia botrana (Lepidoptera: Tortricidae). J. Econ. Entomol. 2011, 104, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Thiéry, D.; Louâpre, P.; Muneret, L.; Rusch, A.; Sentenac, G.; Vogelweith, F.; Iltis, C.; Moreau, J. Biological protection against grape berry moths. A review. Agron. Sustain. Dev. 2018, 38, 15. [Google Scholar] [CrossRef]
- Ioriatti, C.; Lucchi, A.; Varela, L.G. Grape berry moths in Western European vineyards and their recent movement into the New World. In Arthropod Management in Vineyards: Pests, Approaches, and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 339–359. ISBN 978-94-007-4032-7. [Google Scholar]
- Cooper, M.; Varela, L.; Smith, R.; Whitmer, D.; Simmons, G.; Lucchi, A.; Broadway, R.; Steinhauer, R. Growers, scientists and regulators collaborate on European grapevine moth program. Cal. Ag. 2014, 68, 125–133. [Google Scholar] [CrossRef][Green Version]
- CABI Lobesia botrana (European Grapevine Moth). Available online: https://www.cabi.org/isc/datasheet/42794 (accessed on 1 March 2022).
- Marchal, P. Rapport sur les Travaux Accomplis par la Mission D’étude de la Cochylis et de l’Eudémis PENDANT l’année 1911; Ch. Béranger: Paris et Liège, France, 1912. [Google Scholar]
- Maher, N.; Thiéry, D. Daphne gnidium, a possible native host plant of the European grapevine moth Lobesia botrana, stimulates its oviposition. Is a host shift relevant? Chemoecology 2006, 16, 135–144. [Google Scholar] [CrossRef]
- Lucchi, A.; Santini, L. Life history of Lobesia botrana on Daphne gnidium in a Natural Park of Tuscany. IOBC/WPRS Bull. 2011, 67, 197–202. [Google Scholar]
- Tasin, M.; Lucchi, A.; Ioriatti, C.; Mraihi, M.; De Cristofaro, A.; Boger, Z.; Anfora, G. Oviposition response of the moth Lobesia botrana to sensory cues from a host plant. Chem. Senses 2011, 36, 633–639. [Google Scholar] [CrossRef]
- Laccone, G. Prove di lotta contro Lobesia botrana (Schiff.) (Lepid.—Tortricidae) e determinazione della soglia economica sulle uve da tavola in Puglia. Ann. Della Fac. Di Agraria. Dell’università Di Bari 1978, 30, 717–746. [Google Scholar]
- Moleas, T. Essais de lutte dirigée contre la Lobesia botrana Schiff. dans les Pouilles (Italie). In Proceedings of the International Symposium of IOBC/WPRS on Integrated Control in Agriculture and Forestry, Vienna, Austria, 8–12 October 1979; Russ, K., Berger, H., Eds.; 1979; pp. 542–551. [Google Scholar]
- Delrio, G.; Luciano, P.; Prota, R. Researches on grape-vine moths in Sardinia. In Proceedings of the Meeting of E. C. Experts Group “Integrated Pest Control in Viticulture”, Portoferraio, Italy, 26–28 September 1985; Cavalloro, R., Ed.; 1987; pp. 57–67. [Google Scholar]
- Zangheri, S.; Dalla Montà, L.; Duso, C. Observations on biology and control of grape moths in Venetia. In Proceedings of the Meeting of E. C. Expert Group “Integrated Pest Control in Viticulture”, Portoferraio, Italy, 26–28 September 1985; Cavalloro, R., Ed.; 1987; pp. 27–37. [Google Scholar]
- Pinna, M.; Gremo, F.; Scaramozzino, P.L. A preliminary investigation into the influence of biotic and abiotic environmental factors on the winter populations of Lobesia botrana (Den. & Schiff.) in veniyards in Piedmont, Italy (Lepidoptera—Tortricidae). In Proceedings of the Influence of Environmental Factors on the Control of Grape Pests, Diseases and Weeds, Thessaloniki, Greece, 6–8 October 1987; Cavalloro, R., Ed.; 1989; pp. 77–86. [Google Scholar]
- Forti, D. Resultats preliminaires sur l’activité des parasites du ver de la grappe (Lobesia botrana Schiff.) dans le Trentine. Bull. OILB/SROP 1991, XV, 9. [Google Scholar]
- Marchesini, E.; Dalla Montà, L. Observations on natural enemies of Lobesia botrana (Den. & Schiff.) (Lepidoptera Tortricidae) in Venetian vineyards. Boll. Zool. Agr. Bachic. 1994, 26, 201–230. [Google Scholar]
- Roat, C.; Forti, D. Indagine sulla parassitizzazione della tignoletta della vite (Lobesia botrana Den. & Schiff.) in Trentino. Boll. ISMA 1994, 1, 37–41. [Google Scholar]
- Pérez, M.I.; Sáenz de Cabezón, F.J.; Marco, V. Evaluation of natural parasitism on hibernating pupae of the European grape moth (Lobesia botrana Den. & Schiff.) in vineyard of La Rioja. Boletín De Sanid. Veg. Plagas 2000, 26, 715–721. [Google Scholar]
- Colombera, S.; Alma, A.; Arzone, A. Comparison between the parasitoids of Lobesia botrana and Eupoecilia ambiguella in conventional and integrated vineyards. IOBC/WPRS Bull. 2001, 24, 91–96. [Google Scholar]
- Ribeiro, J.J.A.; Martins, F.M.; Mendonça, T.R.; Lavadinho, A.M.P. Natural parasitism of Lobesia botrana during the hibernation period in the Region of “Vinhos Verdes”. IOBC/WPRS Bull. 2001, 24, 117–120. [Google Scholar]
- Thiéry, D.; Xuéreb, A.; Villemant, C.; Sentenac, G.; Delbac, L.; Kuntzman, P. Les parasites larvaires de tordeuses de vignobles: Aperçu de quelques espèces présentes dans 3 régions viticoles françaises. IOBC/WPRS Bull. 2001, 24, 135–142. [Google Scholar]
- Thiéry, D.; Xuéreb, A. Relative abundance of several larval parasitoids of Lobesia botrana on different varieties of grapes. IOBC/WPRS Bull. 2003, 26, 147–150. [Google Scholar]
- Bagnoli, B.; Lucchi, A. Parasitoids of Lobesia botrana (Den. & Schiff.) in Tuscany. IOBC/WPRS Bulletin 2006, 29, 139–142. [Google Scholar]
- Carlos, C.R.; Costa, J.R.; Tão, C.B.; Alves, F.; Torres, L.M. Parasitismo associado à traça-da-uva, Lobesia botrana (Denis & Schiffermüller) na Região Demarcada do Douro. Bol. San. Veg. Plagas 2006, 32, 355–362. [Google Scholar]
- Thiéry, D.; Yoshida, T.; Guisset, M. Phytomyptera nigrina (Meigen), a parasite of first generation European grapevine moth larvae in several vineyards in the Roussillon area. Tachinid Times 2006, 19, 1–4. [Google Scholar]
- Xuéreb, A.; Thiéry, D. Does natural larval parasitism of Lobesia botrana (Lepidoptera: Tortricidae) vary between years, generation, density of the host and vine cultivar? Bull. Entomol. Res. 2006, 96, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bărbuceanu, D.; Jenser, G. The parasitoid complex of Lobesia botrana (Denis et Schiffermüller) (Lep.: Tortricidae) in some vineyards of southern Romania. Acta Phytopathol. Et Entomol. Hung. 2009, 44, 177–184. [Google Scholar] [CrossRef]
- Moreau, J.; Villemant, C.; Benrey, B.; Thiéry, D. Species diversity of larval parasitoids of the European grapevine moth (Lobesia botrana, Lepidoptera: Tortricidae): The influence of region and cultivar. Biol. Control. 2010, 54, 300–306. [Google Scholar] [CrossRef]
- Akbarzadeh, S.G. Larval parasitoids of Lobesia botrana (Denis and Schiffermüller, 1775) (Lepidoptera: Tortricidae) in Orumieh vineyards. J. Agr. Sci. Tech. 2012, 14, 267–274. [Google Scholar]
- Lotfalizadeh, H.; Masnadi-Yazdinejad, A.; Saber, M. New records of the grape berry moth hymenopterous parasitoids in Iran. Mun. Ent. Zool. 2012, 7, 284–291. [Google Scholar]
- Carlos, C.; Gonçalves, F.; Sousa, S.; Salvação, J.; Sharma, L.; Soares, R.; Manso, J.; Nóbrega, M.; Lopes, Á.; Soares, S.; et al. Environmentally safe strategies to control the European Grapevine Moth, Lobesia botrana (Den. & Schiff.), in the Douro Demarcated Region. Ciência E Técnica Vitivinícola 2013, 28, 1006–1011. [Google Scholar]
- Ozsemerci, F.; Altindisli, F.O.; Koclu, T.; Karsavuran, Y. Egg parasitoids of Lobesia botrana (Den. & Schiff.) (Lepidoptera: Tortricidae) in the vineyards of Izmir and Manisa Provinces in Turkey. BIO Web Conf. 2016, 7, 1–4. [Google Scholar] [CrossRef]
- Shapira, I.; Keasar, T.; Harari, A.R.; Gavish-Regev, E.; Kishinevsky, M.; Steinitz, H.; Sofer-Arad, C.; Tomer, M. Does mating disruption of Planococcus ficus and Lobesia botrana affect the diversity, abundance and composition of natural enemies in Israeli vineyards? Pest. Manag. Sci. 2018, 74, 1837–1844. [Google Scholar] [CrossRef]
- Carlos, C.; Gonçalves, F.; Villemant, C.; Paredes, D.; Salvação, J.; Torres, L. Parasitoids of Lobesia botrana (Lepidoptera: Tortricidae) in Douro Demarcated Region vineyards and prospects for enhancing conservation biological control. Bull. Entomol. Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Scaramozzino, P.L.; Di Giovanni, F.; Loni, A.; Gisondi, S.; Lucchi, A.; Cerretti, P. Tachinid (Diptera, Tachinidae) parasitoids of Lobesia botrana (Denis & Schiffermüller, 1775) (Lepidoptera, Tortricidae) and other moths. ZooKeys 2020, 934, 111–140. [Google Scholar] [CrossRef]
- Nuzzaci, G.; Triggiani, O. Note sulla biocenosi in Puglia della Lobesia (Polychrosis) botrana (Schifi.) (Lepidoptera: Tortricidae) infeudata a Daphne gnidium L. Entomologica 1982, 17, 47–52. [Google Scholar] [CrossRef]
- Luciano, P.; Delrio, G.; Prota, R. Osservazioni sulle popolazioni di Lobesia botrana (Den. & Schiff.) su Daphne gnidium L. in Sardegna. Atti XV Congr. Naz. Ital. Di Entomol. 1988, 15, 543–548. [Google Scholar]
- Loni, A.; Samartsev, K.G.; Scaramozzino, P.L.; Belokobylskij, S.A.; Lucchi, A. Braconinae parasitoids (Hymenoptera, Braconidae) emerged from larvae of Lobesia botrana (Denis & Schiffermüller) (Lepidoptera, Tortricidae) feeding on Daphne gnidium L. ZooKeys 2016, 587, 125–150. [Google Scholar] [CrossRef]
- Lucchi, A.; Scaramozzino, P.L.; Michl, G.; Loni, A.; Hoffmann, C. The first record in Italy of Trichogramma cordubense Vargas & Cabello 1985 (Hymenoptera Trichogrammatidae) emerging from the eggs of Lobesia botrana (Denis & Schiffermüller, 1775) (Lepidoptera Tortricidae). VITIS—J. Grapevine Res. 2016, 55, 161–164. [Google Scholar] [CrossRef]
- Scaramozzino, P.L.; Loni, A.; Gandini, L.; Lucchi, A. Who’s who in the nests molded by Lobesia botrana on Daphne gnidium? IOBC/WPRS Bull. 2017, 128, 130–134. [Google Scholar]
- Federici, P.R. Atlante Tematico Della Provincia di Pisa; Pacini Editore: Pisa, Italy, 2003; p. 80. [Google Scholar]
- Bertacchi, A. Dune habitats of the Migliarino—San Rossore—Massaciuccoli Regional Park (Tuscany—Italy). J. Maps 2017, 13, 322–331. [Google Scholar] [CrossRef]
- Bertacchi, A.; Lombardi, T. Aspetti botanici delle pinete litoranee toscane. In Le Pinete Litoranee Come Patrimonio Culturale; Atti della Accademia dei Georgofili. Suppl.; Edizioni Polistampa: Firenze, Italy, 2019; pp. 59–68. [Google Scholar]
- Logli, F. Le pinete del Parco Migliarino San Rossore Massaciuccoli. In Le Pinete Litoranee Come Patrimonio Culturale; I Atti della Accademia dei Georgofili. Suppl.; Edizioni Polistampa: Firenze, Italy, 2019; pp. 43–52. [Google Scholar]
- Scaramozzino, P.L.; Loni, A.; Lucchi, A. A review of insect parasitoids associated with Lobesia botrana (Denis & Schiffermüller, 1775) in Italy. 1. Diptera Tachinidae and Hymenoptera Braconidae (Lepidoptera, Tortricidae). ZooKeys 2017, 647, 67–100. [Google Scholar] [CrossRef]
- Scaramozzino, P.L.; Di Giovanni, F.; Loni, A.; Ricciardi, R.; Lucchi, A. Updated list of the insect parasitoids (Insecta, Hymenoptera) associated with Lobesia botrana (Denis & Schiffermüller, 1775) (Lepidoptera, Tortricidae) in Italy. 2. Hymenoptera, Ichneumonidae, Anomaloninae and Campopleginae. ZooKeys 2018, 772, 47–95. [Google Scholar] [CrossRef]
- Huddleston, T. The Palaearctic species of Ascogaster (Hymenoptera: Braconidae). Bull. Br. Mus. Nat. Hist., Ent. 1984, 49, 341–392. [Google Scholar]
- Peck, O.; Bouček, Z.; Hoffer, A. Keys to the Chalcidoidea of Czechoslovakia (Insecta: Hymenoptera). Mem. Ent. Soc. Can. 1964, S34, 7–121. [Google Scholar] [CrossRef]
- Cerretti, P. I Tachinidi Della Fauna Italiana (Diptera, Tachinidae), con Chiave Interattiva dei Generi Ovest-Paleartici; Centro Nazionale Biodiversità Forestale, Cierre Edizioni: Verona, Italy, 2010; p. 912. [Google Scholar]
- Aeschlimann, J.P. Revision des espèces ouest-palearctiques du genre Triclistus Förster (Hymenoptera: Ichneumonidae). Mitt. Schweiz. Entomol. Ges. 1973, 46, 219–252. [Google Scholar]
- Kasparyan, D.R. A review of the Palearctic ichneumonids of the tribe Pimplini (Hymenoptera, Ichneumonidae). The genera Itoplectis Foerst. and Apechthis Foerst. Entomol. Obozr. 1973, 52, 665–681. [Google Scholar]
- Kasparyan, D.R. A review of the Palearctic ichneumonids of the tribe Pimplini (Hymenoptera, Ichneumonidae). The genus Pimpla Fabricius. Entomol. Obozr. 1974, 53, 102–117. [Google Scholar]
- Gauld, I.D.; Mitchell, P.A. Handbooks for the Identification of British Insects; Vol. VII. Part 2.; Ichneumonidae Orthopelmatinae & Anomaloninae, Royal Entomological Society of London: London, UK, 1977; p. 32. [Google Scholar]
- Horstmann, K. Revision der mit difformis (Gmelin, 1790) verwandten westpaläarktischen Arten der Gattung Campoplex Gravenhorst, 1829 (Hymenoptera, Ichneumonidae). Entomofauna 1985, 6, 129–163. [Google Scholar]
- Tolkanitz, V.I. Ichneumon flies of the genus Exochus Gravenhorst (Hymenoptera: Ichneumonidae: Metopiinae) of the fauna of Palearctic region. Russ. Entomol. J. 2007, 16, 339–358. [Google Scholar]
- Sumer, F.; Tuncbilek, A.S.; Oztemiz, S.; Pintureau, B.; Rugman-Jones, P.F.; Stouthamer, R. A molecular key to the common species of Trichogramma of the Mediterranean region. BioControl 2009, 54, 617–624. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Elsevier/Academic Press: Burlington, NJ, USA, 2006; p. 324. [Google Scholar]
- Gaston, J.K.; He, F. Species occurrence and occupancy. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 141–151. [Google Scholar]
- Mills, N.J. Parasitoid guilds: Defining the structure of the parasitoid communities ofendopterygote insect hosts. Environ. Entomol. 1994, 23, 1066–1083. [Google Scholar] [CrossRef]
- Thompson, W.R. A Catalogue of the Parasites and Predators of Insect Pest; Section 1. Parasite Host Catalogue, Part. 8. Parasites of the Lepidoptera (N-P); Commonwealth Institute of Biological Control, Imperial Agricultural Bureaux, Parasite Service: Belleville, ON, Canada, 1946. [Google Scholar]
- Coscollá, R. La polilla del racimo de la vid (Lobesia botrana Den. y Schiff.). In Consejerfa de Agricultura, Pesca y Alimentación; Generalitat Valenciana: Valencia, Spain, 1997; p. 613. [Google Scholar]
- Hoffmann, C.; Michl, G. Parasitoide von Traubenwicklern—Ein Werkzeug der naturlichen Schadlingsregulation? Dtsch. Weinbaujahrbuch 2003, 55, 1–13. [Google Scholar]
- Yu, D.S.; van Achterberg, C.; Horstmann, K. Taxapad 2016. World Ichneumonoidea (2015). Taxonomy, Biology, Morphology and Distribution; Nepean: Ottawa, ON, Canada, 2016. [Google Scholar]
- Di Giovanni, F.; Scaramozzino, P.L.; Loni, A.; Lucchi, A. Taxonomic revision of the Campoplex difformis group (Ichneumonidae, Campopleginae), with particular reference to species of economic importance. Eur. J. Taxon. 2021, 740, 1–35. [Google Scholar] [CrossRef]
- Villemant, C.; Delvare, G.; Martinez, M.; Sentenac, G.; Kuntzman, P. Parasitoïdes de tordeuses. In La Faune Auxiliaire des Vignobles de France; Sentenac, G., Ed.; Editions France Agricole: Paris, France, 2011; p. 470. [Google Scholar]
- Gordh, G. Goniozus gallicola Fouts, parasite of moth larvae with notes on other bethylids (Hymenoptera: Bethylidae; Lepidoptera: Gelechiidae). USDA Tech. Bull. 1976, 1524, 1–27. [Google Scholar]
- Mellini, E. Studi sui ditteri larvevoridi. XII. Nemorilla maculosa Meig. su Depressaria marcella Rebel (Lepidoptera Gelechiidae). Boll. Dell’istituto Di Entomol. Dell’università Di Bologna 1964, 27, 145–169. [Google Scholar]
- Rohdendorf, B.B. Beiträge zur Kenntnis der Tachinen des Wiesenzünslers (Loxostege sticticalis L.). Izv. Akad. Nauk SSSR 1935, 5, 753–780. [Google Scholar]
- Catoni, G. Die Traubenwikler (Polychrosis botrana Schiff. und Conchylis ambiguella Hübn.) und ihre natürlichen Feinde in Südtyrol. Z. Für Angew. Entomol. 1914, 1, 248–259. [Google Scholar] [CrossRef]
- Schwangart, F. Ueber die Traubenwickler (Clysia [Conchylis] Ambiguella Hübn. und Polychrosis Botrana Schiff.) und ihre Bekämpfung, mit Berücksichtigung Natürlicher Bekämpfungsfaktoren. Zweiter Teil; Verlag von Oustav Fischer: Jena, Germany, 1913. [Google Scholar]
- Bordera, S.; Selfa, J. Datos sobre la fauna ibérica del género Bathythrix Foerster, 1868 (Hym., Ichneumonidae). Bolétin De La Asoc. Española De Entomol. 1992, 16, 193–197. [Google Scholar]
- Sawoniewicz, J. Revision of European species of the genus Bathythrix Förster (Hym., Ichneumonidae). Ann. Zool. 1980, 35, 319–365. [Google Scholar]
- Kasparyan, D.R.; Kopelke, J.P. Taxonomic review and key to European ichneumon flies (Hymenoptera, Ichneumonidae), parasitoids of gall-forming sawflies of the genera Pontania Costa, Phyllocolpa Benson, and Euura Newman (Hymenoptera, Tenthredinidae) on willows: Part I. Entomol. Rev. 2009, 89, 933–957. [Google Scholar] [CrossRef]
- Noyes, J.S. Universal Chalcidoidea Database. Available online: http://www.nhm.ac.uk/chalcidoids (accessed on 8 June 2022).
- Coscollá, R. Aproximación al estudio del parasitismo natural sobre Lobesia botrana Den. y Schiff. en las comarcas vitícolas Valencianas. Boletín De Sanid. Veg.—Plagas 1980, 6, 5–15. [Google Scholar]
- Genini, M. Antagonistes de la cicadelle verte et des vers de la grappe dans le vignoble valaisan et les milieux environnants. Rev. Suisse De Vitic. Arboric. Et Hortic. 2000, 32, 153–162. [Google Scholar]
- Lucchi, A.; Ricciardi, R.; Loni, A.; Cosci, F.; Alvarez, A.R.; Beeche, M.; Scaramozzino, P.L. Rearing Campoplex capitator Aubert in Italy and in Chile: Preliminary achievements. In Proceedings of the Future IPM 3.0 towards a Sustainable Agriculture, IOBC/WPRS General Assembly, Riva del Garda, Italy, 15–20 October 2017; p. 370. [Google Scholar]
- Ibrahim, R.A.E.M.A. Biological control of grape berry moths Eupoecilia ambiguella Hb. and Lobesia botrana Schiff. (Lepidoptera: Tortricidae) by using egg parasitoids of the genus Trichogramma. Ph.D. Thesis, Institute of Phytopathology and Applied Zoology, Justus Liebig University of Giessen, Giessen, Germany, 2004. [Google Scholar]
- Hommay, G.; Gertz, C.; Kienlen, J.C.; Pizzol, J.; Chavigny, P. Comparison between the control efficacy of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae) and two Trichogramma cacoeciae Marchal strains against grapevine moth (Lobesia botrana Den. & Schiff.), depending on their release density. Biocontrol. Sci. Technol. 2002, 12, 569–581. [Google Scholar] [CrossRef]
- Naeem, S. Species redundancy and ecosystem reliability. Conserv. Biol. 1998, 12, 7. [Google Scholar] [CrossRef]
- Mori, A.S.; Furukawa, T.; Sasaki, T. Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. 2013, 88, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Flynn, D.F.; Prager, C.M.; Hart, G.M.; DeVan, C.M.; Ahrestani, F.S.; Palmer, M.I.; Bunker, D.E.; Knops, J.M.; Jouseau, C.F.; et al. The importance of rare species: A trait-based assessment of rare species contributions to functional diversity and possible ecosystem function in tall-grass prairies. Ecol. Evol 2014, 4, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, A.; Loni, A.; Scaramozzino, P.L. Pests in the wild: Life history and behaviour of Lobesia botrana on Daphne gnidium in a natural environment (Lepidoptera Tortricidae; Malvales Thymelaeaceae). IOBC/WPRS Bull. Meet. Integr. Prot. Prod. Vitic. 2017, 128, 84–88. [Google Scholar]
- Moreau, J.; Buffenoir, N.; Thiéry, D.; Vogelweith, F. Lobesia botrana as a preferred host of Campoplex capitator, the most occurring larval parasitoid in European vineyards. Entomol. Gen. 2019, 39, 307–312. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).